Biology and Management of Dedifferentiated Liposarcoma: State of the Art and Perspectives
Abstract
1. Introduction
2. Clinical Characteristics
3. Imaging Features
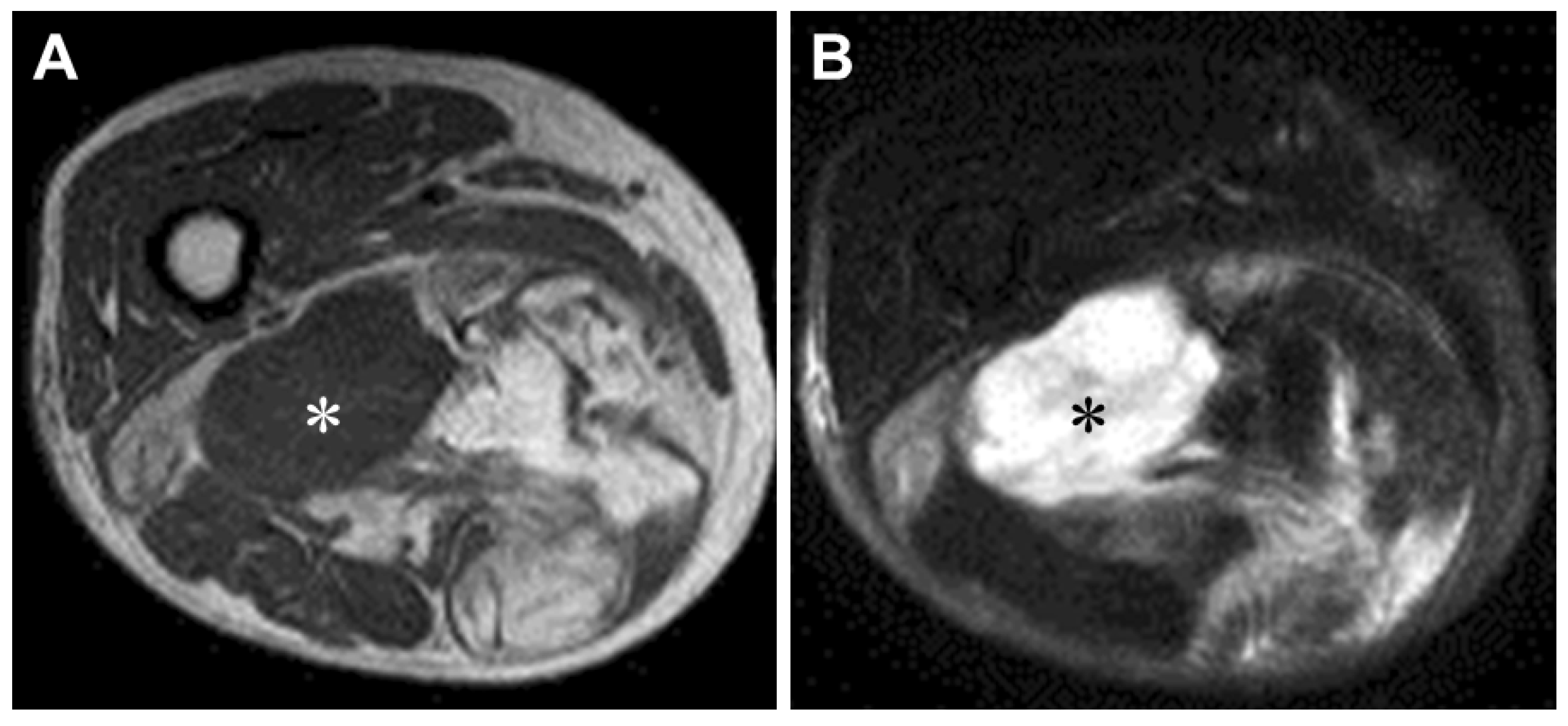
3.1. MRI
3.2. 18F-FDG PET/CT
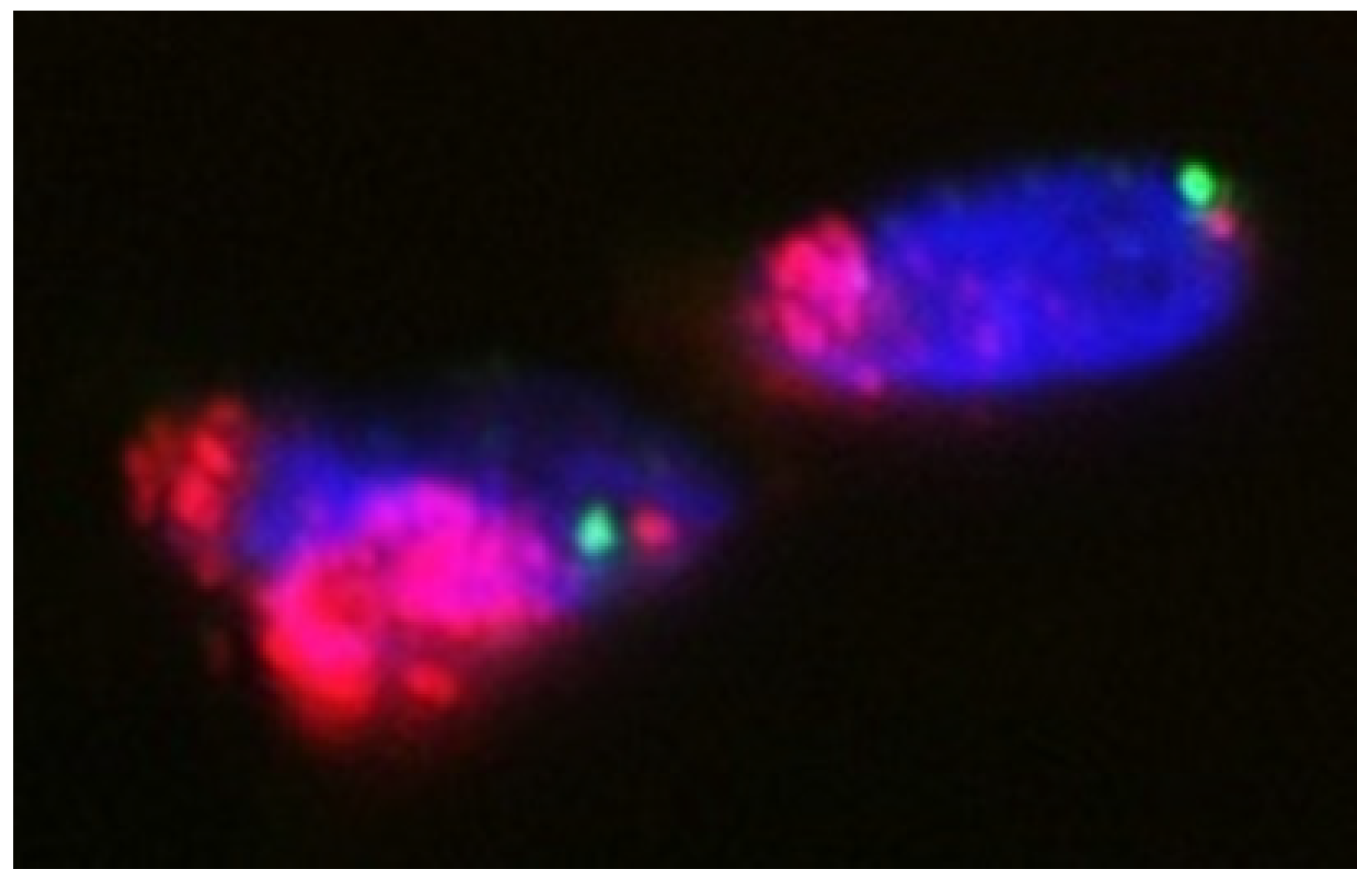
4. Pathogenesis
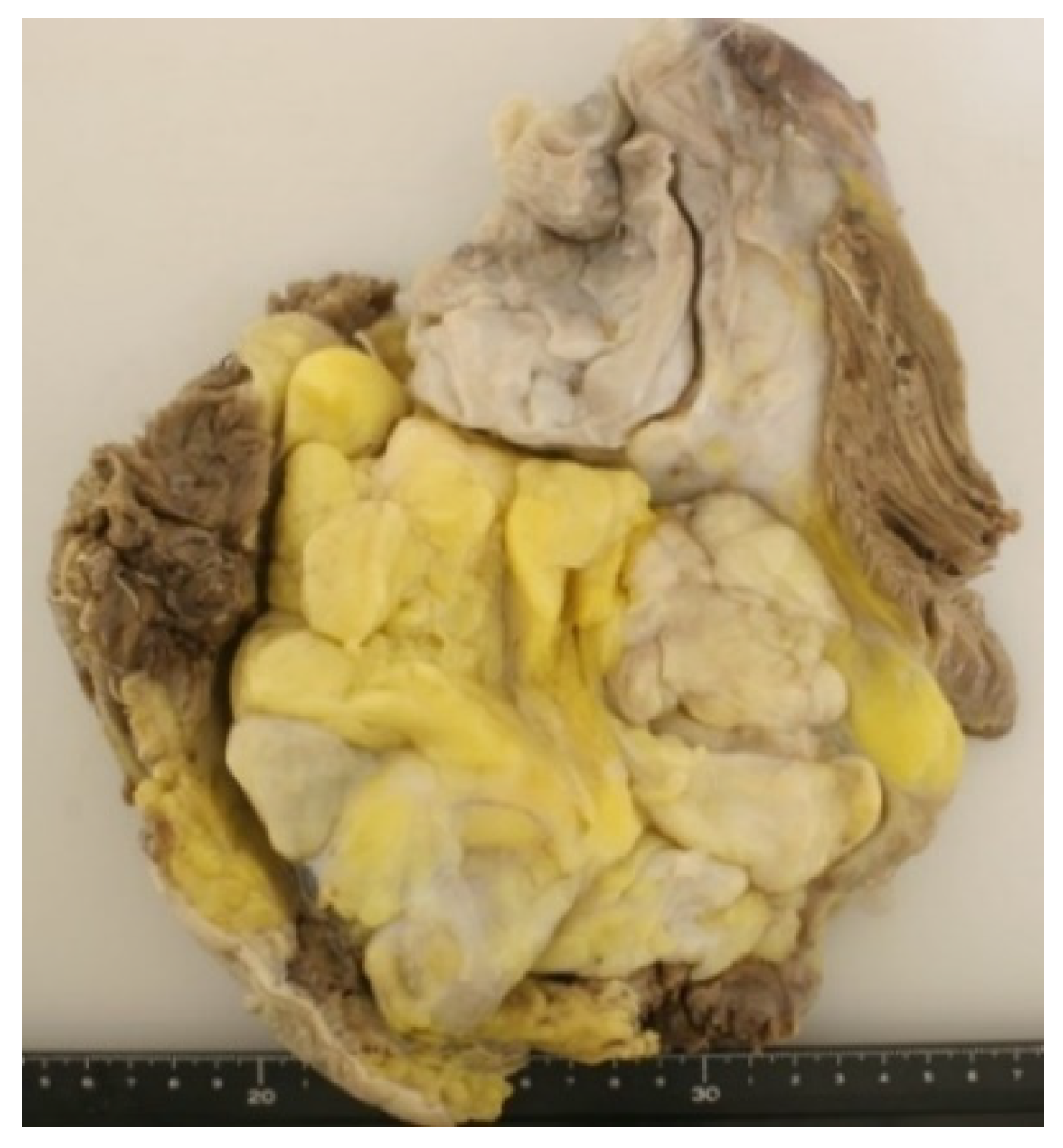
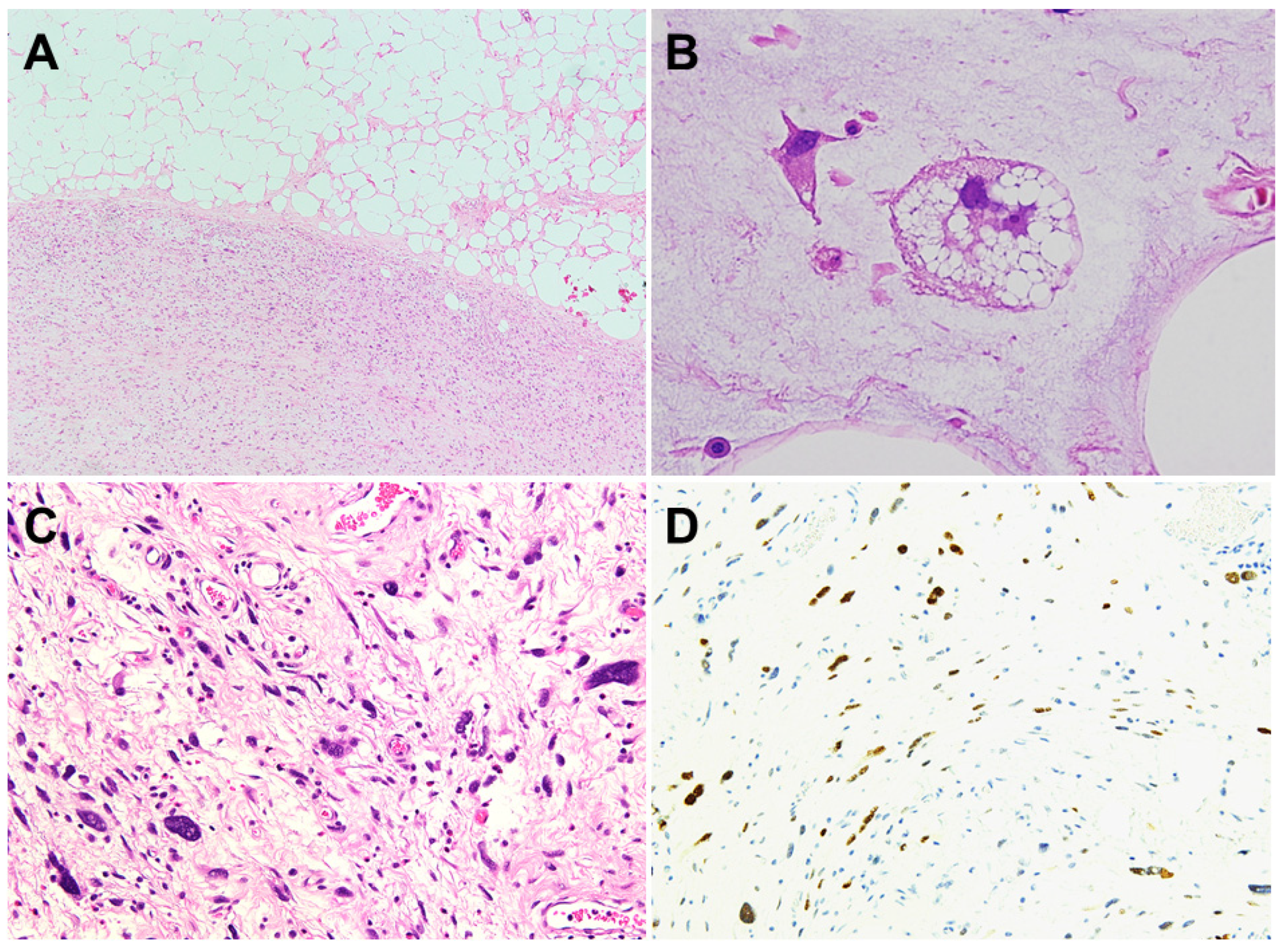
5. Histopathology
6. Management
6.1. Localized Disease
6.2. Advanced Disease
6.2.1. Anthracycline-Based Therapy
6.2.2. Pazopanib
6.2.3. Eribulin
6.2.4. Trabectedin
6.2.5. Gemcitabine and Docetaxel
6.2.6. MDM2-Thargeted Therapy
6.2.7. CDK4-Thageted Therapy
6.2.8. Exportin 1 (XPO1) Inhibitor
6.2.9. Immunotherapy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The WHO Classification of Tumors Editorial Board. WHO Classification of Tumours of Soft Tissue and Bone, 5th ed.; IARC Press: Lyon, France, 2020; pp. 36–48. [Google Scholar]
- Nishio, J. Contributions of Cytogenetics and Molecular Cytogenetics to the Diagnosis of Adipocytic Tumors. J. Biomed. Biotechnol. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Hirata, M.; Asano, N.; Katayama, K.; Yoshida, A.; Tsuda, Y.; Sekimizu, M.; Mitani, S.; Kobayashi, E.; Komiyama, M.; Fujimoto, H.; et al. Integrated exome and RNA sequencing of dedifferentiated liposarcoma. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.; Horvai, A.; Greipp, P.; John, I.; Demicco, E.G.; Dickson, B.C.; Tanas, M.; Larsen, B.T.; Din, N.U.; Creytens, D.H.; et al. Atypical lipomatous tumour/well-differentiated liposarcoma and de-differentiated liposarcoma in patients aged ≤40 years: A study of 116 patients. Histopathology 2019, 75, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Thway, K. Well-differentiated liposarcoma and dedifferentiated liposarcoma: An updated review. Semin. Diagn. Pathol. 2019, 36, 112–121. [Google Scholar] [CrossRef]
- Henricks, W.H.; Chu, Y.C.; Goldblum, J.R.; Weiss, S.W. Dedifferentiated liposarcoma: A clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am. J. Surg. Pathol. 1997, 21, 271–281. [Google Scholar] [CrossRef]
- Thway, K.; Jones, R.L.; Noujaim, J.; Zaidi, S.; Miah, A.B.; Fisher, C. Dedifferentiated liposarcoma: Updates on morphology, genetics and therapeutic strategies. Adv. Anat. Pathol. 2016, 23, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Nakata, E.; Kunisada, T.; Hasei, J.; Nakahara, R.; Yanai, H.; Toji, T.; Inoue, H.; Ozaki, T. What are the results of localized dedifferentiated liposarcomas in the extremities? Clin. Orthop. Relat. Res. 2020, 478, 2550–2561. [Google Scholar] [CrossRef]
- Okada, K.; Hasegawa, T.; Kawai, A.; Ogose, A.; Nishida, J.; Yanagisawa, M.; Morita, T.; Tajino, T.; Tsuchiya, T. Primary (de novo) dedifferentiated liposarcoma in the extremities: A multi-institution Tohoku Musculoskeletal Tumor Society study of 18 cases in northern Japan. Jpn. J. Clin. Oncol. 2011, 41, 1094–1100. [Google Scholar] [CrossRef][Green Version]
- Murphey, M.D.; Arcara, L.K.; Fanburg-Smith, J. From the archives of the AFIP: Imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. RadioGraphics 2005, 25, 1371–1395. [Google Scholar] [CrossRef]
- Parkes, A.; Urquiola, E.; Bhosale, P.; Lin, H.; Watson, K.; Wang, W.-L.; Feig, B.; Torres, K.; Roland, C.L.; Conley, A.P.; et al. PET/CT Imaging as a Diagnostic Tool in Distinguishing Well-Differentiated versus Dedifferentiated Liposarcoma. Sarcoma 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- Baffour, F.I.; Wenger, D.E.; Broski, S.M. 18F-FDG PET/CT imaging features of lipomatous tumors. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 74–82. [Google Scholar]
