The ENDOPAIN 4D Questionnaire: A New Validated Tool for Assessing Pain in Endometriosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. ENDOPAIN-4D Development
2.2. Recruitment Sites and Treatment
2.3. Endometriosis Treatment
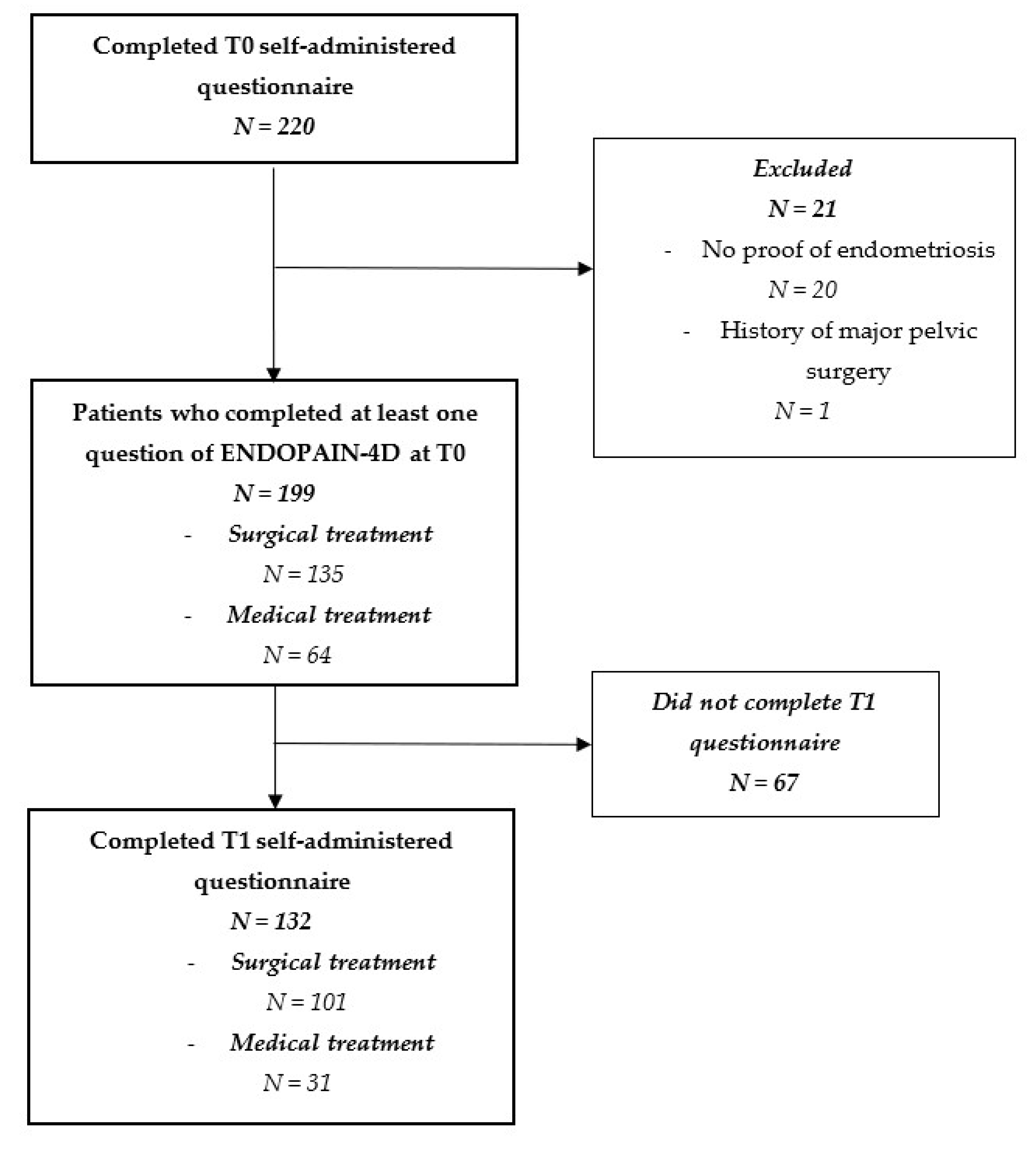
2.4. Study Population
2.5. Design and Data Collection
2.6. Statistical Analysis
2.7. Ethics
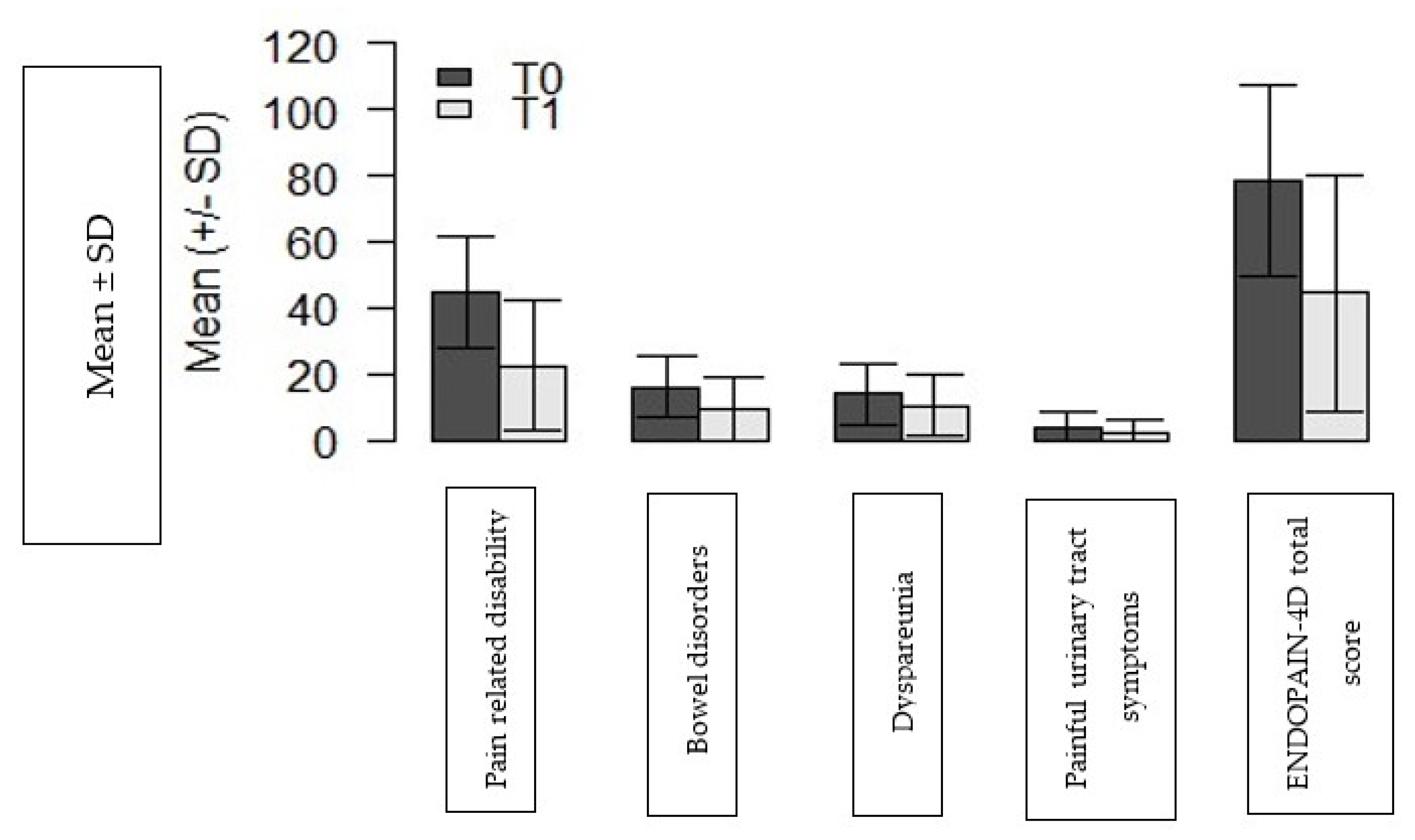
3. Results
Participants
4. Discussion
4.1. Strengths and Weaknesses
4.2. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Facchin, F.; Barbara, G.; Saita, E.; Mosconi, P.; Roberto, A.; Fedele, L.; Vercellini, P. Impact of Endometriosis on Quality of Life and Mental Health: Pelvic Pain Makes the Difference. J. Psychosom. Obstet. Gynaecol. 2015, 36, 135–141. [Google Scholar] [CrossRef]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The Burden of Endometriosis: Costs and Quality of Life of Women with Endometriosis and Treated in Referral Centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef] [Green Version]
- Patrick, D.L.; Burke, L.B.; Powers, J.H.; Scott, J.A.; Rock, E.P.; Dawisha, S.; O’Neill, R.; Kennedy, D.L. Patient-Reported Outcomes to Support Medical Product Labeling Claims: FDA Perspective. Value Health 2007, 10 (Suppl. 2), S125–S137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, J.; Hawe, J.; Hunter, D.; Holmes, M.; Finn, P.; Garry, R. Laparoscopic Excision of Endometriosis: A Randomized, Placebo-Controlled Trial. Fertil. Steril. 2004, 82, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Kosaka, S.; Elliesen, J.; Yasuda, M.; Ito, M.; Momoeda, M. Ethinylestradiol 20 Μg/Drospirenone 3 Mg in a Flexible Extended Regimen for the Management of Endometriosis-Associated Pelvic Pain: A Randomized Controlled Trial. Fertil. Steril. 2017, 108, 798–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodaverdi, S.; Mohammadbeigi, R.; Khaledi, M.; Mesdaghinia, L.; Sharifzadeh, F.; Nasiripour, S.; Gorginzadeh, M. Beneficial Effects of Oral Lactobacillus on Pain Severity in Women Suffering from Endometriosis: A Pilot Placebo-Controlled Randomized Clinical Trial. Int. J. Fertil. Steril. 2019, 13, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Fedele, L.; Arcaini, L.; Bianchi, S.; Baglioni, A.; Vercellini, P. Comparison of Cyproterone Acetate and Danazol in the Treatment of Pelvic Pain Associated with Endometriosis. Obstet. Gynecol. 1989, 73, 1000–1004. [Google Scholar] [CrossRef]
