External Validation of the IOTA Classification in Women with Ovarian Masses Suspected to Be Endometrioma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. TVUS Evaluation of Adnexal Masses
2.3. Laparoscopic Surgeries
2.4. Ethical Aspects
2.5. Statistical Analysis
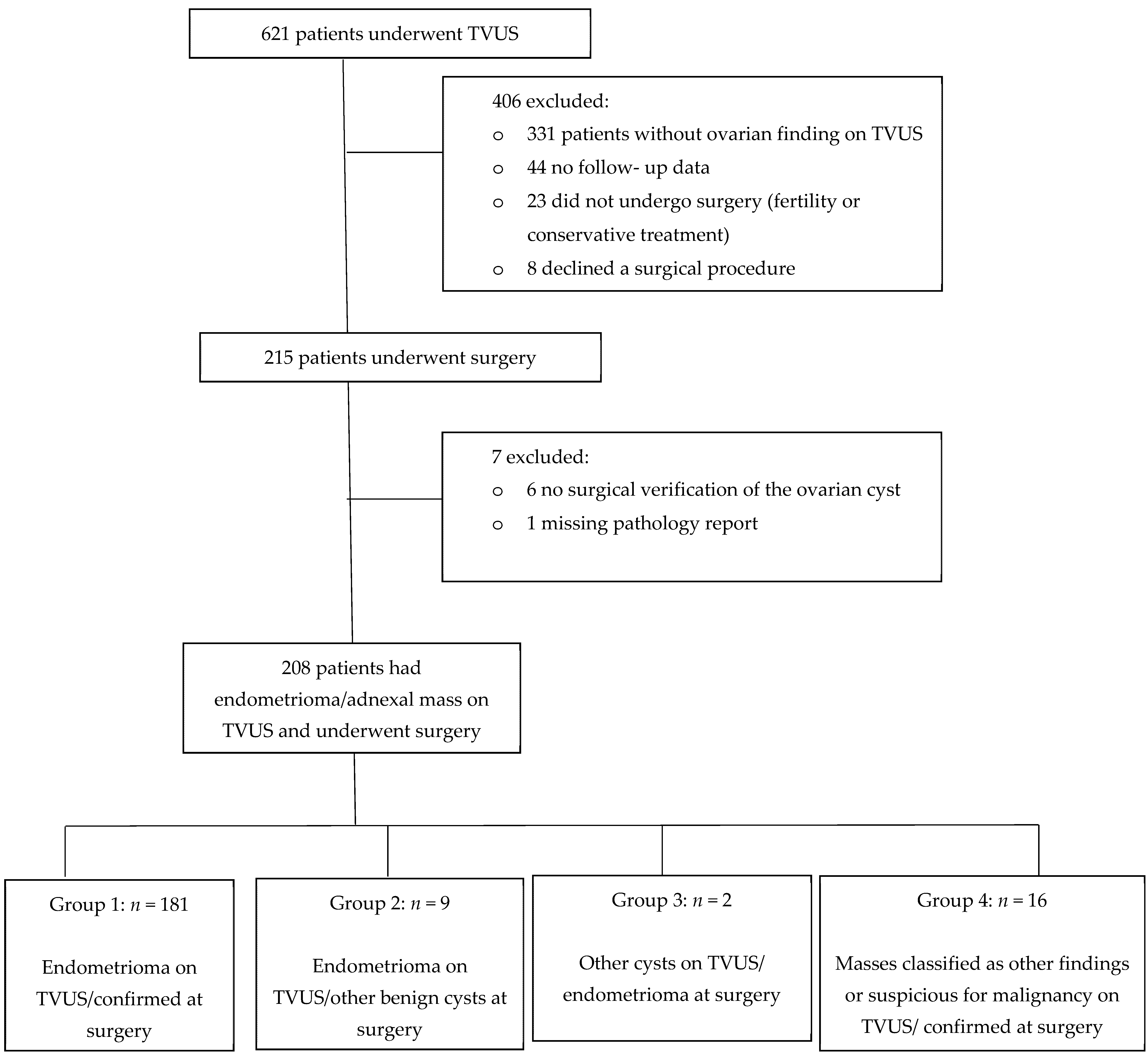
3. Results
3.1. Participants and Clinical Data
3.2. TVUS Evaluation of Adnexal Mass and Surgery Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerriero, S.; Ajossa, S.; Garau, N.; Piras, B.; Paoletti, A.M.; Melis, G.B. Ultrasonography and color Doppler-based triage for adnexal masses to provide the most appropriate surgical approach. Am. J. Obstet. Gynecol. 2005, 192, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, J.L.; Royo, P.; Jurado, M.; Minguez, J.A.; Laparte, C.; Galván, R.; García-Manero, M.; López-García, G. Triage for surgical management of ovarian tumors in asymptomatic women: Assessment of an ultrasound-based scoring system. Ultrasound Obstet. Gynecol. 2008, 32, 220–225. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecoclogists. Practice bulletin No. 174: Evaluation and Management of Adnexal Masses. Obstet. Gynecol. 2016, 128, e210. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Valentin, L.; Bourne, T.; Collins, W.P.; Verrelst, H.; Vergote, I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: A consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet. Gynecol. 2000, 16, 500–505. [Google Scholar] [CrossRef]
- Van Holsbeke, C.; Daemen, A.; Yazbek, J.; Holland, T.K.; Bourne, T.; Mesens, T.; Lannoo, L.; Boes, A.S.; Joos, A.; Van De Vijver, A.; et al. Ultrasound experience substan-tially impacts on diagnostic performance and confidence when adnexal masses are classified using pattern recognition. Gynecol. Obstet. Investig. 2010, 69, 160–168. [Google Scholar] [CrossRef]