- Nishio, J.; Iwasaki, H.; Ishiguro, M.; Ohjimi, Y.; Fujita, C.; Ikegami, H.; Ariyoshi, A.; Naito, M.; Kaneko, Y.; Kikuchi, M. Establishment of a novel human dedifferentiated liposarcoma cell line, FU-DDLS-1: Conventional and molecular cytogenetic characterization. Int. J. Oncol. 2003, 22, 535–542. [Google Scholar]
- Nishio, J.; Aoki, M.; Nabeshima, K.; Iwasaki, H.; Naito, M. Cytogenetic and molecular cytogenetic findings in giant dedifferentiated liposarcoma of the thigh. Oncol. Lett 2012, 27, 764–768. [Google Scholar] [CrossRef]
- Nishio, J.; Iwasaki, H.; Nabeshima, K.; Naito, M. Immunohistochemical, cytogenetic, and molecular cytogenetic characterization of both components of a dedifferentiated liposarcoma: Implications for histogenesis. Anticancer Res. 2015, 35, 345–350. [Google Scholar] [PubMed]
- Sciot, R. MDM2 Amplified Sarcomas: A Literature Review. Diagnostics 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Creytens, D.; Van Gorp, J.; Speel, E.-J.; Ferdinande, L. Characterization of the 12q amplicons in lipomatous soft tissue tumors by multiplex ligation-dependent probe amplification-based copy number analysis. Anticancer. Res. 2015, 35, 1835–1842. [Google Scholar] [PubMed]
- Italiano, A.; Bianchini, L.; Keslair, F.; Bonnafous, S.; Cardot-Leccia, N.; Coindre, J.-M.; Dumollard, J.-M.; Hofman, P.; Leroux, A.; Mainguené, C.; et al. HMGA2 is the partner of MDM2 in well-differentiated and dedifferentiated liposarcomas whereas CDK4 belongs to a distinct inconsistent amplicon. Int. J. Cancer 2008, 122, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Barretina, J.; Taylor, B.S.; Banerji, S.; Ramos, A.H.; Lagos-Quintana, M.; DeCarolis, P.L.; Shah, K.; Socci, N.D.; Weir, B.A.; Ho, A.; et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat. Genet. 2010, 42, 715–721. [Google Scholar] [CrossRef]
- Kanojia, D.; Nagata, Y.; Garg, M.; Lee, D.H.; Sato, A.; Yoshida, K.; Sato, Y.; Sanada, M.; Mayakonda, A.; Bartenhagen, C.; et al. Genomic landscape of liposarcoma. Oncotarget 2015, 6, 42429–42444. [Google Scholar] [CrossRef]
- Lee, A.T.J.; Thway, K.; Huang, P.; Jones, R.L. Clinical and Molecular Spectrum of Liposarcoma. J. Clin. Oncol. 2018, 36, 151–159. [Google Scholar] [CrossRef]
- Tap, W.D.; Eilber, F.C.; Ginther, C.; Dry, S.M.; Reese, N.; Barzan-Smith, K.; Chen, H.W.; Wu, H.; Eilber, F.R.; Slamon, D.J.; et al. Evaluation of well-differentiated/de-differentiated liposarcomas by high-resolution oligonucleotide array-based comparative genomic hybridization. Genes Chromosomes Cancer 2011, 50, 95–112. [Google Scholar] [CrossRef]
- Mariani, O.; Brennetot, C.; Coindre, J.M.; Gruel, N.; Ganem, C.; Delattre, O.; Stern, M.H.; Aurias, A. JUN oncogene amplification and overexpression block adipocytic differentiation in highly aggressive sarcomas. Cancer Cell 2007, 11, 361–374. [Google Scholar] [CrossRef]
- Chibon, F.; Mariani, O.; Derré, J.; Mairal, A.; Coindre, J.-M.; Guillou, L.; Sastre, X.; Pédeutour, F.; Aurias, A. ASK1(MAP3K5) as a potential therapeutic target in malignant fibrous histiocytomas with 12q14-q15 and 6q23 amplifications. Genes Chromosom. Cancer 2004, 40, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Yoshida, A.; Mitani, S.; Kobayashi, E.; Shiotani, B.; Komiyama, M.; Fujimoto, H.; Chuman, H.; Morioka, H.; Matsumoto, M.; et al. Frequent amplification of receptor tyrosine kinase genes in welldifferentiated/dedifferentiated liposarcoma. Oncotarget 2017, 8, 12941–12952. [Google Scholar] [CrossRef] [PubMed]
- Demicco, E.G. Molecular updates in adipocytic neoplasms. Semin. Diagn. Pathol. 2019, 36, 85–94. [Google Scholar] [CrossRef]
- El Azzouzi, H.; Leptidis, S.; Dirkx, E.; Hoeks, J.; Van Bree, B.; Brand, K.; McClellan, E.A.; Poels, E.; Sluimer, J.C.; Van Den Hoogenhof, M.M.; et al. The hypoxia-inducible microRNA cluster miR-199a~214 targets myocardial PPAR γ and impairs mitochondrial fatty acid oxidation. Cell Metab. 2013, 18, 341–354. [Google Scholar] [CrossRef]