- Bourdel, N.; Alves, J.; Pickering, G.; Ramilo, I.; Roman, H.; Canis, M. Systematic Review of Endometriosis Pain Assessment: How to Choose a Scale? Hum. Reprod. Update 2015, 21, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Fauconnier, A.; Staraci, S.; Huchon, C.; Roman, H.; Panel, P.; Descamps, P. Comparison of Patient- and Physician-Based Descriptions of Symptoms of Endometriosis: A Qualitative Study. Hum. Reprod. 2013, 28, 2686–2694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauconnier, A.; Staraci, S.; Daraï, E.; Descamps, P.; Nisolle, M.; Panel, P.; Roman, H.; Boulkedid, R. A Self-Administered Questionnaire to Measure the Painful Symptoms of Endometriosis: Results of a Modified DELPHI Survey of Patients and Physicians. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 69–79. [Google Scholar] [CrossRef]
- Guillemin, F.; Bombardier, C.; Beaton, D. Cross-Cultural Adaptation of Health-Related Quality of Life Measures: Literature Review and Proposed Guidelines. J. Clin. Epidemiol. 1993, 46, 1417–1432. [Google Scholar] [CrossRef]
- Chapron, C.; Fauconnier, A.; Vieira, M.; Barakat, H.; Dousset, B.; Pansini, V.; Vacher-Lavenu, M.C.; Dubuisson, J.B. Anatomical Distribution of Deeply Infiltrating Endometriosis: Surgical Implications and Proposition for a Classification. Hum. Reprod. 2003, 18, 157–161. [Google Scholar] [CrossRef]
- Bazot, M.; Daraï, E. Diagnosis of Deep Endometriosis: Clinical Examination, Ultrasonography, Magnetic Resonance Imaging, and Other Techniques. Fertil. Steril. 2017, 108, 886–894. [Google Scholar] [CrossRef] [Green Version]
- Ballester, M.; Chereau, E.; Dubernard, G.; Coutant, C.; Bazot, M.; Daraï, E. Urinary Dysfunction after Colorectal Resection for Endometriosis: Results of a Prospective Randomized Trial Comparing Laparoscopy to Open Surgery. Am. J. Obstet. Gynecol. 2011, 204, 303.e1–303.e6. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, A.; Panel, P.; Rouquette, A.; du Cheyron, J.; Deffieux, X.; Fauconnier, A. Validation of the Sexual Activity Questionnaire in Women with Endometriosis. Hum. Reprod. 2019, 34, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Aubry, G.; Panel, P.; Thiollier, G.; Huchon, C.; Fauconnier, A. Measuring Health-Related Quality of Life in Women with Endometriosis: Comparing the Clinimetric Properties of the Endometriosis Health Profile-5 (EHP-5) and the EuroQol-5D (EQ-5D). Hum. Reprod. 2017, 32, 1258–1269. [Google Scholar] [CrossRef]