- Ameye, L.; Timmerman, D.; Valentin, L.; Paladini, D.; Zhang, J.; Van Holsbeke, C.; Lissoni, A.A.; Savelli, L.; Veldman, J.; Testa, A.C.; et al. Clinically oriented three-step strategy for assessment of adnexal pathology. Ultrasound Obstet. Gynecol. 2012, 40, 582–591. [Google Scholar] [CrossRef]
- Ueland, F.R.; DePriest, P.D.; Pavlik, E.J.; Kryscio, R.J.; Van Nagell, J.R. Preoperative differentiation of malignant from benign ovar-ian tumors: The efficacy of morphology indexing and Doppler flow sonography. Gynecol. Oncol. 2003, 91, 46–50. [Google Scholar] [CrossRef]
- Timmerman, D.; Testa, A.C.; Bourne, T.; Ameye, L.; Jurkovic, D.; Van Holsbeke, C.; Paladini, D.; Van Calster, B.; Vergote, I.; Van Huffel, S.; et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet. Gynecol. 2008, 31, 681–690. [Google Scholar] [CrossRef]
- Timmerman, D.; Ameye, L.; Fischerova, D.; Epstein, E.; Melis, G.B.; Guerriero, S.; Van Holsbeke, C.; Savelli, L.; Fruscio, R.; Lissoni, A.A.; et al. Simple ultrasound rules to distinguish between be-nign and malignant adnexal masses before surgery: Prospective validation by IOTA group. Bmj 2011, 342, 94. [Google Scholar]
- Froyman, W.; Wynants, L.; Landolfo, C.; Bourne, T.; Valentin, L.; Testa, A.; Sladkevicius, P.; Franchi, D.; Fischerova, D.; Savelli, L.; et al. Validation of the performance of International Ovarian Tumor Analysis (IOTA) methods in the diagnosis of early stage ovarian cancer in a non-screening population. Diagnostics 2017, 7, 32. [Google Scholar] [CrossRef]
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 2005, 20, 2698–2704. [Google Scholar] [CrossRef]
- Kontoravdis, A.; Chryssikopoulos, A.; Hassiakos, D.; Liapis, A.; Zourlas, P.A. The diagnostic value of laparoscopy in 2365 patients with acute and chronic pelvic pain. Int. J. Obstet. Gynaecol. 1996, 52, 243–248. [Google Scholar] [CrossRef]