- Gronchi, A.; Collini, P.; Miceli, R.; Valeri, B.; Renne, S.L.; Dagrada, G.; Fiore, M.; Sanfilippo, R.; Barisella, M.; Colombo, C.; et al. Myogenic differentiation and histologic grading are major prognostic determinants in retroperitoneal liposarcoma. Am. J. Surg. Pathol. 2015, 39, 383–393. [Google Scholar] [CrossRef]
- Kurzawa, P.; Mullen, J.T.; Chen, Y.L.; Johnstone, S.E.; Deshpande, V.; Chebib, I.; Nielsen, G.P. Prognostic Value of Myogenic differentiation in dedifferentiated liposarcoma. Am. J. Surg. Pathol. 2020, 44, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Yoon, R.S.; Benevenia, J.; Beebe, K.S.; Hameed, M. Dedifferentiated liposarcoma of the thigh with chondrosarocomatous dedifferentiated component. Am. J. Orthop. 2010, 39, E114–E118. [Google Scholar] [PubMed]
- Nascimento, A.G.; Kurtin, P.J.; Guillou, L.; Fletcher, C.D. Dedifferentiated liposarcoma: A report of nine cases with a peculiar neurallike whorling pattern associated with metaplastic bone formation. Am. J. Surg. Pathol. 1998, 22, 945–955. [Google Scholar] [CrossRef]
- Fanburg-Smith, J.C.; Miettinen, M. Liposarcoma with meningothelial-like whorls: A study of 17 cases of a distinctive histological pattern associated with dedifferentiated liposarcoma. Histopathology 1998, 33, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Binh, M.B.; Sastre-Garau, X.; Guillou, L.; De Pinieux, G.; Terrier, P.; Lagacé, R.; Aurias, A.; Hostein, I.; Coindre, J.M. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: A comparative analysis of 559 soft tissue neoplasms with genetic data. Am. J. Surg. Pathol. 2005, 29, 1340–1347. [Google Scholar] [CrossRef]
- Kammerer-Jacquet, S.F.; Thierry, S.; Cabillic, F.; Lannes, M.; Burtin, F.; Henno, S.; Dugay, F.; Bouzillé, G.; Rioux-Leclercq, N.; Belaud-Rotureau, M.A.; et al. Differential diagnosis of atypical lipomatous tumor/well-differentiated liposarcoma and dedifferentiated liposarcoma: Utility of p16 in combination with MDM2 and CDK4 immunohistochemistry. Hum. Pathol. 2017, 59, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Thway, K.; Flora, R.; Shah, C.; Olmos, D.; Fisher, C. Diagnostic Utility of p16, CDK4, and MDM2 as an Immunohistochemical Panel in Distinguishing Well-differentiated and Dedifferentiated Liposarcomas From Other Adipocytic Tumors. Am. J. Surg. Pathol. 2012, 36, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Suster, S.; Fisher, C. Immunoreactivity for the Human Hematopoietic Progenitor Cell Antigen (CD34) in Lipomatous Tumors. Am. J. Surg. Pathol. 1997, 21, 195–200. [Google Scholar] [CrossRef]
- Weaver, J.; Downs-Kelly, E.; Goldblum, J.R.; Turner, S.; Kulkarni, S.; Tubbs, R.R.; Rubin, B.P.; Skacel, M. Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasms. Mod. Pathol. 2008, 21, 943–949. [Google Scholar] [CrossRef]
- Kimura, H.; Dobashi, Y.; Nojima, T.; Nakamura, H.; Yamamoto, N.; Tsuchiya, H.; Ikeda, H.; Sawada-Kitamura, S.; Oyama, T.; Ooi, A. Utility of fluorescence in situ hybridization to detect MDM2 amplification in liposarcomas and their morphological mimics. Int. J. Clin. Exp. Pathol. 2013, 15, 1306–1316. [Google Scholar]
- Sirvent, N.; Coindre, J.M.; Maire, G.; Hostein, I.; Keslair, F.; Guillou, L.; Ranchere-Vince, D.; Terrier, P.; Pedeutour, F. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: Utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. Am. J. Surg. Pathol. 2007, 31, 1476–1489. [Google Scholar] [CrossRef]