- Crosby, R.D.; Kolotkin, R.L.; Williams, G.R. Defining Clinically Meaningful Change in Health-Related Quality of Life. J. Clin. Epidemiol. 2003, 56, 395–407. [Google Scholar] [CrossRef]
- Scholtes, V.A.; Terwee, C.B.; Poolman, R.W. What Makes a Measurement Instrument Valid and Reliable? Injury 2011, 42, 236–240. [Google Scholar] [CrossRef]
- Revised American Society for Reproductive Medicine Classification of Endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [CrossRef]
- Chapron, C.; Dubuisson, J.B.; Chopin, N.; Foulot, H.; Jacob, S.; Vieira, M.; Barakat, H.; Fauconnier, A. Deep pelvic endometriosis: Management and proposal for a “surgical classification”. Gynecol. Obstet. Fertil. 2003, 31, 197–206. [Google Scholar] [CrossRef]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of Health Status. Ascertaining the Minimal Clinically Important Difference. Control Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef]
- Brozek, J.L.; Guyatt, G.H.; Schünemann, H.J. How a Well-Grounded Minimal Important Difference Can Enhance Transparency of Labelling Claims and Improve Interpretation of a Patient Reported Outcome Measure. Health Qual. Life Outcomes 2006, 4, 69. [Google Scholar] [CrossRef] [Green Version]
- Terwee, C.B.; Bot, S.D.M.; de Boer, M.R.; van der Windt, D.A.W.M.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C.W. Quality Criteria Were Proposed for Measurement Properties of Health Status Questionnaires. J. Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, T.D.; Hodge, D.O.; Christian, T.F.; Milavetz, J.J.; Bailey, K.R.; Gibbons, R.J. Effects of Adjustment for Referral Bias on the Sensitivity and Specificity of Single Photon Emission Computed Tomography for the Diagnosis of Coronary Artery Disease. Am. J. Med. 2002, 112, 290–297. [Google Scholar] [CrossRef]
- Fedele, L.; Parazzini, F.; Bianchi, S.; Arcaini, L.; Candiani, G.B. Stage and Localization of Pelvic Endometriosis and Pain. Fertil. Steril. 1990, 53, 155–158. [Google Scholar] [CrossRef]
- Vercellini, P.; Trespidi, L.; De Giorgi, O.; Cortesi, I.; Parazzini, F.; Crosignani, P.G. Endometriosis and Pelvic Pain: Relation to Disease Stage and Localization. Fertil. Steril. 1996, 65, 299–304. [Google Scholar] [CrossRef]
