Pulmonary Complications in Hematopoietic Stem Cell Transplant Recipients—A Clinician Primer
Abstract
1. Introduction
2. Hematopoietic Stem Cell Transplant Overview
2.1. Autologous versus Allogeneic Hematopoietic Stem Cell Transplantation (HSCT)
2.2. Engraftment
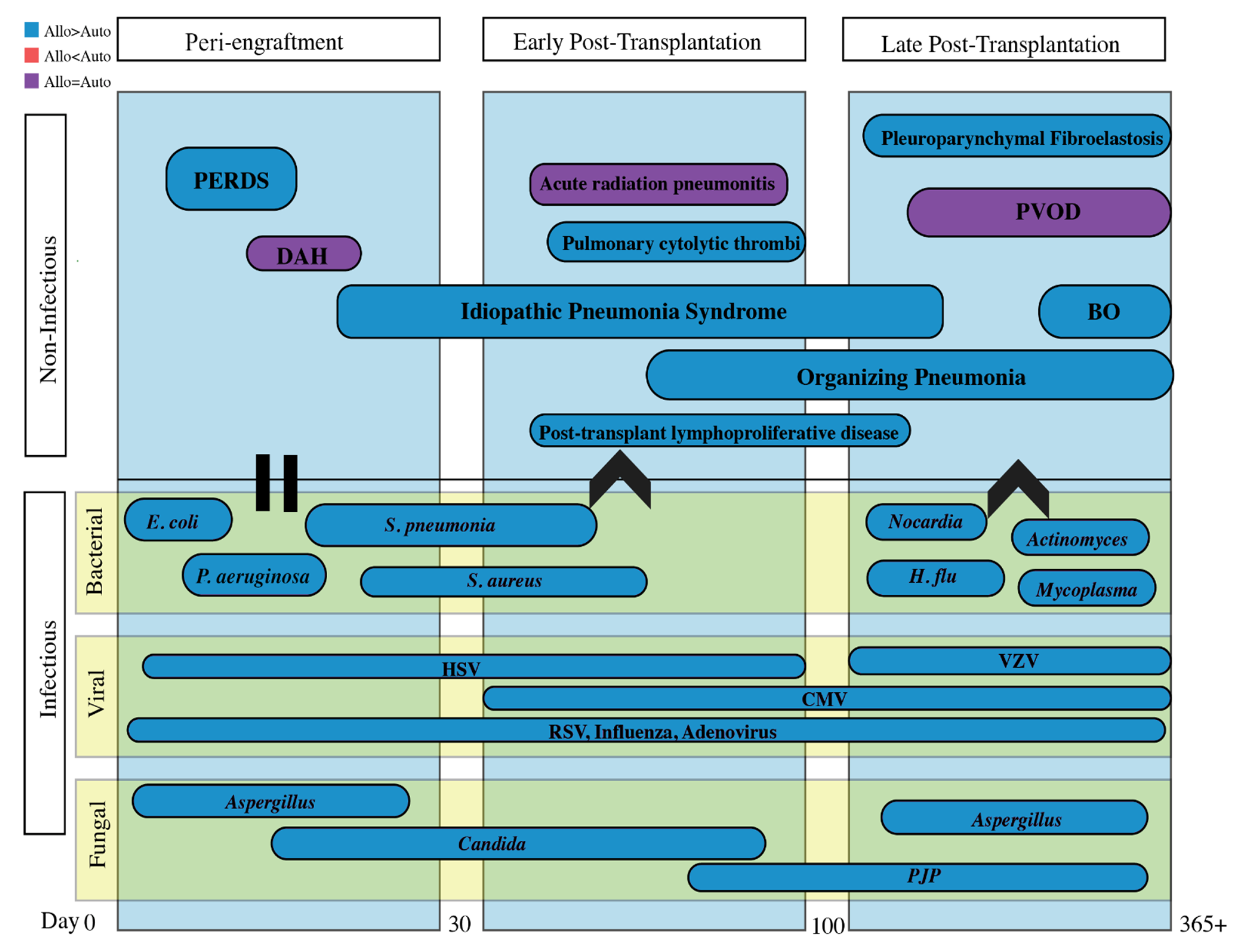
3. Timeline of Complications Following HSCT
4. Peri-Engraftment Period (0–30 Days Post-Transplant)
4.1. Peri-Engraftment Respiratory Distress Syndrome (PERDS)
4.2. Diffuse Alveolar Hemorrhage (DAH)
5. Early Post-Transplantation Period (31–100 Days Post-Transplant)
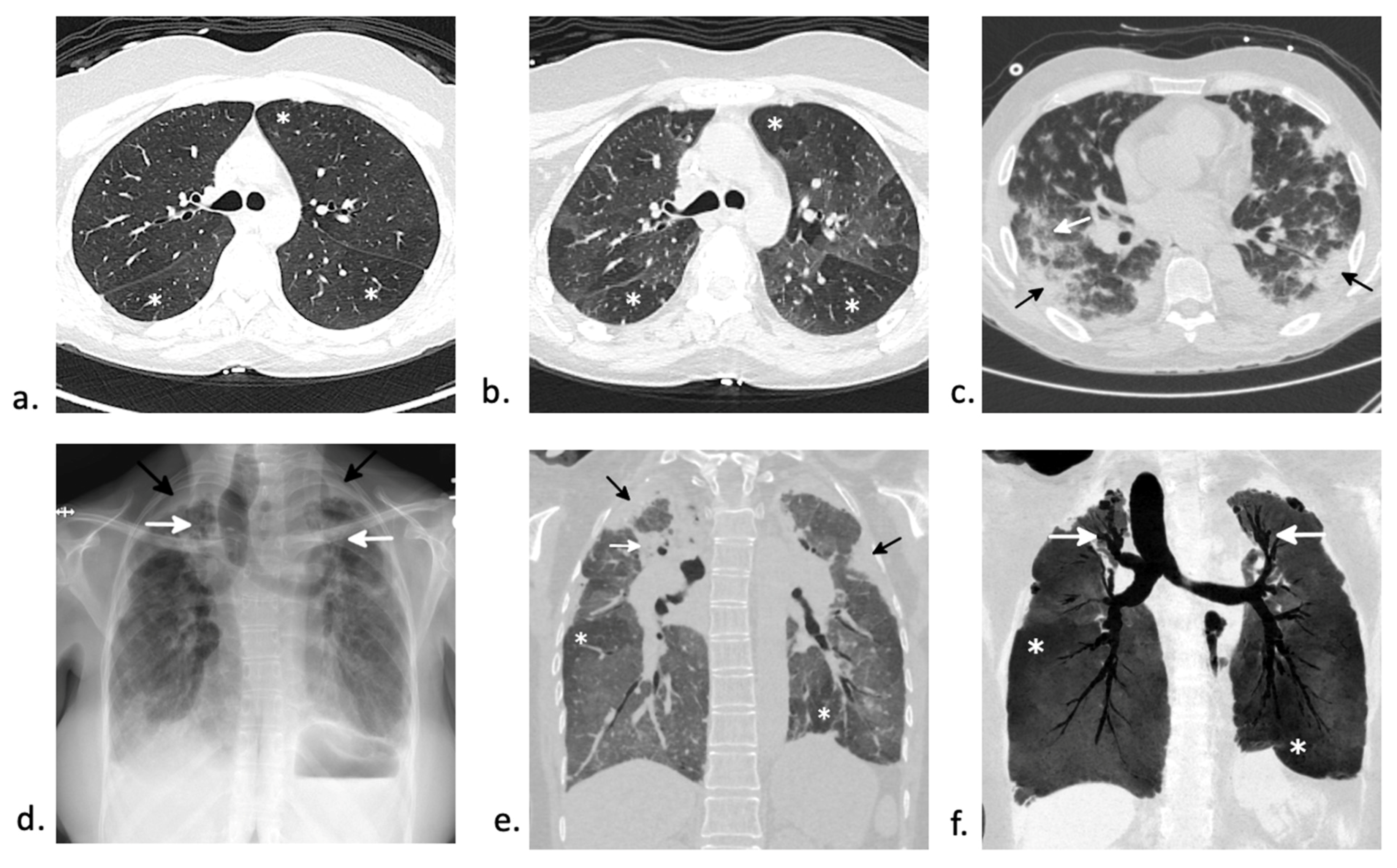
5.1. Idiopathic Pneumonia Syndrome (IPS)
5.2. Pulmonary Cytolytic Thrombi
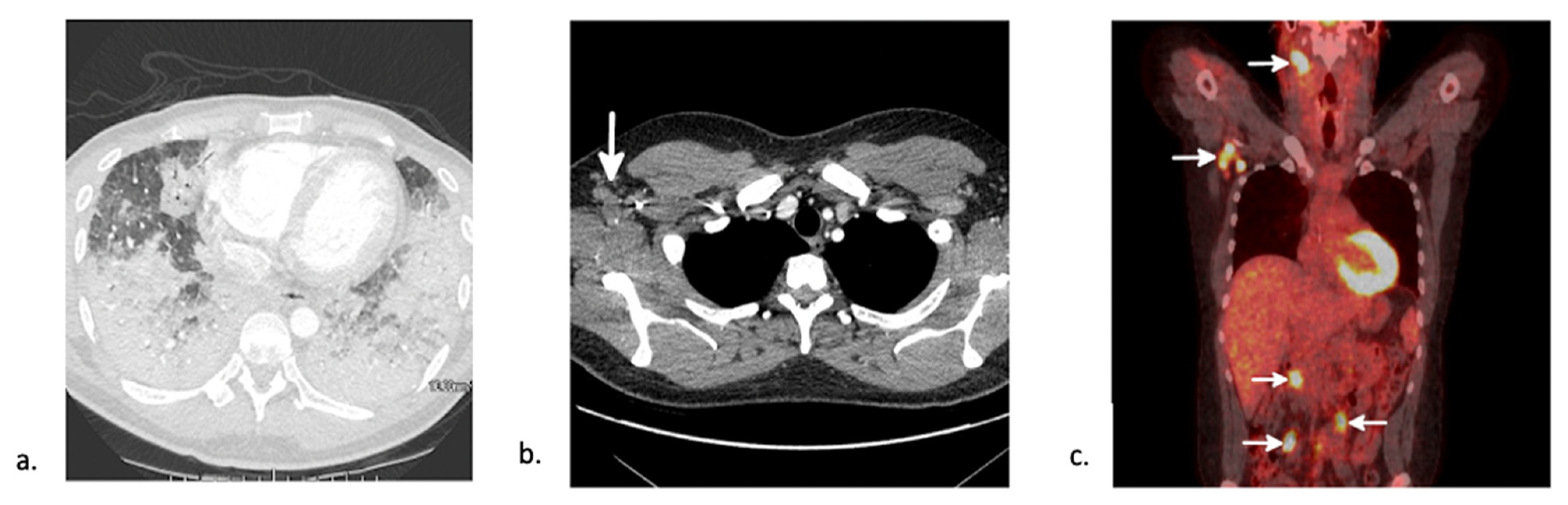
5.3. Post-Transplant Lymphoproliferative Disease (PTLD)
6. Late Post-Transplantation Period (>100 Days Post-Transplant)
6.1. Bronchiolitis Obliterans Syndrome (BOS)
6.2. Organizing Pneumonia
6.3. Interstitial Lung Disease
6.4. Pulmonary Veno-Occlusive Disease (PVOD)
7. Infectious Complications
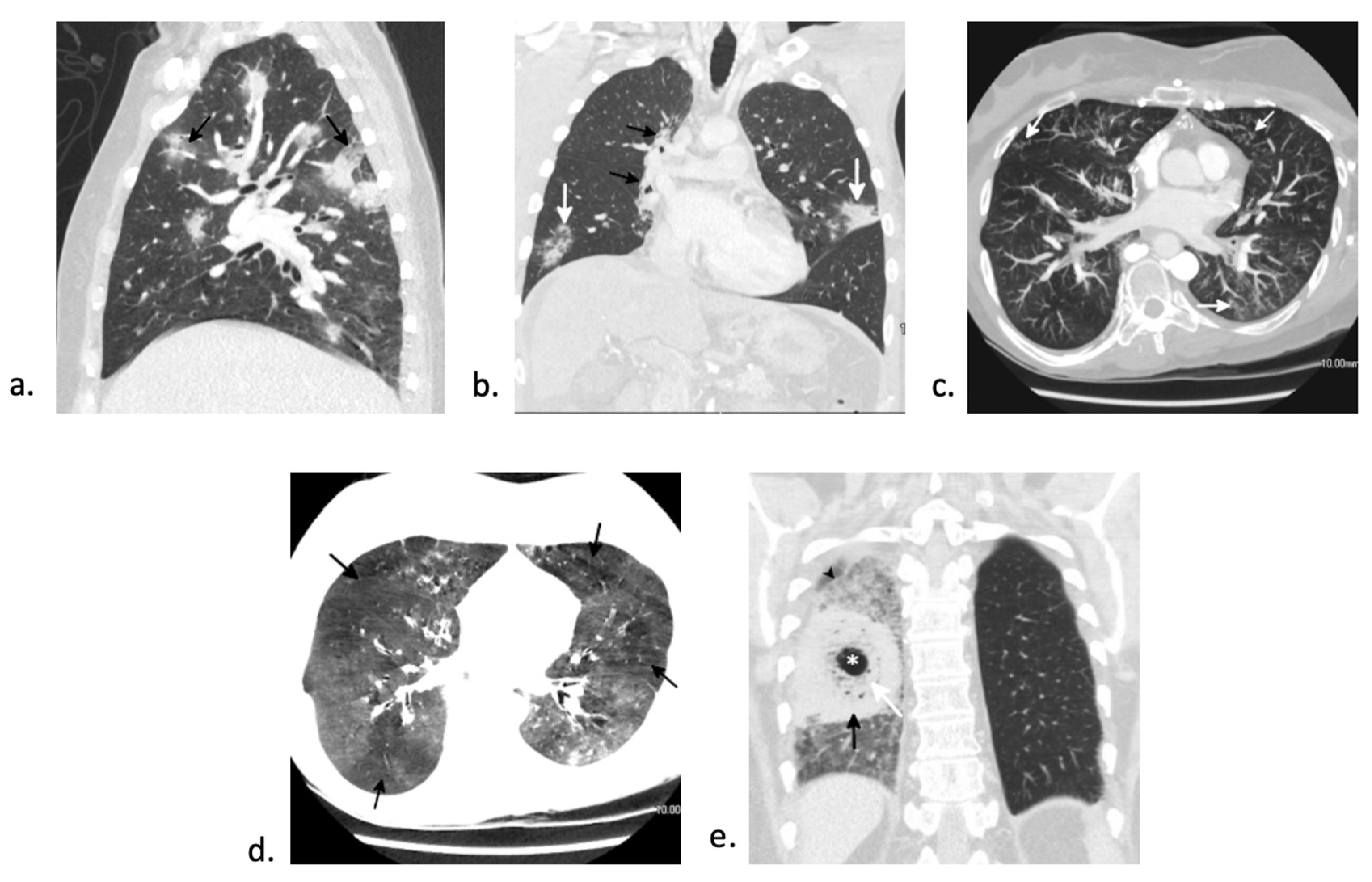
7.1. Bacterial Pneumonia
7.2. Fungal Pneumonia
7.3. Viral Pneumonia
8. Diagnostic Tools
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barriga, F.; Ramirez, P.; Wietstruck, A.; Rojas, N. Hematopoietic stem cell transplantation: Clinical use and perspectives. Biol. Res. 2012, 45, 307–316. [Google Scholar] [CrossRef]
- Cordonnier, C.; Bernaudin, J.F.; Bierling, P.; Huet, Y.; Vernant, J.P. Pulmonary complications occurring after allogeneic bone marrow transplantation. A study of 130 consecutive transplanted patients. Cancer 1986, 58, 1047–1054. [Google Scholar] [CrossRef]
- Jules-Elysee, K.; Stover, D.E.; Yahalom, J.; White, D.A.; Gulati, S.C. Pulmonary complications in lymphoma patients treated with high-dose therapy autologous bone marrow transplantation. Am. Rev. Respir Dis. 1992, 146, 485–491. [Google Scholar] [CrossRef]
- Shorr, A.F.; Moores, L.K.; Edenfield, W.J.; Christie, R.J.; Fitzpatrick, T.M. Mechanical ventilation in hematopoietic stem cell transplantation: Can We effectively predict outcomes? Chest 1999, 116, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Forman, S.J.; Negrin, R.S.; Antin, J.H.; Appelbaum, F.R. Thomas’ Hematopoietic Cell Transplantation; Wiley Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Copelan, E.A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 2006, 354, 1813–1826. [Google Scholar] [CrossRef]
- Majhail, N.S.; Farnia, S.H.; Carpenter, P.A.; Champlin, R.E.; Crawford, S.; Marks, D.I.; Omel, J.L.; Orchard, P.J.; Palmer, J.; Saber, W.; et al. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 1863–1869. [Google Scholar] [CrossRef]
- Hatzimichael, E.; Tuthill, M. Hematopoietic stem cell transplantation. Stem Cells Cloning 2010, 3, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, T.R. Engraftment syndrome: Double-edged sword of hematopoietic cell transplants. Bone Marrow Transplant. 2015, 50, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, A.; Popradi, G. A general practitioner’s guide to hematopoietic stem-cell transplantation. Curr. Oncol. 2019, 26, 187–191. [Google Scholar] [CrossRef]
- Quesenberry, P.J.; Stewart, F.M.; Becker, P.; D’Hondt, L.; Frimberger, A.; Lambert, J.F.; Colvin, G.A.; Miller, C.; Heyes, C.; Abedi, M.; et al. Stem cell engraftment strategies. Ann. N. Y. Acad Sci. 2001, 938, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, T.R. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001, 27, 893–898. [Google Scholar] [CrossRef]
- Azar, M.M.; Gaston, D.C.; Kotton, C.N.; Malinis, M.F. Emerging Microbiology Diagnostics for Transplant Infections: On the Cusp of a Paradigm Shift. Transplantation 2020, 104, 1358–1384. [Google Scholar] [CrossRef] [PubMed]
- Cooke, K.R.; Yanik, G.A. Lung Injury Following Hematopoietic Cell Transplantation. Thomas’ Hematop. Cell Transplant. 2009, 95, 1456–1472. [Google Scholar]
- Ahya, V.N. Noninfectious Acute Lung Injury Syndromes Early After Hematopoietic Stem Cell Transplantation. Clin. Chest Med. 2017, 38, 595–606. [Google Scholar] [CrossRef]
- Capizzi, S.A.; Kumar, S.; Huneke, N.E.; Gertz, M.A.; Inwards, D.J.; Litzow, M.R.; Lacy, M.Q.; Gastineau, D.A.; Prakash, U.B.S.; Tefferi, A. Peri-engraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001, 27, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Afessa, B.; Tefferi, A.; Litzow, M.R.; Krowka, M.J.; Wylam, M.E.; Peters, S.G. Diffuse Alveolar Hemorrhage in Hematopoietic Stem Cell Transplant Recipients. Am. J. Respir. Crit. Care Med. 2002, 166, 641–645. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Peters, S.G.; Hogan, W.J.; Hashmi, S.K.; Litzow, M.R.; Patnaik, M.S.; Niven, A.S.; Yadav, H. Epidemiology, Risk Factors, and Outcomes of Diffuse Alveolar Hemorrhage After Hematopoietic Stem Cell Transplantation. Chest 2021, 159, 2325–2333. [Google Scholar] [CrossRef]