- Rogers, P.A.; Adamson, G.D.; Al-Jefout, M.; Becker, C.M.; D’Hooghe, T.M.; Dunselman, G.A.; Fazleabas, A.; Giudice, L.C.; Hoenr, A.W.; Hull, M.L.; et al. Research priorities for endometriosis: Recommendations from a global consortium of investigators in en-dometriosis and for the WES/WERF consortium for research priorities in endometriosis. Reprod. Sci. 2017, 24, 202–226. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, V.H.; Weil, C.; Chodick, G.; Shalev, V. Epidemiology of endometriosis: A large population-based database study from a healthcare provider with 2 million members. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Sumimoto, K.; Kitanaka, T.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; et al. Ovarian endometrioma—Risks factors of ovarian cancer development. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 138, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.A.; Gridley, G.; Persson, I.; Baron, J.; Bergqvist, A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am. J. Obstet. Gynecol. 1997, 176, 572–579. [Google Scholar] [CrossRef]
- Jimbo, H.; Yoshikawa, H.; Onda, T.; Yasugi, T.; Sakamoto, A.; Taketani, Y. Prevalence of ovarian endometriosis in epithelial ovar-ian cancer. Int. J. Gynecol. Obstet. 1997, 59, 245–250. [Google Scholar] [CrossRef]
- Crozier, M.A.; Copeland, L.J.; Silva, E.G.; Gershenson, D.M.; Stringer, C. Clear cell carcinoma of the ovary: A study of 59 cases. Gynecol. Oncol. 1989, 35, 199–203. [Google Scholar] [CrossRef]
- DePriest, P.D.; Banks, E.R.; Powell, D.E.; van Nagell, J.R.; Gallion, H.H.; Puls, L.E.; Hunter, J.E.; Kryscio, R.J.; Royalty, M.B. Endometrioid carcinoma of the ovary and endometriosis: The association in postmenopausal women. Gynecol. Oncol. 1992, 47, 71–75. [Google Scholar] [CrossRef]
- Thomsen, L.H.; Schnack, T.H.; Buchardi, K.; Hummelshoj, L.; Missmer, S.A.; Forman, A.; Blaakaer, J. Risk factors of epithelial ovarian carcinomas among women with endometriosis: A systematic review. Acta Obstet. Gynecol. Scand. 2016, 96, 761–778. [Google Scholar] [CrossRef]