- Italiano, A.; Bianchini, L.; Gjernes, E.; Keslair, F.; Ranchere-Vince, D.; Dumollard, J.M.; Haudebourg, J.; Leroux, A.; Mainguené, C.; Terrier, P.; et al. Clinical and biological significance of CDK4 amplification in well-differentiated and dedifferentiated liposarcomas. Clin. Cancer Res. 2009, 15, 5696–5703. [Google Scholar] [CrossRef]
- Jour, G.; Gullet, A.; Liu, M.; Hoch, B.L. Prognostic relevance of Fédération Nationale des Centres de Lutte Contre le Cancer grade and MDM2 amplification levels in dedifferentiated liposarcoma: A study of 50 cases. Mod. Pathol. 2015, 28, 37–47. [Google Scholar] [CrossRef]
- Ricciotti, R.W.; Baraff, A.J.; Jour, G.; Kyriss, M.; Wu, Y.; Liu, Y.; Li, S.-C.; Hoch, B.; Liu, Y.J. High amplification levels of MDM2 and CDK4 correlate with poor outcome in patients with dedifferentiated liposarcoma: A cytogenomic microarray analysis of 47 cases. Cancer Genet. 2017, 218–219, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, Y.J.; Kwon, M.J.; Choi, D.I.; Lee, J.; Cho, J.; Seo, S.W.; Kim, S.J.; Shin, Y.K.; Choi, Y.-L. High level of CDK4 amplification is a poor prognostic factor in well-differentiated and dedifferentiated liposarcoma. Histol. Histopathol. 2013, 29, 127–138. [Google Scholar]
- Gronchi, A.; Vullo, S.L.; Fiore, M.; Mussi, C.; Stacchiotti, S.; Collini, P.; Lozza, L.; Pennacchioli, E.; Mariani, L.; Casali, P.G. Aggressive Surgical Policies in a Retrospectively Reviewed Single-Institution Case Series of Retroperitoneal Soft Tissue Sarcoma Patients. J. Clin. Oncol. 2009, 27, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, D.P.; Rushing, C.N.; O Lane, W.; Cardona, D.; Kirsch, D.G.; Peterson, B.L.; Blazer, D.G., 3rd. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: A case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol. 2016, 17, 966–975. [Google Scholar] [CrossRef]
- Haas, R.L.M.; Bonvalot, S.; Miceli, R.; Strauss, D.C.; Swallow, C.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Callegaro, D.; Hayes, A.J.; et al. Radiotherapy for retroperitoneal liposarcoma: A report from the Transatlantic Retroperitoneal Sarcoma Working Group. Cancer 2019, 125, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Bonvalot, S.; Gronchi, A.; Le Péchoux, C.; Swallow, C.J.; Strauss, D.; Meeus, P.; Van Coevorden, F.; Stoldt, S.; Stoeckle, E.; Rutkowski, P.; et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): A multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2020, 21, 1366–1377. [Google Scholar] [CrossRef]
- Bramwell, V.H.; Anderson, D.; Charette, M.L. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft-tissue sarcoma: A meta-analysis and clinical practice guideline. Sarcoma 2000, 4, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Gahvari, Z.; Parkes, A. Dedifferentiated liposarcoma: Systemic therapy options. Curr. Treat. Options Oncol. 2020, 21, 15. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Ryan, C.W.; Merimsky, O.; Agulnik, M.; Blay, J.-Y.; Schuetze, S.M.; Van Tine, B.A.; Jones, R.L.; Elias, A.D.; Choy, E.; Alcindor, T.; et al. PICASSO III: A Phase III, Placebo-Controlled Study of Doxorubicin With or Without Palifosfamide in Patients With Metastatic Soft Tissue Sarcoma. J. Clin. Oncol. 2016, 34, 3898–3905. [Google Scholar] [CrossRef]
- Tap, W.D.; Papai, Z.; Van Tine, B.A.; Attia, S.; Ganjoo, K.N.; Jones, R.L.; Schuetze, S.; Reed, D.; Chawla, S.P.; Riedel, R.F.; et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): An international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2017, 18, 1089–1103. [Google Scholar] [CrossRef]
- Young, R.J.; Litière, S.; Lia, M.; Hogendoorn, P.C.W.; Fisher, C.; Mechtersheimer, G.; Daugaard, S.; Sciot, R.; Collin, F.; Messiou, C.; et al. Predictive and prognostic factors associated with soft tissue sarcoma response to chemotherapy: A subgroup analysis of the European Organisation for Research and Treatment of Cancer 62012 study. Acta Oncol. 2017, 56, 1013–1020. [Google Scholar] [CrossRef]