- Keklik, F.; Alrawi, E.B.; Cao, Q.; Bejanyan, N.; Rashidi, A.; Lazaryan, A.; Arndt, P.; Dincer, E.H.; Bachanova, V.; Warlick, E.D.; et al. Diffuse alveolar hemorrhage is most often fatal and is affected by graft source, conditioning regimen toxicity, and engraftment kinetics. Haematologica 2018, 103, 2109–2115. [Google Scholar] [CrossRef]
- Majhail, N.S.; Parks, K.; Defor, T.E.; Weisdorf, D.J. Diffuse Alveolar Hemorrhage and Infection-Associated Alveolar Hemorrhage following Hematopoietic Stem Cell Transplantation: Related and High-Risk Clinical Syndromes. Biol. Blood Marrow Transplant. 2006, 12, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jain, A.; Warneke, C.L.; Gupta, A.; Shannon, V.R.; Morice, R.C.; Onn, A.; Jimenez, C.A.; Bashoura, L.; Giralt, S.A.; et al. Outcome of alveolar hemorrhage in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2007, 40, 71–78. [Google Scholar] [CrossRef]
- Wenger, D.S.; Triplette, M.; Crothers, K.; Cheng, G.-S.; Hill, J.A.; Milano, F.; Shahrir, S.; Schoch, G.; Vusse, L.K.V. Incidence, Risk Factors, and Outcomes of Idiopathic Pneumonia Syndrome after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2020, 26, 413–420. [Google Scholar] [CrossRef]
- Gao, R.W.; Weisdorf, D.J.; DeFor, T.E.; Ehler, E.; Dusenbery, K.E. Influence of Total Body Irradiation Dose Rate on Idiopathic Pneumonia Syndrome in Acute Leukemia Patients Undergoing Allogeneic Hematopoietic Cell Transplantation. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 180–189. [Google Scholar] [CrossRef]
- Zhu, K.E.; Hu, J.Y.; Zhang, T.; Chen, J.; Zhong, J.; Lu, Y.H. Incidence, risks, and outcome of idiopathic pneumonia syndrome early after allogeneic hematopoietic stem cell transplantation. Eur. J. Haematol. 2008, 81, 461–4666. [Google Scholar] [CrossRef]
- Fukuda, T.; Hackman, R.C.; Guthrie, K.A.; Sandmaier, B.M.; Boeckh, M.; Maris, M.B.; Maloney, D.G.; Deeg, H.J.; Martin, P.J.; Storb, R.F.; et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 2003, 102, 2777–2785. [Google Scholar] [CrossRef]
- Panoskaltsis-Mortari, A.; Griese, M.; Madtes, D.K.; Belperio, J.A.; Haddad, I.Y.; Folz, R.J.; Cooke, K.R. An official American Thoracic Society research statement: Noninfectious lung injury after hematopoietic stem cell transplantation: Idiopathic pneumonia syndrome. Am. J. Respir. Crit. Care Med. 2011, 183, 1262–1279. [Google Scholar] [CrossRef]
- Spira, D.; Faul, C.; Schaup, V.; Wirths, S.; Schulze, M.; Sauter, A.; Horger, M. HRCT findings in idiopathic pneumonia syndrome with documentation of the disease course. Eur. J. Radiol. 2012, 81, e147–e152. [Google Scholar] [CrossRef]
- Kantrow, S.P.; Hackman, R.C.; Boeckh, M.; Myerson, D.; Crawford, S.W. Idiopathic pneumonia syndrome: Changing spectrum of lung injury after marrow transplantation. Transplantation 1997, 63, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Bilgrami, S.F.; Metersky, M.L.; McNally, D.; Naqvi, B.H.; Kapur, D.; Raible, D.; Bona, R.D.; Edwards, R.L.; Feingold, J.M.; Clive, J.M.; et al. Idiopathic pneumonia syndrome following myeloablative chemotherapy and autologous transplantation. Ann. Pharmacother. 2001, 35, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Yanik, G.A.; Horowitz, M.M.; Weisdorf, D.J.; Logan, B.R.; Ho, V.T.; Soiffer, R.J.; Carter, S.L.; Wu, J.; Wingard, J.R.; DiFronzo, N.L.; et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: Enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: Blood and marrow transplant clinical trials network Protocol. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2014, 20, 858–864. [Google Scholar] [CrossRef]
- Thompson, J.; Yin, Z.; D’Souza, A.; Fenske, T.; Hamadani, M.; Hari, P.; Rizzo, J.D.; Pasquini, M.; Saber, W.; Shah, N.; et al. Etanercept and Corticosteroid Therapy for the Treatment of Late-Onset Idiopathic Pneumonia Syndrome. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2017, 23, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Tizon, R.; Frey, N.; Heitjan, D.F.; Tan, K.S.; Goldstein, S.C.; O Hexner, E.; Loren, A.W.; Luger, S.M.; Reshef, R.; Tsai, D.; et al. High-dose corticosteroids with or without etanercept for the treatment of idiopathic pneumonia syndrome after allo-SCT. Bone Marrow Transplant. 2012, 47, 1332–1337. [Google Scholar] [CrossRef]
- Woodard, J.P.; Gulbahce, E.; Shreve, M.; Steiner, M.; Peters, C.; Hite, S.; Ralmsay, N.; DeFor, T.; Baker, K. Pulmonary cytolytic thrombi: A newly recognized complication of stem cell transplantation. Bone Marrow Transplant. 2000, 25, 293–300. [Google Scholar] [CrossRef]
- Gulbahce, H.E.; Manivel, J.C.; Jessurun, J. Pulmonary Cytolytic Thrombi: A Previously Unrecognized Complication of Bone Marrow Transplantation. Am. J. Surg. Pathol. 2000, 24, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansour, Z.; Nelson, B.P.; Evens, A.M. Post-Transplant Lymphoproliferative Disease (PTLD): Risk Factors, Diagnosis, and Current Treatment Strategies. Curr. Hematol. Malig. Rep. 2013, 8, 173–183. [Google Scholar] [CrossRef]