- Testa, A.C.; Van Holsbeke, C.; Zannoni, G.F.; Fransis, S.; Moerman, P.; Vellone, V.G.; Mascilini, F.; Licameli, A.; Ludovisi, M.; Di Legge, A.; et al. Ovarian cancer arising in endometrioid cysts: Ultrasound findings. Ultrasound Obstet. Gynecol. 2011, 38, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, S.; Condous, G.; Bosch, T.V.D.; Valentin, L.; Leone, F.P.G.; Van Schoubroeck, D.; Exacoustos, C.; Installé, A.J.F.; Martins, W.P.; Abrao, M.S.; et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: A consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet. Gynecol. 2016, 48, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Thomassin, I.; Hourani, R.; Cortez, A.; Darai, E. Diagnostic accuracy of transvaginal sonography for deep pelvic en-dometriosis. Ultrasound Obstet. Gynecol. 2004, 24, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Hudelist, G.; English, J.; Thomas, A.; Tinelli, A.; Singer, C.F.; Keckstein, J. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2011, 37, 257–263. [Google Scholar] [CrossRef]
- Piketty, M.; Chopin, N.; Dousset, B.; Millischer-Bellaische, A.-E.; Roseau, G.; Leconte, M.; Borghese, B.; Chapron, C. Preoperative work-up for patients with deeply infiltrating endometriosis: Transvaginal ultrasonography must definitely be the first-line imaging examination. Hum. Reprod. 2008, 24, 602–607. [Google Scholar] [CrossRef] [Green Version]
- Menakaya, U.; Reid, S.; Infante, F.; Condous, G. Systematic evaluation of women with suspected endometriosis using a 5-domain sonographically based approach. J. Ultrasound Med. 2015, 34, 937–947. [Google Scholar] [CrossRef]
- Abrao, M.S.; Goncalves, M.O.D.C.; Dias, J.A., Jr.; Podgaec, S.; Chamie, L.P.B.R. Comparison between clinical examination, transvagi-nal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum. Reprod. 2007, 22, 3092–3097. [Google Scholar] [CrossRef] [Green Version]
- Guerriero, S.; Ajossa, S.; Gerada, M.; Virgilio, B.; Angioni, S.; Melis, G.B. Diagnostic value of transvaginal ’tenderness-guided’ ultrasonography for the prediction of location of deep endometriosis. Hum. Reprod. 2008, 23, 2452–2457. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.C.; Batt, R.E. Retrocervical, retrovaginal pouch, and rectovaginal septum endometriosis. J. Am. Assoc. Gynecol. Laparosc. 2001, 8, 12–17. [Google Scholar] [CrossRef]