- Jones, R.L.; Fisher, C.; Al-Muderis, O.; Judson, I.R. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur. J. Cancer 2005, 41, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Toulmonde, M.; Cioffi, A.; Penel, N.; Isambert, N.; Bompas, E.; Duffaud, F.; Patrikidou, A.; Lortal, B.; Le Cesne, A.; et al. Advanced well-differentiated/dedifferentiated liposarcomas: Role of chemotherapy and survival. Ann. Oncol. 2012, 23, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Livingston, J.A.; Bugano, D.; Barbo, A.; Lin, H.; Madewell, J.E.; Wang, W.L.; Lazar, A.J.; Tseng, W.W.; Roland, C.L.; Feig, B.W.; et al. Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: Defining the benefit and challenges of the standard. Sci. Rep. 2017, 19, 11836. [Google Scholar] [CrossRef]
- Sleijfer, S.; Ray-Coquard, I.; Papai, Z.; Le Cesne, A.; Scurr, M.; Schöffski, P.; Collin, F.; Pandite, L.; Marreaud, S.; De Brauwer, A.; et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J. Clin. Oncol. 2009, 27, 3126–3132. [Google Scholar]
- Van der Graaf, W.T.; Blay, J.-Y.; Chawla, S.P.; Kim, D.-W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Samuels, B.L.; Chawla, S.P.; Somaiah, N.; Staddon, A.P.; Skubitz, K.M.; Milhem, M.M.; Kaiser, P.E.; Portnoy, D.C.; Priebat, D.A.; Walker, M.S.; et al. Results of a prospective phase 2 study of pazopanib in patients with advanced intermediate-grade or high-grade liposarcoma. Cancer 2017, 123, 4640–4647. [Google Scholar] [CrossRef]
- Valverde, C.M.; Martin-Broto, J.; Lopez-Martin, J.A.; Romagosa, C.; Sancho-Marquez, M.P.; Carrasco, J.A.; Poveda, A.; Bauer, S.; Martinez-Trufero, J.; Cruz, J.; et al. Phase II clinical trial evaluating the activity and tolerability of pazopanib in patients (pts) with advanced and/or metastatic liposarcoma (LPS): A joint Spanish Sarcoma Group (GEIS) and German Interdisciplinary Sarcoma Group (GISG) Study—NCT01692496. J. Clin. Oncol. 2016, 34 (Suppl. S15), 11039. [Google Scholar] [CrossRef]
- Grünwald, V.; Kunitz, A.; Schuler, M.K.; Schoffski, P.; Kopp, H.-G.; Bauer, S.; Kasper, B.; Lindner, L.H.; Chemnitz, J.-M.; Crysandt, M.M.; et al. Randomized comparison of pazopanib (PAZ) and doxorubicin (DOX) in the first line treatment of metastatic soft tissue sarcoma (STS) in elderly patients (pts): Results of a phase II study (EPAZ). J. Clin. Oncol. 2018, 36 (Suppl. S15), 11506. [Google Scholar] [CrossRef]
- Suehara, Y.; Kohsaka, S.; Yamaguchi, S.; Hayashi, T.; Kurihara, T.; Sano, K.; Sasa, K.; Akaike, K.; Ueno, T.; Kojima, S.; et al. Assessment of Predictive Biomarkers of the Response to Pazopanib Based on an Integrative Analysis of High-grade Soft-tissue Sarcomas: Analysis of a Tumor Sample from a Responder and Patients with Other Soft-tissue Sarcomas. Clin. Orthop. Relat. Res. 2020, 478, 2461–2476. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.T.; Agresta, S.; Vigil, C.E.; Zhao, X.; Han, G.; D’Amato, G.; Calitri, C.E.; Dean, M.; Garrett, C.; Schell, M.J.; et al. Phase II study of sunitinib malate, a multitargeted tyrosine kinase inhibitor in patients with relapsed or refractory soft tissue sarcomas. Focus on three prevalent histologies: Leiomyosarcoma, liposarcoma and malignant fibrous histiocytoma. Int. J. Cancer 2011, 129, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Brodowicz, T.; Italiano, A.; Wallet, J.; Blay, J.-Y.; Bertucci, F.; Chevreau, C.; Piperno-Neumann, S.; Bompas, E.; Salas, S.; et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1732–1742. [Google Scholar] [CrossRef]