- Naik, S.; Riches, M.; Hari, P.; Kim, S.; Chen, M.; Bachier, C.; Shaughnessy, P.; Hill, J.; Ljungman, P.; Battiwalla, M.; et al. Survival outcomes of allogeneic hematopoietic cell transplants with EBV-positive or EBV-negative post-transplant lymphoproliferative disorder, A CIBMTR study. Transpl. Infect. Dis. 2019, 21, e13145. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Uhlin, M.; Wikell, H.; Sundin, M.; Blennow, O.; Maeurer, M.; Ringden, O.; Winiarski, J.; Ljungman, P.; Remberger, M.; Mattsson, J.; et al. Risk factors for Epstein-Barr virus-related post-transplant lymphoproliferative disease after allogeneic hematopoietic stem cell transplantation. Haematologica 2014, 99, 346. [Google Scholar] [CrossRef] [PubMed]
- Sundin, M.; Le Blanc, K.; Ringdén, O.; Barkholt, L.; Omazic, B.; Lergin, C.; Levitsky, V.; Remberger, M. The role of HLA mismatch, splenectomy and recipient Epstein-Barr virus seronegativity as risk factors in post-transplant lymphoproliferative disorder following allogeneic hematopoietic stem cell transplantation. Haematologica 2006, 91, 1059–1067. [Google Scholar] [PubMed]
- Zallio, F.; Primon, V.; Tamiazzo, S.; Pini, M.; Baraldi, A.; Corsetti, M.T.; Gotta, F.; Bertassello, C.; Salvi, F.; Rocchetti, A.; et al. Epstein-Barr virus reactivation in allogeneic stem cell transplantation is highly related to cytomegalovirus reactivation. Clin. Transplant. 2013, 27, E491–E497. [Google Scholar] [CrossRef]
- Styczynski, J.; van der Velden, W.; Fox, C.P.; Engelhard, D.; de la Camara, R.; Cordonnier, C.; Ljungman, P. Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica 2016, 101, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, F.; Poletti, V.; Costabel, U.; Battista, M.; Sperotto, A.; Medeot, M.; Toffoletti, E.; Falnin, R. Clinical presentation, outcome and risk factors of late-onset non-infectious pulmonary complications after allogeneic stem cell transplantation. Curr. Stem Cell Res. Ther. 2009, 4, 161–167. [Google Scholar] [CrossRef]
- Majhail, N.S.; Rizzo, J.D.; Lee, S.J.; Aljurf, M.; Atsuta, Y.; Bonfim, C.; Burns, L.J.; Chaudhri, N.; Davies, S.; Okamoto, S.; et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2012, 18, 348–371. [Google Scholar] [CrossRef]
- Wingard, J.R.; Majhail, N.S.; Brazauskas, R.; Wang, Z.; Sobocinski, K.A.; Jacobsohn, D.; Sorror, M.L.; Horowitz, M.M.; Bolwell, B.; Rizzo, J.D.; et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 2230–2239. [Google Scholar] [CrossRef]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.-S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 2015, 21, 389–401.e1. [Google Scholar] [CrossRef]
- Yoshihara, S.; Yanik, G.; Cooke, K.R.; Mineishi, S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2007, 13, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Soubani, A.O.; Uberti, J.P. Bronchiolitis obliterans following haematopoietic stem cell transplantation. Eur. Respir. J. 2007, 29, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, A.; Chevret, S.; Chagnon, K.; Godet, C.; Bergot, E.; De Latour, R.P.; Dominique, S.; De Revel, T.; Juvin, K.; Maillard, N.; et al. Budesonide/Formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am. J. Respir. Crit. Care Med. 2015, 191, 1242–1249. [Google Scholar] [CrossRef]
- Williams, K.M.; Cheng, G.-S.; Pusic, I.; Jagasia, M.; Burns, L.; Ho, V.T.; Pidala, J.; Palmer, J.; Johnston, L.; Mayer, S.; et al. Fluticasone, Azithromycin, and Montelukast Treatment for New-Onset Bronchiolitis Obliterans Syndrome after Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Penack, O.; Marchetti, M.; Ruutu, T.; Aljurf, M.; Bacigalupo, A.; Bonifazi, F.; Ciceri, F.; Cornelissen, J.; Malladi, R.; Duarte, R.F.; et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: Updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020, 7, e157–e167. [Google Scholar] [CrossRef]
- Lam, D.C.; Lam, B.; Wong, M.K.; Lu, C.; Au, W.Y.; Tse, E.; Leung, A.Y.H.; Kwong, Y.L.; Liang, R.H.S.; Lam, W.K.; et al. Effects of azithromycin in bronchiolitis obliterans syndrome after hematopoietic SCT—A randomized double-blinded placebo-controlled study. Bone Marrow Transplant. 2011, 46, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shi, J.; Luo, Y.; Tan, Y.; Zhang, M.; Lai, X.; Yu, J.; Liu, L.; Fu, H.; Huang, H.; et al. Evaluation of Ruxolitinib for Steroid-Refractory Chronic Graft-vs-Host Disease After Allogeneic Hematopoietic Stem Cell Transplantation. JAMA Netw. Open 2021, 4, e2034750. [Google Scholar] [CrossRef]