- Leon, M.; Alcazar, J.L. High sliding sign: A new soft marker of uterine fundus compromise in deep infiltrating endometriosis. Ultrasound Obstet. Gynecol. 2015, 45, 624. [Google Scholar] [CrossRef]
- Guerriero, S.; Alcazar, J.L.; Ajossa, S.; Pilloni, M.; Melis, G.B. Three-dimensional sonographic characteristics of deep endometrio-sis. J. Ultrasound Med. 2009, 28, 1061–1066. [Google Scholar] [CrossRef]
- Sayasneh, A.; Kaijser, J.; Preisler, J.; Johnson, S.; Stalder, C.; Husicka, R.; Guha, S.; Naji, O.; Abdallah, Y.; Raslan, F.; et al. A multicenter prospective external vali-dation of the diagnostic performance of IOTA simple descriptors and rules to characterize ovarian masses benign simple descriptor malignant simple descriptor. Gynecol. Oncol. 2013, 130, 140–146. [Google Scholar] [CrossRef]
- Testa, A.; Kaijser, J.; Wynants, L.; Fischerová, D.; Van Holsbeke, C.; Franchi, D.; Savelli, L.; Epstein, E.; Czekierdowski, A.; Guerriero, S.; et al. Strategies to diagnose ovarian cancer: New evidence from phase 3 of the multicentre international IOTA study. Br. J. Cancer 2014, 111, 680–688. [Google Scholar] [CrossRef] [Green Version]
- Alcázar, J.L.; Pascual, M.A.; Graupera, B.; Aubá, M.; Errasti, T.; Olartecoechea, B.; Ruiz-Zambrana, A.; Hereter, L.; Ajossa, S.; Guerriero, S. External validation of IOTA simple descriptors and simple rules for classifying adnexal masses. Ultrasound Obstet. Gynecol. 2016, 48, 397–402. [Google Scholar] [CrossRef]
- Van Holsbeke, C.; Van Calster, B.; Guerriero, S.; Savelli, L.; Paladini, D.; Lissoni, A.A.; Czekierdowski, A.; Fischerova, D.; Zhang, J.; Mestdagh, G.; et al. Endometriomas: Their ultrasound characteristics. Ultrasound Obstet. Gynecol. 2010, 35, 730–740. [Google Scholar] [CrossRef]