- Chi, Y.; Fang, Z.; Hong, X.-N.; Yao, Y.; Sun, P.; Wang, G.; Du, F.; Sun, Y.; Wu, Q.; Qu, G.; et al. Safety and Efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in Patients with Refractory Metastatic Soft-Tissue Sarcoma. Clin. Cancer Res. 2018, 24, 5233–5238. [Google Scholar] [CrossRef] [PubMed]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- Demetri, G.D.; Schöffski, P.; Grignani, G.; Blay, J.-Y.; Maki, R.G.; Van Tine, B.A.; Alcindor, T.; Jones, R.L.; D’Adamo, D.R.; Guo, M.; et al. Activity of Eribulin in Patients With Advanced Liposarcoma Demonstrated in a Subgroup Analysis From a Randomized Phase III Study of Eribulin Versus Dacarbazine. J. Clin. Oncol. 2017, 35, 3433–3439. [Google Scholar] [CrossRef]
- Demetri, G.D.; Von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef]
- Patel, S.; Von Mehren, M.; Reed, D.R.; Kaiser, P.; Charlson, J.; Ryan, C.W.; Rushing, D.; Livingston, M.; Singh, A.; Seth, R.; et al. Overall survival and histology-specific subgroup analyses from a phase 3, randomized controlled study of trabectedin or dacarbazine in patients with advanced liposarcoma or leiomyosarcoma. Cancer 2019, 125, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Fabbroni, C.; Fucà, G.; Ligorio, F.; Fumagalli, E.; Barisella, M.; Collini, P.; Morosi, C.; Gronchi, A.; Dei Tos, A.P.; Casali, P.G.; et al. Impact of pathological stratification on the clinical outcomes of advanced well-differentiated/dedifferentiated liposarcoma treated with trabectedin. Cancers 2021, 13, 1453. [Google Scholar] [CrossRef]
- Maki, R.G. Gemcitabine and docetaxel in metastatic sarcoma: Past, present, and feature. Oncologist 2007, 12, 999–1006. [Google Scholar] [CrossRef]
- Maki, R.G.; Wathen, J.K.; Patel, S.R.; Priebat, D.A.; Okuno, S.H.; Samuels, B.; Fanucchi, M.; Harmon, D.C.; Schuetze, S.M.; Reinke, D.; et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft-tissue sarcomas. J. Clin. Oncol. 2007, 25, 2755–2763. [Google Scholar] [CrossRef]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef]
- Bill, K.L.J.; Garnett, J.; Meaux, I.; Ma, X.; Creighton, C.J.; Bolshakov, S.; Barriere, C.; Debussche, L.; Lazar, A.; Prudner, B.C.; et al. SAR405838: A Novel and Potent Inhibitor of the MDM2:p53 Axis for the Treatment of Dedifferentiated Liposarcoma. Clin. Cancer Res. 2016, 22, 1150–1160. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Blay, J.Y.; Italiano, A.; Le Cesne, A.; Penel, N.; Zhi, J.; Heil, F.; Rueger, R.; Graves, B.; Ding, M.; et al. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: An exploratory proof-of-mechanism study. Lancet Oncol. 2012, 13, 1133–1140. [Google Scholar] [CrossRef]
- De Jonge, M.; de Weger, V.A.; Dickson, M.A.; Langenberg, M.; Le Cesne, A.; Wagner, A.J.; Hsu, K.; Zheng, W.; Macé, S.; Tuffal, G.; et al. A phase I study of SAR405838, a novel human double minute 2 (HDM2) antagonist, in patients with solid tumours. Eur. J. Cancer 2017, 76, 144–151. [Google Scholar] [CrossRef]
- Wagner, A.J.; Banerji, U.; Mahipal, A.; Somaiah, N.; Hirsch, H.; Fancourt, C.; Johnson-Levonas, A.O.; Lam, R.; Meister, A.K.; Russo, G.; et al. Phase I Trial of the Human Double Minute 2 Inhibitor MK-8242 in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2017, 35, 1304–1311. [Google Scholar] [CrossRef]
- Bauer, T.M.; Gounder, M.M.; Weise, A.M.; Schwartz, G.K.; Carvajal, R.D.; Kumar, P.; Zernovak, O.; Beck, A.; Doyle, J.; Mendell-Harary, J.; et al. A phase 1 study of MDM2 inhibitor DS-3032b in patients with well/de-differentiated liposarcoma (WD/DD LPS), solid tumors (ST) and lymphomas (L). J. Clin. Oncol. 2018, 36 (Suppl. S15), 11514. [Google Scholar] [CrossRef]