- Malik, M.I.; Litzow, M.; Hogan, W.; Patnaik, M.; Murad, M.H.; Prokop, L.J.; Wintetrs, J.L.; Halshmi, S. Extracorporeal photopheresis for chronic graft-versus-host disease: A systematic review and meta-analysis. Blood Res. 2014, 49, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Ozeki, K.; Ukai, S.; Sagou, K.; Fukushima, N.; Kohno, A. Patterns of onset and outcome of cryptogenic organizing pneumonia after allogeneic hematopoietic stem cell transplantation. Int. J. Hematol. 2019, 109, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Watadani, T.; Maeda, E.; Ota, S.; Kataoka, K.; Seo, S.; Kumano, K.; Hangaishi, A.; Takahashi, T.; Imai, Y.; et al. Outcome and treatment of late-onset noninfectious pulmonary complications after allogeneic haematopoietic SCT. Bone Marrow Transplant. 2010, 45, 1719–1727. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freudenberger, T.D.; Madtes, D.K.; Curtis, J.R.; Cummings, P.; Storer, B.E.; Hackman, R.C. Association between acute and chronic graft-versus-host disease and bronchiolitis obliterans organizing pneumonia in recipients of hematopoietic stem cell transplants. Blood 2003, 102, 3822–3828. [Google Scholar] [CrossRef]
- Afessa, B.; Litzow, M.R.; Tefferi, A. Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001, 28, 425–434. [Google Scholar] [CrossRef]
- Pipavath, S.N.; Chung, J.H.; Chien, J.W.; Godwin, J.D. Organizing pneumonia in recipients of hematopoietic stem cell transplantation: CT features in 16 patients. J. Comput. Assist. Tomogr. 2012, 36, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, F.; Chevret, S.; Lorillon, G.; De Bazelaire, C.; de Latour, R.P.; Meignin, V.; Michallet, M.; Hermet, E.; Wyplosz, B.; Houdouin, V.; et al. Late-onset noninfectious interstitial lung disease after allogeneic hematopoietic stem cell transplantation. Respir. Med. 2014, 108, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, H.; Ishii, M.; Mori, T.; Sugiura, H.; Tasaka, S.; Sakurai, M.; Koda, Y.; Kato, J.; Hasegawa, N.; Okamoto, S.; et al. Clinical and radiological characteristics of patients with late-onset severe restrictive lung defect after hematopoietic stem cell transplantation. BMC Pulm. Med. 2017, 17, 123. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, A.; Chevret, S.; Peffault de Latour, R.P.; Chagnon, K.; De Margerie-Mellon, C.; Rivière, F.; Robin, M.; Mani, J.; Lorillon, G.; Socié, G.; et al. Noninfectious lung complications after allogeneic haematopoietic stem cell transplantation. Eur. Respir. J. 2018, 51, 1702617. [Google Scholar] [CrossRef]
- Meignin, V.; Thivolet-Bejui, F.; Kambouchner, M.; Hussenet, C.; Bondeelle, L.; Mitchell, A.; Chagnon, K.; Begueret, H.; Segers, V.; Cottin, V.; et al. Lung histopathology of non-infectious pulmonary complications after allogeneic haematopoietic stem cell transplantation. Histopathology. 2018, 73, 832–842. [Google Scholar] [CrossRef]
- Chua, F.; Desai, S.R.; Nicholson, A.G.; Detvaraj, A.; Renzoni, E.; Rice, A.; Wells, A.U. Pleuroparenchymal Fibroelastosis. A Review of Clinical, Radiological, and Pathological Characteristics. Ann. Am. Thorac. Soc. 2019, 16, 1351–1359. [Google Scholar] [CrossRef]
- Mariani, F.; Gatti, B.; Rocca, A.; Bonifazi, F.; Cavazza, A.; Fanti, S.; Tomassetti, S.; Piciucchi, S.; Poletti, V.; Zompaltori, M. Pleuroparenchymal fibroelastosis: The prevalence of secondary forms in hematopoietic stem cell and lung transplantation recipients. Diagn. Interv. Radiol. 2016, 22, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Bondeelle, L.; Gras, J.; Michonneau, D.; Houdouin, V.; Hermet, E.; Blin, N.; Nicolini, F.; Michallet, M.; Dominique, S.; Huynh, A.; et al. Pleuroparenchymal fibroelastosis after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020, 55, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Higo, H.; Miyahara, N.; Taniguchi, A.; Maeda, Y.; Kiura, K. Cause of pleuroparenchymal fibroelastosis following allogeneic hematopoietic stem cell transplantation. Respir. Investig. 2019, 57, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Miyagawa-Hayashino, A.; Chen, F.; Kubo, T.; Handa, T.; Datet, H.; Halga, H. Pleuroparenchymal fibroelastosis and non-specific interstitial pneumonia: Frequent pulmonary sequelae of haematopoietic stem cell transplantation. Histopathology 2015, 66, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Gazourian, L.; Spring, L.; Meserve, E.; Hwang, D.; Diaz, A.A.; Ash, S.Y.; Ho, V.T.; Sholl, L.M.; Washko, G.R. Pulmonary Clinicopathological Correlation after Allogeneic Hematopoietic Stem Cell Transplantation: An Autopsy Series. Biol Blood Marrow Transplant. 2017, 23, 1767–1772. [Google Scholar] [CrossRef]
- Bunte, M.C.; Patnaik, M.M.; Pritzker, M.R.; Burns, L.J. Pulmonary veno-occlusive disease following hematopoietic stem cell transplantation: A rare model of endothelial dysfunction. Bone Marrow Transplant. 2008, 41, 677–686. [Google Scholar] [CrossRef]
- Mandel, J.; Mark, E.J.; Hales, C.A. Pulmonary Veno-occlusive Disease. Am. J. Respir. Crit. Care Med. 2000, 162, 1964–1973. [Google Scholar] [CrossRef]
- Resten, A.; Maitre, S.; Humbert, M.; Rabiller, A.; Sitbon, O.; Capron, F.; Simonneau, G.; Musset, D. Pulmonary Hypertension: CT of the Chest in Pulmonary Venoocclusive Disease. Am. J. Roentgenol. 2004, 183, 65–70. [Google Scholar] [CrossRef]
- Dykewicz, C.A. Summary of the Guidelines for Preventing Opportunistic Infections among Hematopoietic Stem Cell Transplant Recipients. Clin. Infect. Dis. 2001, 33, 139–144. [Google Scholar] [CrossRef]