- Moore, J.; Copley, S.; Morris, J.; Lindsell, D.; Golding, S.; Kennedy, S. A systematic review of the accuracy of ultrasound in the diagnosis of endometriosis. Ultrasound Obstet. Gynecol. 2002, 20, 630–634. [Google Scholar] [CrossRef]
- Alcazar, J.L.; Pascual, M.A.; Olartecoechea, B.; Graupera, B.; Auba, M.; Ajossa, S.; Hereter, L.; Julve, R.; Gaston, B.; Peddes, C.S.F. IOTA simple rules for discriminating between benign and malignant adnexal masses: Prospective external validation. Ultrasound Obstet. Gynecol. 2013, 4, 467–471. [Google Scholar] [CrossRef]
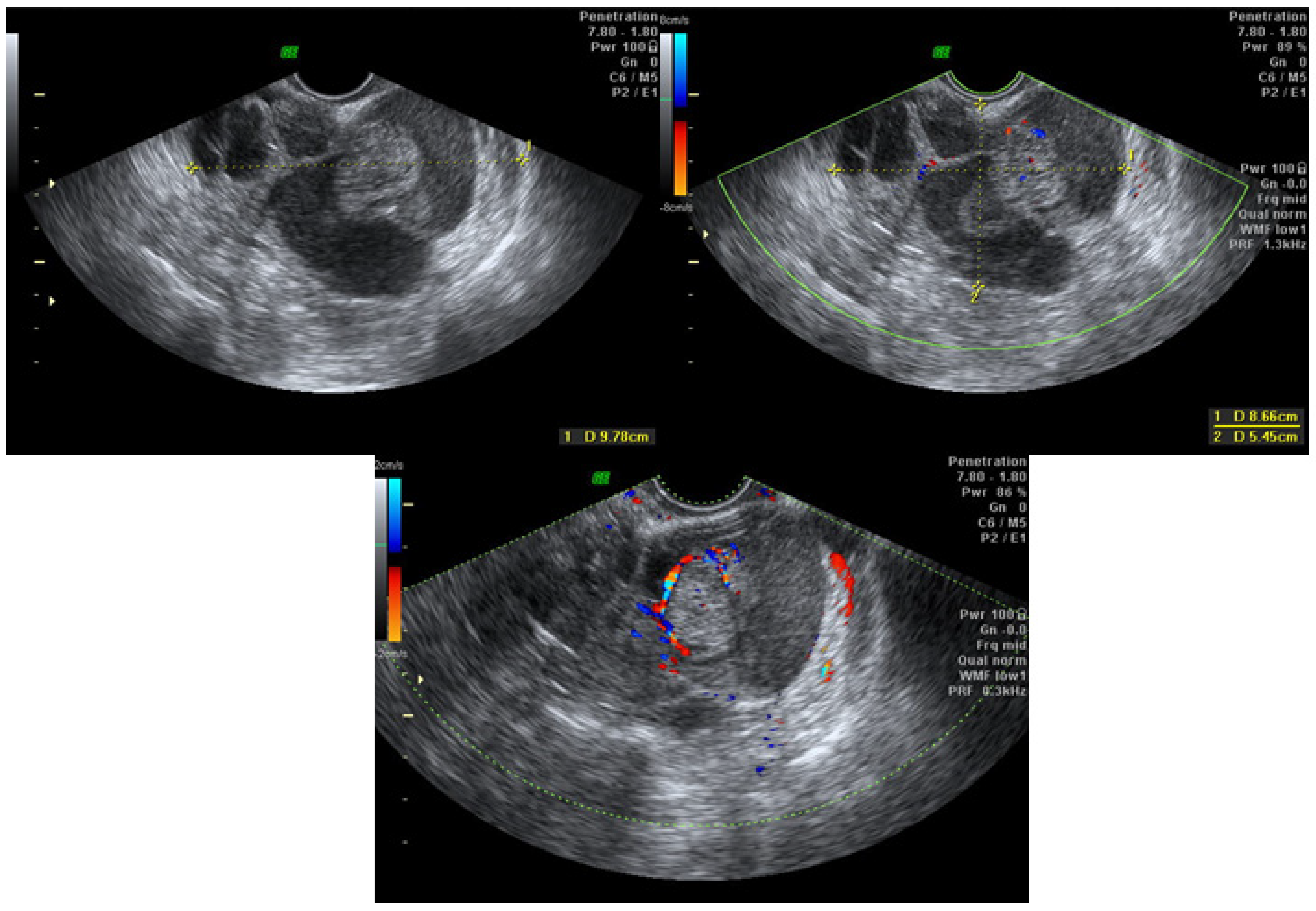
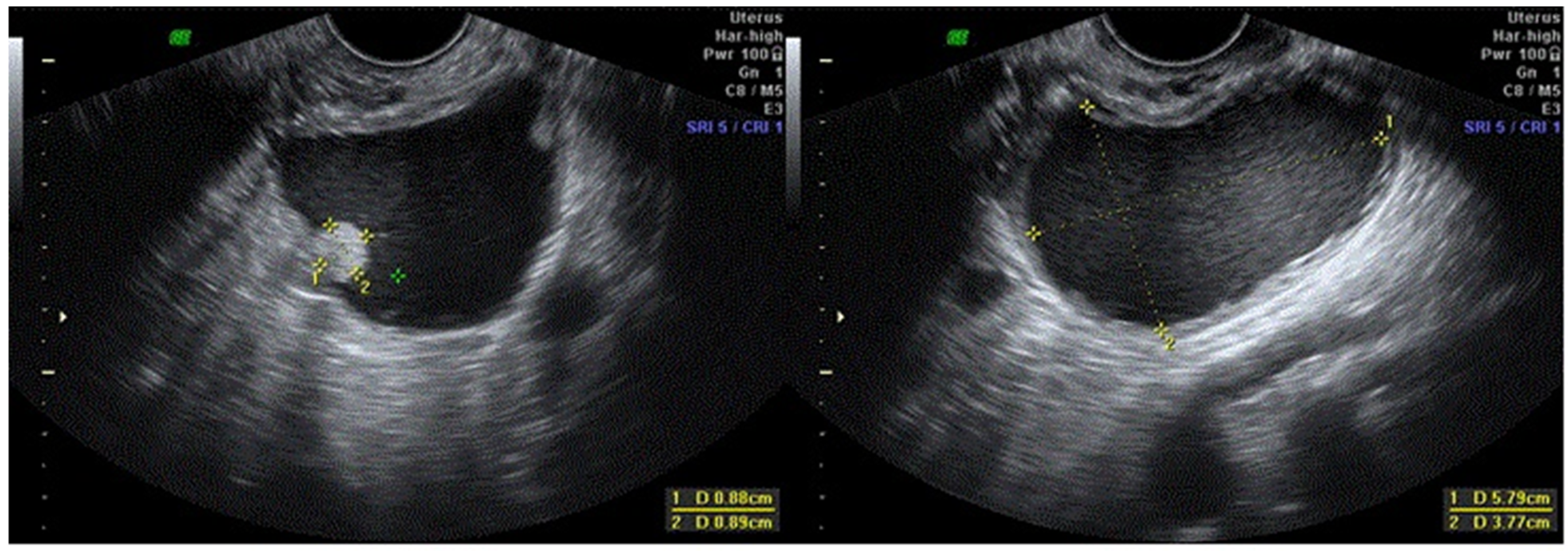

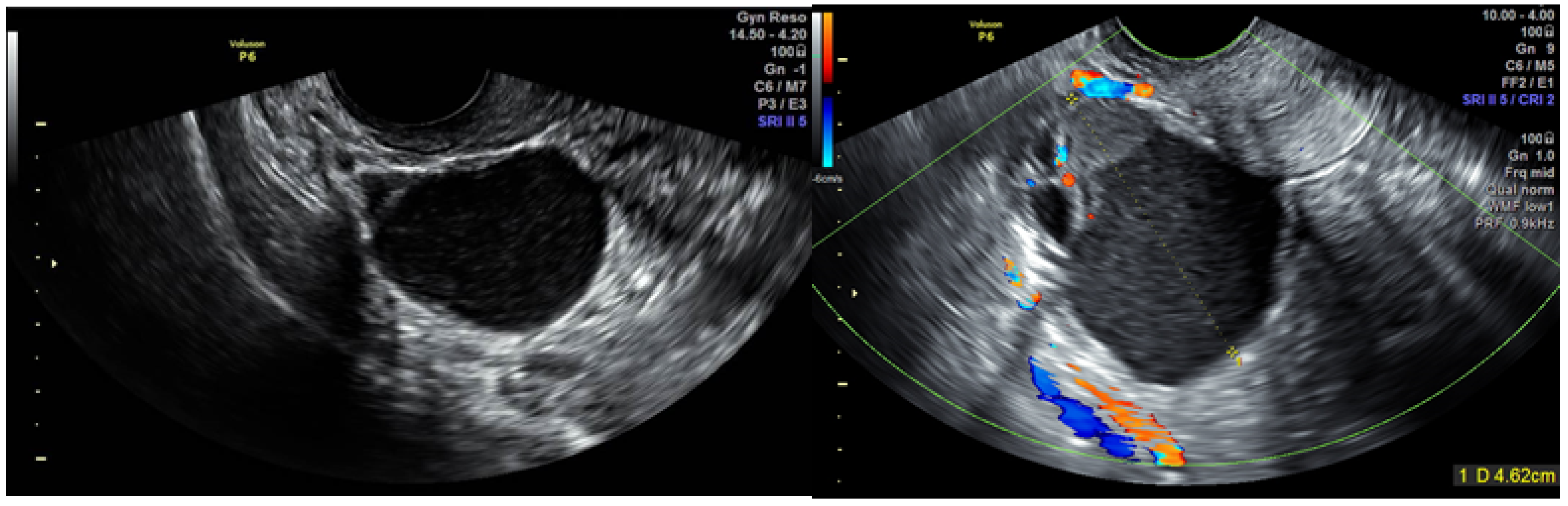
| Parameter | Group 1 (n = 181) | Group 2 (n = 9) | Group 3 (n = 2) | Group 4 (n = 16) | Total (n = 208) |
|---|---|---|---|---|---|
| Age, mean ± SD, years | 33.6 ± 6.1 | 30.4 ± 10.4 | 43 ± 1.4 | 34.1 ± 7.2 | 33.6 ± 6.5 |
| BMI, median (IQR) | 22.5 (19.9–25.6) | 22.3 (20.7–22.8) | 23 | 23.4 (20.6–26.6) | 22.5 (20.1–25.5) |
| Smoking (%) | 56 (30.9%) | 4 (44.4%) | 1 (50%) | 6 (37.5%) | 67 (32.2%) |
| Nulliparous (%) | 115 (63.5%) | 5 (55.6%) | 0 | 10 (62.5%) | 130 (62.5%) |
| Previous Cesarean section (%) | 17 (9.4%) | 1 (11.1%) | 0 | 2 (12.5%) | 20 (9.6%) |
| Previous surgery (%) | 74 (40.9%) | 4 (44.4%) | 1 (50%) | 10 (62.5%) | 89 (42.8%) |
| Additional TVUS findings (%) | |||||
| Maximal size of cyst, median (IQR), mm | 50 (41–66) | 44.5 (39.5–53) | 40 | 46 (38.5–64.7) | 49 (40.5–65.5) |
| Kissing ovaries | 53 (29.8%) | 2 (22.2%) | 0 | 3 (18.8%) | 58 (28.3%) |
| Uterosacral ligaments nodule | 85 (47.2%) | 5 (55.6%) | 0 | 2 (12.5%) | 92 (44.4%) |
| Retro cervical nodule | 46 (25.4%) | 0 | 0 | 0 | 46 (22.1%) |
| Recto sigmoid nodule | 57 (31.5%) | 3 (33.3%) | 0 | 2 (12.5%) | 62 (29.8%) |
| Bladder nodule | 8 (4.4%) | 0 | 0 | 1 (6.3%) | 9 (4.3%) |
| Intestinal nodule | 26 (14.4%) | 1 (11.1%) | 0 | 1 (6.3%) | 28 (13.5%) |
| Pouch of Douglas obliteration | 85 (47%) | 4 (44.4%) | 0 | 3 (18.8%) | 92 (44.2%) |