- Ballester, M.; Santulli, P.; Bazot, M.; Coutant, C.; Rouzier, R.; Daraï, E. Preoperative Evaluation of Posterior Deep-Infiltrating Endometriosis Demonstrates a Relationship with Urinary Dysfunction and Parametrial Involvement. J. Minim. Invasive Gynecol. 2011, 18, 36–42. [Google Scholar] [CrossRef]
- Laganà, A.S.; La Rosa, V.L.; Rapisarda, A.M.C.; Valenti, G.; Sapia, F.; Chiofalo, B.; Rossetti, D.; Ban Frangež, H.; Vrtačnik Bokal, E.; Vitale, S.G. Anxiety and Depression in Patients with Endometriosis: Impact and Management Challenges. Int. J. Womens Health 2017, 9, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Arena, A.; Orsini, B.; Degli Esposti, E.; Manzara, F.; Ambrosio, M.; Raimondo, D.; Lenzi, J.; Casadio, P.; Seracchioli, R. The Unbearable Burden of Endometriosis: Results from a Large Cohort about Anxiety Reduction during the First Outpatient Evaluation. J. Psychosom. Res. 2021, 147, 110512. [Google Scholar] [CrossRef]
- Hackethal, A.; Luck, C.; von Hobe, A.-K.; Eskef, K.; Oehmke, F.; Konrad, L. A Structured Questionnaire Improves Preoperative Assessment of Endometriosis Patients: A Retrospective Analysis and Prospective Trial. Arch. Gynecol. Obstet. 2011, 284, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Gruppo Italiano per lo Studio dell’Endometriosi Relationship between Stage, Site and Morphological Characteristics of Pelvic Endometriosis and Pain. Hum. Reprod. 2001, 16, 2668–2671. [CrossRef] [PubMed]
- Chapron, C.; Barakat, H.; Fritel, X.; Dubuisson, J.-B.; Bréart, G.; Fauconnier, A. Presurgical Diagnosis of Posterior Deep Infiltrating Endometriosis Based on a Standardized Questionnaire. Hum. Reprod. 2005, 20, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Fedele, L.; Bianchi, S.; Carmignani, L.; Berlanda, N.; Fontana, E.; Frontino, G. Evaluation of a New Questionnaire for the Presurgical Diagnosis of Bladder Endometriosis. Hum. Reprod. 2007, 22, 2698–2701. [Google Scholar] [CrossRef] [Green Version]
- Center for Drug Evaluation and Research. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Guidance for Industry; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2009. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims (accessed on 8 July 2021).