- Omrani, A.S.; Almaghrabi, R.S. Complications of hematopoietic stem cell transplantation: Bacterial infections. Hematol. Oncol. Stem Cell Ther. 2017, 10, 228–232. [Google Scholar] [CrossRef]
- Einsele, H.; Bertz, H.; Beyer, J.; Kiehl, M.G.; Runde, V.; Kolb, H.-J.; Holler, E.; Beck, R.; Schwerdfeger, R.; Schumacher, U.; et al. Infectious complications after allogeneic stem cell transplantation: Epidemiology and interventional therapy strategies—guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann. Hematol. 2003, 82 (Suppl. 2), S175–S185. [Google Scholar] [CrossRef]
- Balletto, E.; Mikulska, M. Bacterial Infections in Hematopoietic Stem Cell Transplant Recipients. Mediterr. J. Hematol. Infect. Dis. 2015, 7, e2015045. [Google Scholar] [CrossRef]
- Sahin, U.; Toprak, S.K.; Atilla, P.A.; Atilla, E.; Demirer, T. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J. Infect. Chemother. 2016, 22, 505–514. [Google Scholar] [CrossRef]
- Diab, M.; ZazaDitYafawi, J.; Soubani, A.O. Major Pulmonary Complications After Hematopoietic Stem Cell Transplant. Exp. Clin. Transplant. 2016, 14, 259–270. [Google Scholar] [CrossRef][Green Version]
- Kotloff, R.M.; Ahya, V.N.; Crawford, S.W. Pulmonary Complications of Solid Organ and Hematopoietic Stem Cell Transplantation. Am. J. Respir. Crit. Care Med. 2004, 170, 22–48. [Google Scholar] [CrossRef] [PubMed]
- Lossos, I.S.; Breuer, R.; Or, R.; Strauss, N.; Elishoov, H.; Naparstek, E.; Aker, M.; Nagler, A.; Moses, A.E.; Shapiro, M.; et al. Bacterial pneumonia in recipients of bone marrow transplantation. A five-year prospective study. Transplantation 1995, 60, 672–678. [Google Scholar] [CrossRef]
- Aguilar-Guisado, M.; Jiménez-Jambrina, M.; Espigado, I.; Rovira, M.; Martino, R.; Oriol, A.; Borrell, N.; Ruiz, I.; Martín-Dávila, P.; de la Camara, R.; et al. Pneumonia in allogeneic stem cell transplantation recipients: A multicenter prospective study. Clin. Transplant. 2011, 25, E629–E638. [Google Scholar] [CrossRef]
- Cordonnier, C.; Martino, R.; Trabasso, P.; Held, T.K.; Akan, H.; Ward, M.S.; Fabian, K.; Ullmann, A.J.; Wulffraat, N.; Ljungman, P.; et al. Mycobacterial Infection: A Difficult and Late Diagnosis in Stem Cell Transplant Recipients. Clin. Infect. Dis. 2004, 38, 1229–1236. [Google Scholar] [CrossRef]
- Gea-Banacloche, J. Pulmonary infectious complications after hematopoietic stem cell transplantation: A practical guide to clinicians. Curr. Opin. Organ. Transplant. 2018, 23, 375–380. [Google Scholar] [CrossRef]
- De La Cruz, O.; Silveira, F.P. Respiratory Fungal Infections in Solid Organ and Hematopoietic Stem Cell Transplantation. Clin. Chest Med. 2017, 38, 727–739. [Google Scholar] [CrossRef]
- Gea-Banacloche, J. Risks and Epidemiology of Infections After Hematopoietic Stem Cell Transplantation. In Transplant Infections, 4th ed.; Ljungman, P., Snydman, D., Boeckh, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 81–99. [Google Scholar]
- Chuzi, S.; Tavora, F.; Cruz, M.; Costa, R.; Chaet, Y.K.; A Carneiro, B.; Giles, F.J. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag. Res. 2017, 9, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Henzler, C.; Henzler, T.; Buchheidt, D.; Nance, J.W.; Weis, C.A.; Vogelmann, R.; Benck, U.; Viergutz, T.; Becher, T.; Boch, T.; et al. Diagnostic Performance of Contrast Enhanced Pulmonary Computed Tomography Angiography for the Detection of Angioinvasive Pulmonary Aspergillosis in Immunocompromised Patients. Sci. Rep. 2017, 7, 4483. [Google Scholar] [CrossRef] [PubMed]
- Young, J.-A.H. Infectious complications of acute and chronic GVHD. Best Pract. Res. Clin. Haematol. 2008, 21, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Limper, A.H.; Knox, K.S.; Sarosi, G.A.; Ampel, N.M.; Bennett, J.E.; Catanzaro, A.; Davies, S.F.; Dismukes, W.E.; Hage, C.A.; Marr, K.A.; et al. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am. J. Respir. Crit. Care Med. 2011, 183, 96–128. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Sun, H.-Y.; Ribaud, P.; Singh, N.; Kontoyiannis, D.P.; Lortholary, O. Mucormycosis in Organ and Stem Cell Transplant Recipients. Clin. Infect. Dis. 2012, 54, 1–8. [Google Scholar] [CrossRef]
- Jung, J.; Kim, M.Y.; Lee, H.J.; Park, Y.; Lee, S.-O.; Choi, S.-H.; Kim, Y.; Woo, J.; Kim, S.-H. Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin. Microbiol. Infect. 2015, 21, 684.e11–684.e18. [Google Scholar] [CrossRef]
- Tacke, D.; Buchheidt, D.; Karthaus, M.; Krause, S.; Maschmeyer, G.; Neumann, S.; Ostermann, H.; Penack, O.; Rieger, C.; Ruhnke, M.; et al. Primary prophylaxis of invasive fungal infections in patients with haematologic malignancies. 2014 update of the recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Ann. Hematol. 2014, 93, 1449–1456. [Google Scholar] [CrossRef]