| Case Number | Age (Years) | Parity | Side of Finding | Maximal Size of Finding (mm) | TVUS * Description | Suspected Malignancy | Additional Findings | Pathology Result of Adnexal Mass | Follow-Up (Months) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | 1 | Left | 98 | Multilocular cystic | Yes | Uterus myomatosus, right endometrioma, rectosigmoid nodule | Borderline mucinous tumor | 53 | Normal follow-up, no recurrence. |
| 2 | 32 | 2 | Right | 100 | Multilocular cystic | Yes | None | Borderline mucinous tumor | 5 | Normal follow-up, no recurrence. |
| 3 | 37 | 0 | Left | 58 | Unilocular solid | Yes | None | Borderline serous tumor | 92 | Recurrence after 4 years, underwent USO **, normal follow-up. |
| 4 | 28 | 0 | Right | 40 | Unilocular cystic low-level echogenicity | Yes | None | Borderline serous tumor | 54 | Normal follow-up, no recurrence. Spontaneous pregnancy. |
| 5 | 36 | 0 | Right | 47 | Unilocular cystic low-level echogenicity | Yes | Rectovaginal nodule | Endometrioid lesion (complex hyperplasia without atypia) | 34 | Normal follow-up. Infertility-repeated IVF cycles, no pregnancy. |
| 6 | 22 | 0 | Left | 46 | Multilocular solid cyst with papillations | Yes | Uterosacral ligament nodules | Hydrosalpinx | 22 | Normal follow-up, no recurrence. |
| 7 | 36 | 3 | Left | 45 | Multilocular cystic | No | Uterosacral ligament nodules | Serous cystadenoma | 15 | Normal follow-up, no recurrence. |
| 8 | 30 | 0 | Right | 66 | Multilocular cystic | No | None | Mucinous cystadenoma | 31 | Recurrence after 2.5 years, underwent cystectomy for the third time, normal follow-up. |
| 9 | 38 | 0 | Left | 33 | Unilocular cystic low-level echogenicity | No | Deep lesion around right ureter | Mucinous cystadenoma | 45 | Normal follow-up, no recurrence. Conceived twice with fertility treatments. |
| 10 | 32 | 0 | Left | 98 | Unilocular cystic | No | None | Mucinous cystadenoma | 19 | Normal follow-up, no recurrence. Spontaneous pregnancy. |