- Jung, J.; Lee, J.S.; Dickson, M.A.; Schwartz, G.K.; Le Cesne, A.; Varga, A.; Bahleda, R.; Wagner, A.J.; Choy, E.; De Jonge, M.J.; et al. TP53 mutations emerge with HDM2 inhibitor SAR405838 treatment in de-differentiated liposarcoma. Nat. Commun. 2016, 7, 12609. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.A.; Schwartz, G.K.; Keohan, M.L.; D’Angelo, S.P.; Gounder, M.M.; Chi, P.; Antonescu, C.R.; Landa, J.; Qin, L.X.; Crago, A.M.; et al. Progression-free survival among patients with well-differentiated or dedifferentiated liposarcoma treated with CDK4 inhibitor palbociclib: A phase 2 clinical trial. JAMA Oncol. 2016, 2, 937–940. [Google Scholar] [CrossRef]
- Infante, J.R.; Cassier, P.A.; Gerecitano, J.F.; Witteveen, P.O.; Chugh, R.; Ribrag, V.; Chakraborty, A.; Matano, A.; Dobson, J.R.; Crystal, A.S.; et al. A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 2016, 22, 5696–5705. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.A.; Koff, A.; D’Angelo, S.P.; Gounder, M.M.; Keohan, M.L.; Kelly, C.M.; Chi, P.; Antonescu, C.R.; Landa, J.; Qin, L.X.; et al. Phase 2 study of the CDK4 inhibitor abemaciclib in dedifferentiated liposarcoma. J. Clin. Oncol. 2019, 37 (Suppl. S15), 11004. [Google Scholar] [CrossRef]
- Laroche-Clary, A.; Chaire, V.; Algeo, M.P.; Derieppe, M.A.; Loarer, F.L.; Italiano, A. Combined targeting of MDM2 and CDK4 is synergistic in dedifferentiated liposarcomas. J. Hematol. Oncol. 2017, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Kanojia, D.; Mayakonda, A.; Said, J.W.; Doan, N.B.; Chien, W.; Ganesan, T.S.; Chuang, L.S.; Venkatachalam, N.; Baloglu, E.; et al. Molecular mechanism and therapeutic implications of selinexor (KPT-330) in liposarcoma. Oncotarget 2017, 8, 7521–7532. [Google Scholar] [CrossRef] [PubMed]
- Zuco, V.; Pasquali, S.; Tortoreto, M.; Brich, S.; Percio, S.; Dagrada, G.P.; Colombo, C.; Sanfilippo, R.; Lauricella, C.; Gounder, M.; et al. Selinexor versus doxorubicin in dedifferentiated liposarcoma PDXs: Evidence of greater activity and apoptotic response dependent on p53 nuclear accumulation and surviving down-regulation. J. Exp. Clin. Cancer Res. 2021, 40, 83. [Google Scholar] [CrossRef]
- Gounder, M.M.; Zer, A.; Tap, W.D.; Salah, S.; Dickson, M.A.; Gupta, A.A.; Keohan, M.L.; Loong, H.; D’Angelo, S.P.; Baker, S.; et al. Phase IB Study of Selinexor, a First-in-Class Inhibitor of Nuclear Export, in Patients With Advanced Refractory Bone or Soft Tissue Sarcoma. J. Clin. Oncol. 2016, 34, 3166–3174. [Google Scholar] [CrossRef]
- Gounder, M.; Razak, A.A.; Somaiah, N.; Martin-Broto, J.; Schuetze, S.; Grignani, G.; Chawla, S.P.; Chmielowski, B.; Vincenzi, B.; Stacchiotti, S.; et al. A phase 2/3, randomized, double-Blind, cross-over study of selinexor versus placebo in advanced unresectable dedifferentiated liposarcoma (DDLS). In Proceedings of the Connective Tissue Oncology Society (CTOS) Virtual Annual Meeting, Online, 18–21 November 2020. [Google Scholar]
- Gounder, M.; Razak, A.R.A.; Gilligan, A.M.; Leong, H.; Ma, X.; Somaiah, N.; Chawla, S.P.; Martin-Broto, J.; Grignani, G.; Schuetze, S.M.; et al. Health-related quality of life and pain with selinexor in patients with advanced dedifferentiated liposarcoma. Futur. Oncol. 2021. [Google Scholar] [CrossRef]
- Wisdom, A.J.; Mowery, Y.M.; Riedel, R.F.; Kirsch, D.G. Rationale and emerging strategies for immune checkpoint blockade in soft tissue sarcoma. Cancer 2018, 124, 3819–3829. [Google Scholar] [CrossRef]
- Park, H.K.; Kim, M.; Sung, M.; Lee, S.E.; Kim, Y.J.; Choi, Y.-L. Status of programmed death-ligand 1 expression in sarcomas. J. Transl. Med. 2018, 16, 303. [Google Scholar] [CrossRef]