- Stern, A.; Green, H.; Paul, M.; Vidal, L.; Leibovici, L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst. Rev. 2014, Cd005590. [Google Scholar] [CrossRef]
- Bondeelle, L.; Bergeron, A. Managing pulmonary complications in allogeneic hematopoietic stem cell transplantation. Expert Rev. Respir. Med. 2019, 13, 105–119. [Google Scholar] [CrossRef]
- Tomblyn, M.; Chiller, T.; Einsele, H.; Gress, R.; Sepkowitz, K.; Storek, J.; Wingalrd, J.R.; Young, J.-A.; Boeckh, M.A. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: A global perspective. Biol. Blood Marrow Transplant. 2009, 15, 1143–1238. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.M.; Dulley, F.L.; Boas, L.S.; Castetlli, J.B.; A Macedo, M.C.; Silva, R.L.; Pallota, R.; Saboya, R.S.; Pannuti, C.S. CMV pneumonia in allogeneic BMT recipients undergoing early treatment of pre-emptive ganciclovir therapy. Bone Marrow Transplant. 2000, 26, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, S.; Champlin, R.E.; Giralt, S.; Ueno, N.T.; Khouri, I.; Raad, I.; Rolston, K.; Jacobson, K.; Tarrand, J.; Luna, M.; et al. Cytomegalovirus pneumonia in adult autologous blood and marrow transplant recipients. Bone Marrow Transplant. 2001, 27, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Wieruszewski, P.M.; Herasevich, S.; Gajic, O.; Yadav, H. Respiratory failure in the hematopoietic stem cell transplant recipient. World J. Crit. Care Med. 2018, 7, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Travi, G.; Pergam, S.A. Cytomegalovirus pneumonia in hematopoietic stem cell recipients. J. Intensive Care Med. 2014, 29, 200–212. [Google Scholar] [CrossRef]
- Ison, M.G.; Fishman, J.A. Cytomegalovirus pneumonia in transplant recipients. Clin. Chest Med. 2005, 26, 691–705. [Google Scholar] [CrossRef]
- Tamm, M.; Traenkle, P.; Grilli, B.; Solèr, M.; Bolliger, C.T.; Grilli, B.; et Dalquen, P.; Cathomas, G. Pulmonary cytomegalovirus infection in immunocompromised patients. Chest 2001, 119, 838–843. [Google Scholar] [CrossRef]
- Franquet, T.; Lee, K.S.; Müller, N.L. Thin-section CT findings in 32 immunocompromised patients with cytomegalovirus pneumonia who do not have AIDS. AJR Am. J. Roentgenol. 2003, 181, 1059–1063. [Google Scholar] [CrossRef]
- Meng, X.Y.; Fu, H.X.; Zhu, X.L.; Wang, J.-Z.; Liu, X.; Yan, C.-H.; Zhang, Y.-Y.; Mo, X.-D.; Wang, Y.; Han, W.; et al. Comparison of different cytomegalovirus diseases following haploidentical hematopoietic stem cell transplantation. Ann. Hematol. 2020, 99, 2659–2670. [Google Scholar] [CrossRef]
- Erard, V.; Guthrie, K.A.; Seo, S.; Smith, J.; Huang, M.; Chietn, J.; Flowers, M.E.D.; Corey, L.; Boeckh, M. Reduced Mortality of Cytomegalovirus Pneumonia After Hematopoietic Cell Transplantation Due to Antiviral Therapy and Changes in Transplantation Practices. Clin. Infect. Dis. 2015, 61, 31–39. [Google Scholar] [CrossRef]
- Ljungman, P.; Ellis, M.N.; Hackman, R.C.; Shepp, D.H.; Meyers, J.D. Acyclovir—resistant herpes simplex virus caresuepneumonia after marrow transplantation. J. Infect. Dis. 1990, 162, 244–248. [Google Scholar] [CrossRef]
- Jellinge, M.E.; Hansen, F.; Coia, J.E.; Song, Z. Herpes Simplex Virus Type 1 Pneumonia-A Review. J. Intensive Care Med. 2021, 885066620965941. [Google Scholar] [CrossRef]
- Taplitz, R.A.; Jordan, M.C. Pneumonia caused by herpesviruses in recipients of hematopoietic cell transplants. Semin. Respir. Infect. 2002, 17, 121–129. [Google Scholar] [CrossRef]
- Dadwal, S.S. Herpes Virus Infections Other than Cytomegalovirus in the Recipients of Hematopoietic Stem Cell Transplantation. Infect. Dis. Clin. N. Am. 2019, 33, 467–484. [Google Scholar] [CrossRef]
- Chemaly, R.F.; Ghosh, S.; Bodey, G.P.; Rohatgi, N.; Safdar, A.; Keating, M.J.; Champlin, R.E.; Aguilera, E.A.; Tarrand, J.J.; Raad, I.I. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: A retrospective study at a major cancer center. Medicine 2006, 85, 278–287. [Google Scholar] [CrossRef]
- Wang, L.; Allen, J.; Diong, C.; Goh, Y.-T.; Gopalakrishnan, S.; Ho, A.; Hwang, W.; Lim, F.; Oon, L.; Tan, T.-T.; et al. Respiratory virus infection after allogeneic hematopoietic stem cell transplant in a tropical center: Predictive value of the immunodeficiency scoring index. Transpl. Infect. Dis. 2017, 19, e12693. [Google Scholar] [CrossRef]
- Schiffer, J.T.; Kirby, K.; Sandmaier, B.; Storb, R.; Corey, L.; Boeckh, M. Timing and severity of community acquired respiratory virus infections after myeloablative versus non-myeloablative hematopoietic stem cell transplantation. Haematologica 2009, 94, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clin. Infect. Dis. 2014, 58, E44–E100. [Google Scholar] [CrossRef]
- Ljungman, P.; Ward, K.N.; Crooks, B.N.; Parker, A.; Martino, R.; Shaw, P.; Brinch, L.; Brune, M.; De La Camara, R.; Dekker, A.W.; et al. Respiratory virus infections after stem cell transplantation: A prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001, 28, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.P.; Ghantoji, S.S.; Shah, J.N.; El Taoum, K.K.; Jiang, Y.; Popat, U.; Hosing, C.; Rondon, G.; Tarrand, J.J.; Champlin, R.E.; et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J. Antimicrob. Chemother. 2013, 68, 1872–1880. [Google Scholar] [CrossRef]