| 11 | 40 | 3 | Left | 61 | Unilocular solid | No | None | Fibro-thecoma | 4 | Normal follow-up, no recurrence. |
| 12 | 24 | 0 | Right | 53 | Multilocular cystic | No | Bladder nodule, left endometrioma. | Multi-cystic benign mesothelioma with decidual changes | 11 | Normal follow-up, no recurrence. |
| 13 | 39 | 2 | Right | 20 | Unilocular solid | No | Bladder nodule, uterosacral ligament nodule | Mature cystic teratoma | 25 | Normal follow-up, no recurrence. |
| 14 | 43 | 1 | Right | 74 | Multilocular cystic | No | Intestinal adhesions | Hemorrhagic corpus luteum | 5 | Normal follow-up, no recurrence. |
| 15 | 40 | 2 | Left | 35 | Unilocular cystic | No | Uterosacral ligament nodule | Hemorrhagic corpus luteum | 78 | Normal follow-up, no recurrence. |
| 16 | 23 | 0 | Left | 69 | Unilocular cystic low-level echogenicity with papillation (para ovarian) | No | None | Para-ovarian cyst: Serous Cyst adenofibroma | 31 | Normal follow-up, no recurrence. Spontaneous pregnancy. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen Ben-Meir, L.; Mashiach, R.; Eisenberg, V.H. External Validation of the IOTA Classification in Women with Ovarian Masses Suspected to Be Endometrioma. J. Clin. Med. 2021, 10, 2971. https://doi.org/10.3390/jcm10132971
Cohen Ben-Meir L, Mashiach R, Eisenberg VH. External Validation of the IOTA Classification in Women with Ovarian Masses Suspected to Be Endometrioma. Journal of Clinical Medicine. 2021; 10(13):2971. https://doi.org/10.3390/jcm10132971
Chicago/Turabian StyleCohen Ben-Meir, Lee, Roy Mashiach, and Vered H. Eisenberg. 2021. "External Validation of the IOTA Classification in Women with Ovarian Masses Suspected to Be Endometrioma" Journal of Clinical Medicine 10, no. 13: 2971. https://doi.org/10.3390/jcm10132971
APA StyleCohen Ben-Meir, L., Mashiach, R., & Eisenberg, V. H. (2021). External Validation of the IOTA Classification in Women with Ovarian Masses Suspected to Be Endometrioma. Journal of Clinical Medicine, 10(13), 2971. https://doi.org/10.3390/jcm10132971