- Deal, L.S.; DiBenedetti, D.B.; Williams, V.S.; Fehnel, S.E. The Development and Validation of the Daily Electronic Endometriosis Pain and Bleeding Diary. Health Qual. Life Outcomes 2010, 8, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nooten, F.E.; Cline, J.; Elash, C.A.; Paty, J.; Reaney, M. Development and Content Validation of a Patient-Reported Endometriosis Pain Daily Diary. Health Qual. Life Outcomes 2018, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Wyrwich, K.W.; O’Brien, C.F.; Soliman, A.M.; Chwalisz, K. Development and Validation of the Endometriosis Daily Pain Impact Diary Items to Assess Dysmenorrhea and Nonmenstrual Pelvic Pain. Reprod. Sci. 2018, 25, 1567–1576. [Google Scholar] [CrossRef]
- Jacobson, T.Z.; Duffy, J.M.N.; Barlow, D.; Koninckx, P.R.; Garry, R. Laparoscopic Surgery for Pelvic Pain Associated with Endometriosis. Cochrane Database Syst. Rev. 2009, 4, CD001300. [Google Scholar] [CrossRef]
- Brown, J.; Crawford, T.J.; Datta, S.; Prentice, A. Oral Contraceptives for Pain Associated with Endometriosis. Cochrane Database Syst. Rev. 2018, 5, CD001019. [Google Scholar] [CrossRef]
- Biberoglu, K.O.; Behrman, S.J. Dosage Aspects of Danazol Therapy in Endometriosis: Short-Term and Long-Term Effectiveness. Am. J. Obstet. Gynecol. 1981, 139, 645–654. [Google Scholar] [CrossRef]
- Strowitzki, T.; Marr, J.; Gerlinger, C.; Faustmann, T.; Seitz, C. Detailed Analysis of a Randomized, Multicenter, Comparative Trial of Dienogest versus Leuprolide Acetate in Endometriosis. Int. J. Gynaecol. Obstet. 2012, 117, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Vincent, K.; Kennedy, S.; Stratton, P. Pain Scoring in Endometriosis: Entry Criteria and Outcome Measures for Clinical Trials. Report from the Art and Science of Endometriosis Meeting. Fertil. Steril. 2010, 93, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| All Patients n = 199 | Patients Who Completed ENDOPAIN-4D at T1 n = 132 | Patients Who Did Not Complete ENDOPAIN-4D at T1 n = 67 | p Value | |
|---|---|---|---|---|
| Center n (%) | ||||
| 102 (51.0) | 65 (49.2) | 37 (55.2) | |
| 66 (33.0) | 47 (35.6) | 19 (28.4) | 0.20 | |
| 31 (16.0) | 20 (15.2) | 11 (16.4) | ||
| Main indications for endometriosis treatment n (%) | ||||
| 180 (90.5) | 118 (89.4) | 62 (92.5) | |
| 17 (8.5) | 13 (9.8) | 4 (6.0) | 0.20 | |
| 1 (0.5) | 1 (0.8) | 0 (0) | ||
| 1 (0.5) | 0 | 1 (1.5) | ||
| Age—years mean (sd) | 33.4 (6.8) | 34.9 (7.2) | 33.4 (7.7) | 0.17 |
| BMI—kg/m2 mean (sd) | 24.0 (5.2) | 24.7 (4.7) | 23.1 (4.5) | 0.04 |
| Gravidity—mean (sd) | 0.9 (1.32) | 0.8 (1.3) | 1.0 (1.4) | 0.32 |
| Parity—mean (sd) | 0.6 (0.9) | 0.5 (0.9) | 0.7 (1.0) | 0.47 |
| Medical treatment only—n (%) | 55 (27.6) | 27 (20.5) | 28 (41.8) | |
| Medical treatment and ART n (%) | 9 (4.6) | 4 (0.03) | 5 (7.5) | 0.20 |
| Surgical treatment—n (%) | 135 (67.8) | 101(76.5) | 34 (50.7) | |