- Vargas, A.C.; MacLean, F.M.; Sioson, L.; Tran, D.; Bonar, F.; Mahar, A.; Cheah, A.L.; Russell, P.; Grimison, P.; Richardson, L.; et al. Prevalence of PD-L1 expression in matched recurrent and/or metastatic sarcoma samples and in a range of selected sarcomas subtypes. PLoS ONE 2020, 15, e0222551. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Oda, Y.; Nishimura, N.; Morizawa, Y.; Ohnishi, S.; Hatakeyama, K.; Fujii, T.; Hori, S.; Gotoh, D.; Nakai, Y.; et al. Integrative assessment of clinicopathological parameters and the expression of PD-L1, PD-L2 and PD-1 in tumor cells of retroperitoneal sarcoma. Oncol. Lett. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- A Tawbi, H.; Burgess, M.; Bolejack, V.; A Van Tine, B.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; A Priebat, D.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Burgess, M.A.; Bolejack, V.; Schuetze, S.; Van Tine, B.A.; Attia, S.; Riedel, R.F.; Hu, J.S.; Daivs, L.E.; Okuno, S.H.; Priebat, D.A.; et al. Clinical activity of pembrolizumab (P) in undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated/pleomorphic liposarcoma (LPS): Final results of SARC028 expansion cohorts. J. Clin. Oncol. 2019, 37 (Suppl. S15), 11015. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Mahoney, M.R.; A Van Tine, B.; Atkins, J.; Milhem, M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.H.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Chen, J.L.; Mahoney, M.R.; George, S.; Antonescu, C.R.; Liebner, D.A.; Van Tine, B.A.; Milhem, M.M.; Tap, W.D.; Streicher, H.; Schwartz, G.K.; et al. A multicenter phase II study of nivolumab +/− ipilimumab for patients with metastatic sarcoma (Alliance A091401): Results of expansion cohorts. J. Clin. Oncol. 2020, 38 (Suppl. S15), 11511. [Google Scholar] [CrossRef]
- Keung, E.Z.; Lazar, A.J.; E Torres, K.; Wang, W.-L.; Cormier, J.N.; Guadagnolo, B.A.; Bishop, A.; Lin, H.; Hunt, K.K.; Bird, J.; et al. Phase II study of neoadjuvant checkpoint blockade in patients with surgically resectable undifferentiated pleomorphic sarcoma and dedifferentiated liposarcoma. BMC Cancer 2018, 18, 913. [Google Scholar] [CrossRef] [PubMed]
- Roland, C.L.; Keung, E.Z.; Lazar, A.J.; Torres, K.E.; Wang, W.L.; Ashleigh Guadagnolo, B.; Bishop, A.J.; Lin, H.Y.; Hunt, K.; Feig, B.W.; et al. Preliminary results of a phase II study of neoadjuvant checkpoint blockade for surgically resectable undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated liposarcoma (DDLPS). J. Clin. Oncol. 2020, 38 (Suppl. S15), 11505. [Google Scholar] [CrossRef]
- Mowery, Y.M.; Ballman, K.V.; Riedel, R.F.; Brigman, B.E.; Attia, S.; Meyer, C.F.; Schuetze, S.; Burgess, M.A.; Chmielowski, B.; Dickson, M.A.; et al. SU2C-SARC032: A phase II randomized controlled trial of neoadjuvant pembrolizumab with radiotherapy and adjuvant pembrolizumab for high-risk soft tissue sarcoma. J. Clin. Oncol. 2018, 36 (Suppl. S15), 11588. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishio, J.; Nakayama, S.; Nabeshima, K.; Yamamoto, T. Biology and Management of Dedifferentiated Liposarcoma: State of the Art and Perspectives. J. Clin. Med. 2021, 10, 3230. https://doi.org/10.3390/jcm10153230
Nishio J, Nakayama S, Nabeshima K, Yamamoto T. Biology and Management of Dedifferentiated Liposarcoma: State of the Art and Perspectives. Journal of Clinical Medicine. 2021; 10(15):3230. https://doi.org/10.3390/jcm10153230
Chicago/Turabian StyleNishio, Jun, Shizuhide Nakayama, Kazuki Nabeshima, and Takuaki Yamamoto. 2021. "Biology and Management of Dedifferentiated Liposarcoma: State of the Art and Perspectives" Journal of Clinical Medicine 10, no. 15: 3230. https://doi.org/10.3390/jcm10153230
APA StyleNishio, J., Nakayama, S., Nabeshima, K., & Yamamoto, T. (2021). Biology and Management of Dedifferentiated Liposarcoma: State of the Art and Perspectives. Journal of Clinical Medicine, 10(15), 3230. https://doi.org/10.3390/jcm10153230