- Kim, Y.J.; Guthrie, K.A.; Waghmare, A.; Walsh, E.E.; Falsey, A.R.; Kuypers, J.; Cent, A.; Etnglund, J.A.; Boeckh, M. Respiratory syncytial virus in hematopoietic cell transplant recipients: Factors determining progression to lower respiratory tract disease. J. Infect. Dis. 2014, 209, 1195–1204. [Google Scholar] [CrossRef]
- Pilie, P.; Werbel, W.A.; Riddell Jt Shu, X.; Schaubel, D.; Gregg, K.S. Adult patients with respiratory syncytial virus infection: Impact of solid organ and hematopoietic stem cell transplantation on outcomes. Transpl. Infect. Dis. 2015, 17, 551–557. [Google Scholar] [CrossRef]
- Renaud, C.; Xie, H.; Seo, S.; Kuypers, J.; Cent, A.; Corey, L.; Leisenring, W.; Boeckh, M.; Etnglund, J.A. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol. Blood Marrow Transplant. 2013, 19, 1220–1226. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Kingman, H.M.; Kelly, C.; Taylor, G.S.; O Caul, E.; Grier, D.; Moppett, J.; Foot, A.B.M.; Cornish, J.M.; Oakhill, A.; et al. The outcome of 26 patients with respiratory syncytial virus infection following allogeneic stem cell transplantation. Bone Marrow Transplant. 1999, 24, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Campbell, A.P.; Xie, H.; Chien, J.W.; Leisenring, W.; Etnglund, J.A.; Boeckh, M. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: Significance of stem cell source and oxygen requirement. Biol. Blood Marrow Transplant. 2013, 19, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bhatt, N.S.; St Martin, A.; Abid, M.B.; Bloomquist, J.; Chemaly, R.F.; Dandoy, C.; Gauthier, J.; Gowda, L.; Perales, M.-A.; et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: An observational cohort study. Lancet Haematol. 2021, 8, e185–e193. [Google Scholar] [CrossRef]
- Varma, A.; Kosuri, S.; Ustun, C.; lbrahim, U.; Moreira, J.; Bishop, M.R.; Nathan, S.; Mehta, J.; Moncayo, D.; Heng, J.; et al. COVID-19 infection in hematopoietic cell transplantation: Age, time from transplant and steroids matter. Leukemia 2020, 34, 2809–2812. [Google Scholar] [CrossRef]
- Shah, G.L.; DeWolf, S.; Lee, Y.J.; Tamari, R.; Dahi, P.B.; Lavery, J.A.; Ruiz, J.D.; Devlin, S.M.; Cho, C.; Peled, J.U.; et al. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J. Clin. Investig. 2020, 130, 6656–6667. [Google Scholar] [CrossRef]
- Altuntas, F.; Ata, N.; Yigenoglu, T.N.; Bascı, S.; Dal, M.S.; Korkmaz, S.; Namdaroglu, S.; Basturk, A.; Hacıbekiroglu, T.; Dogu, M.H.; et al. COVID-19 in hematopoietic cell transplant recipients. Bone Marrow Transplant. 2021, 56, 952–955. [Google Scholar] [CrossRef]
- Piñana, J.L.; Martino, R.; García-García, I.; Parody, R.; Morales, M.D.; Benzo, G.; Gómez-Catalan, I.; Coll, R.; De La Fuente, I.; Luna, A.; et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp. Hematol. Oncol. 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, A.; Abidi, M.Z.; Boeckh, M.; Chemaly, R.F.; Dadwal, S.; EBoghdadly, Z.; Kamboj, M.; Papanicolaou, G.A.; Pergam, S.A.; Shahid, Z.; et al. Guidelines for COVID-19 Management in Hematopoietic Cell Transplantation and Cellular Therapy Recipients. Biol. Blood Marrow Transplant. 2020, 26, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Mikulska, M.; de la Camara, R.; Basak, G.W.; Chabannon, C.; Corbacioglu, S.; Duarte, R.; Dolstra, H.; Lankester, A.C.; Mohty, M.; et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020, 55, 2071–2076. [Google Scholar] [CrossRef]
- Sakata, K.K.; Klassen, C.L.; Bollin, K.B.; Grys, T.E.; Slack, J.L.; Wesselius, L.J.; Vikra, H.R. Microbiologic yield of bronchoalveolar lavage specimens from stem cell transplant recipients. Transpl. Infect. Dis. 2017, 19, e12684. [Google Scholar] [CrossRef] [PubMed]
- Wahla, A.S.; Chatterjee, A.; Khan, I.I.; Conforti, J.F.; Haponik, E. Survey of academic pulmonologists, oncologists, and infectious disease physicians on the role of bronchoscopy in managing hematopoietic stem cell transplantation patients with pulmonary infiltrates. J. Bronchol. Interv. Pulmonol. 2014, 21, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Shannon, V.R.; Andersson, B.S.; Lei, X.; Champlin, R.E.; Kontoyiannis, D.P. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010, 45, 647–655. [Google Scholar] [CrossRef]
- Jorge, L.; Torres, D.; Languasco, A.; Rodriguez, P.; Bonvehí, P.; Temporiti, E.; Relloso, S.; Videlal, C.; Herrera, F. Clinical Usefulness of Bronchoalveolar Lavage in the Management of Pulmonary Infiltrates in Adults with Hematological Malignancies and Stem Cell Transplantation. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020025. [Google Scholar] [CrossRef]