| Usual pain (NRS)—mean (SD) | 5.1 (1.6) | 5.0 (2.4) | 5.3 (2.2) | 0.45 |
| Worst case pain (NRS)—mean (SD) | 8.6 (1.6) | 8.6 (1.6) | 8.7 (1.5) | 0.77 |
| rASRM stage * | ||||
| 28 (21.9) | 22 (23.2) | 6 (9.0) | |
| 28 (21.9) | 21 (22.1) | 7 (10.4) | 0.24 | |
| 24 (18.8) | 16 (16.8) | 8 (11.9) | ||
| 48 (37.4) | 36 (37.9) | 12 (17.9) | ||
| Anterior DIE ** | ||||
| 123 (69.9) | 83 (65.4) | 40 (63.5) | 0.21 |
| 45 (25.6) | 33 (26.0) | 12 (19.0) | ||
| 8 (4.5 | 7 (5.5) | 1 (1.5) | ||
| Posterior DIE ** | ||||
| 16 (9.0) | 10 (7.9) | 6 (9.2) | 0.22 |
| 100 (56.2) | 72 (56.7) | 28 (43.1) | ||
| 8 (4.5) | 6 (4.7) | 2 (3.1) | ||
| 4 (2.2) | 3 (2.4) | 1 (1.5) | ||
| 50 (28.1) | 32 (25.2) | 18 (27.7) | ||
| Item (Condensed) | Pain-Related Disability | Painful Bowel Symptoms | Dyspareunia | Painful Urinary Tract Symptoms |
|---|---|---|---|---|
| Dysmenorrhoea | 0.40 | 0.27 | 0.14 | 0.11 |
| Non-menstrual pelvic pain | 0.15 | 0.43 | 0.30 | −0.01 |
| Intense pain | 0.68 | 0.14 | 0.18 | 0.19 |
| Worsening pain | 0.44 | 0.12 | 0.27 | 0.27 |
| Pain before period | 0.41 | 0.13 | 0.19 | 0.23 |
| Stabbing pain | 0.49 | 0.19 | 0.14 | 0.17 |
| Lower back pain | 0.30 | 0.17 | 0.15 | 0.14 |
| Leg/hip pain | 0.44 | 0.32 | −0.07 | 0.05 |
| Disabling pain | 0.78 | 0.10 | 0.01 | 0.05 |
| Pain affects mobility | 0.71 | 0.06 | 0.07 | −0.01 |
| Dyspareunia | 0.05 | 0.25 | 0.79 | 0.13 |
| Positional dyspareunia | 0.22 | 0.15 | 0.68 | 0.02 |
| Interruption of sexual intercourse | 0.11 | −0.02 | 0.76 | 0.21 |
| Painful bowel movements | 0.20 | 0.82 | 0.10 | 0.05 |
| Bowel spasms | 0.13 | 0.79 | 0.10 | 0.05 |
| Diarrhea/constipation | 0.27 | 0.55 | 0.04 | 0.16 |
| Pain when urinating | 0.17 | 0.06 | 0.07 | 0.75 |
| Bladder pain | 0.21 | 0.16 | 0.24 | 0.80 |
| Sciatica | 0.35 | 0.26 | 0.05 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puchar, A.; Panel, P.; Oppenheimer, A.; Du Cheyron, J.; Fritel, X.; Fauconnier, A. The ENDOPAIN 4D Questionnaire: A New Validated Tool for Assessing Pain in Endometriosis. J. Clin. Med. 2021, 10, 3216. https://doi.org/10.3390/jcm10153216
Puchar A, Panel P, Oppenheimer A, Du Cheyron J, Fritel X, Fauconnier A. The ENDOPAIN 4D Questionnaire: A New Validated Tool for Assessing Pain in Endometriosis. Journal of Clinical Medicine. 2021; 10(15):3216. https://doi.org/10.3390/jcm10153216
Chicago/Turabian StylePuchar, Anne, Pierre Panel, Anne Oppenheimer, Joseph Du Cheyron, Xavier Fritel, and Arnaud Fauconnier. 2021. "The ENDOPAIN 4D Questionnaire: A New Validated Tool for Assessing Pain in Endometriosis" Journal of Clinical Medicine 10, no. 15: 3216. https://doi.org/10.3390/jcm10153216
APA StylePuchar, A., Panel, P., Oppenheimer, A., Du Cheyron, J., Fritel, X., & Fauconnier, A. (2021). The ENDOPAIN 4D Questionnaire: A New Validated Tool for Assessing Pain in Endometriosis. Journal of Clinical Medicine, 10(15), 3216. https://doi.org/10.3390/jcm10153216