- Feinstein, M.B.; Habtes, I.; Giralt, S.; Stover, D.E. Utility of Bronchoscopy with Bronchoalveolar Lavage among Hematologic Transplant Recipients in the Era of Noninvasive Testing. Respiration 2021, 100, 339–346. [Google Scholar] [CrossRef]
- Harris, B.; Lowy, F.D.; Stover, D.E.; Arcasoy, S.M. Diagnostic bronchoscopy in solid-organ and hematopoietic stem cell transplantation. Ann. Am. Thorac. Soc. 2013, 10, 39–49. [Google Scholar] [CrossRef]
- Gilbert, C.R.; Lerner, A.; Baram, M.; Awsare, B.K. Utility of flexible bronchoscopy in the evaluation of pulmonary infiltrates in the hematopoietic stem cell transplant population—A single center fourteen year experience. Arch. Bronconeumol. 2013, 49, 189–195. [Google Scholar] [CrossRef]
- Mulabecirovic, A.; Gaulhofer, P.; Auner, H.W.; Popper, H.; Krause, R.; Hesse, C.; Sill, H. Pulmonary infiltrates in patients with haematologic malignancies: Transbronchial lung biopsy increases the diagnostic yield with respect to neoplastic infiltrates and toxic pneumonitis. Ann. Hematol. 2004, 83, 420–422. [Google Scholar] [CrossRef]
- O’Dwyer, D.N.; Duvall, A.S.; Xia, M.; Hoffman, T.C.; Bloye, K.S.; Bulte, C.A.; Zhou, X.; Murray, S.; Moore, B.; Yanik, G.A. Transbronchial biopsy in the management of pulmonary complications of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2018, 53, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Ikezawa, Y.; Shinagawa, N.; Sukoh, N.; Morimoto, M.; Kikuchi, H.; Watanabe, M.; Nakano, K.; Oizumi, S.; Nishimura, M. Usefulness of Endobronchial Ultrasonography With a Guide Sheath and Virtual Bronchoscopic Navigation for Ground-Glass Opacity Lesions. Ann. Thorac. Surg. 2017, 103, 470–475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hayama, M.; Okamoto, N.; Suzuki, H.; Tamiya, M.; Shiroyama, T.; Tanaka, A.; Nishida, T.; Nishihara, T.; Uehara, N.; Morishita, N.; et al. Radial endobronchial ultrasound with a guide sheath for diagnosis of peripheral cavitary lung lesions: A retrospective study. BMC Pulm. Med. 2016, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Bouso, J.M.; Yendur, O.; Hysinger, E.; Planet, P.J.; Haas, A.; Goldfarb, S.; Piccione, J. Endobronchial Ultrasound–guided Biopsy Is Feasible, Safe, and Improves Diagnostic Yields in Immunocompromised Children. Am. J. Respir. Crit. Care Med. 2019, 201, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.; Geyer, A.I. Diagnostic Evaluation of Pulmonary Abnormalities in Patients with Hematologic Malignancies and Hematopoietic Cell Transplantation. Clin. Chest Med. 2017, 38, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Carrafiello, G.; Laganà, D.; Nosari, A.M.; Guffanti, C.; Morra, E.; Recaldini, C.; D’Alba, M.J.; Sonvico, U.; Vanzulli, A.; Fugazzola, C. Utility of computed tomography (CT) and of fine needle aspiration biopsy (FNAB) in early diagnosis of fungal pulmonary infections. Study of infections from filamentous fungi in haematologically immunodeficient patients. Radiol. Med. 2006, 111, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.L.; Ramsay, N.K.; McGlave, P.B.; Ferrell, K.L.; Leonard, A.S. Diagnostic open-lung biopsy after bone marrow transplantation. J. Pediatr. Surg. 1990, 25, 871–876. [Google Scholar] [CrossRef]
- Chellapandian, D.; Lehrnbecher, T.; Phillips, B.; Fisher, B.T.; Zaoutis, T.E.; Steinbach, W.J.; Beyene, J.; Sung, L. Bronchoalveolar lavage and lung biopsy in patients with cancer and hematopoietic stem-cell transplantation recipients: A systematic review and meta-analysis. J. Clin. Oncol. 2015, 33, 501–509. [Google Scholar] [CrossRef]
- Wingard, J.R.; Hiemenz, J.W.; Jantz, M.A. How I manage pulmonary nodular lesions and nodular infiltrates in patients with hematologic malignancies or undergoing hematopoietic cell transplantation. Blood 2012, 120, 1791–1800. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astashchanka, A.; Ryan, J.; Lin, E.; Nokes, B.; Jamieson, C.; Kligerman, S.; Malhotra, A.; Mandel, J.; Joshua, J. Pulmonary Complications in Hematopoietic Stem Cell Transplant Recipients—A Clinician Primer. J. Clin. Med. 2021, 10, 3227. https://doi.org/10.3390/jcm10153227
Astashchanka A, Ryan J, Lin E, Nokes B, Jamieson C, Kligerman S, Malhotra A, Mandel J, Joshua J. Pulmonary Complications in Hematopoietic Stem Cell Transplant Recipients—A Clinician Primer. Journal of Clinical Medicine. 2021; 10(15):3227. https://doi.org/10.3390/jcm10153227
Chicago/Turabian StyleAstashchanka, Anna, Joseph Ryan, Erica Lin, Brandon Nokes, Catriona Jamieson, Seth Kligerman, Atul Malhotra, Jess Mandel, and Jisha Joshua. 2021. "Pulmonary Complications in Hematopoietic Stem Cell Transplant Recipients—A Clinician Primer" Journal of Clinical Medicine 10, no. 15: 3227. https://doi.org/10.3390/jcm10153227
APA StyleAstashchanka, A., Ryan, J., Lin, E., Nokes, B., Jamieson, C., Kligerman, S., Malhotra, A., Mandel, J., & Joshua, J. (2021). Pulmonary Complications in Hematopoietic Stem Cell Transplant Recipients—A Clinician Primer. Journal of Clinical Medicine, 10(15), 3227. https://doi.org/10.3390/jcm10153227





