Associates of Insomnia in People with Chronic Spinal Pain: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Identification and Selection of Studies
2.1.1. Eligibility Criteria
2.1.2. Information Sources
2.1.3. Study Selection
2.2. Data Collection Process
2.3. Risk of Bias Assessment of Individual Studies
2.4. Summary Measures
2.5. Methods of Analysis
2.6. Quality of Evidence
3. Results
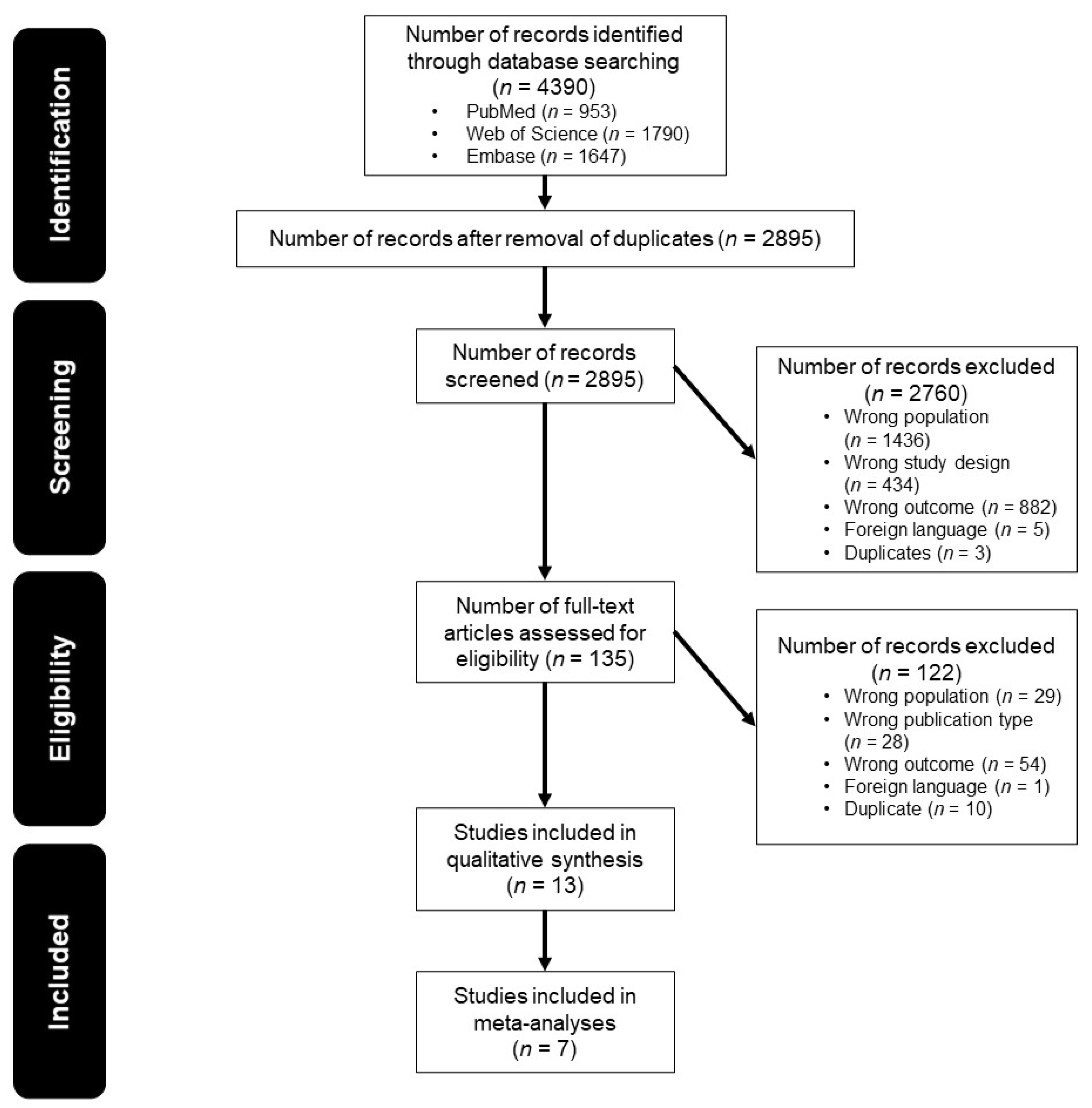
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias within Studies
3.4. Synthesis of Results
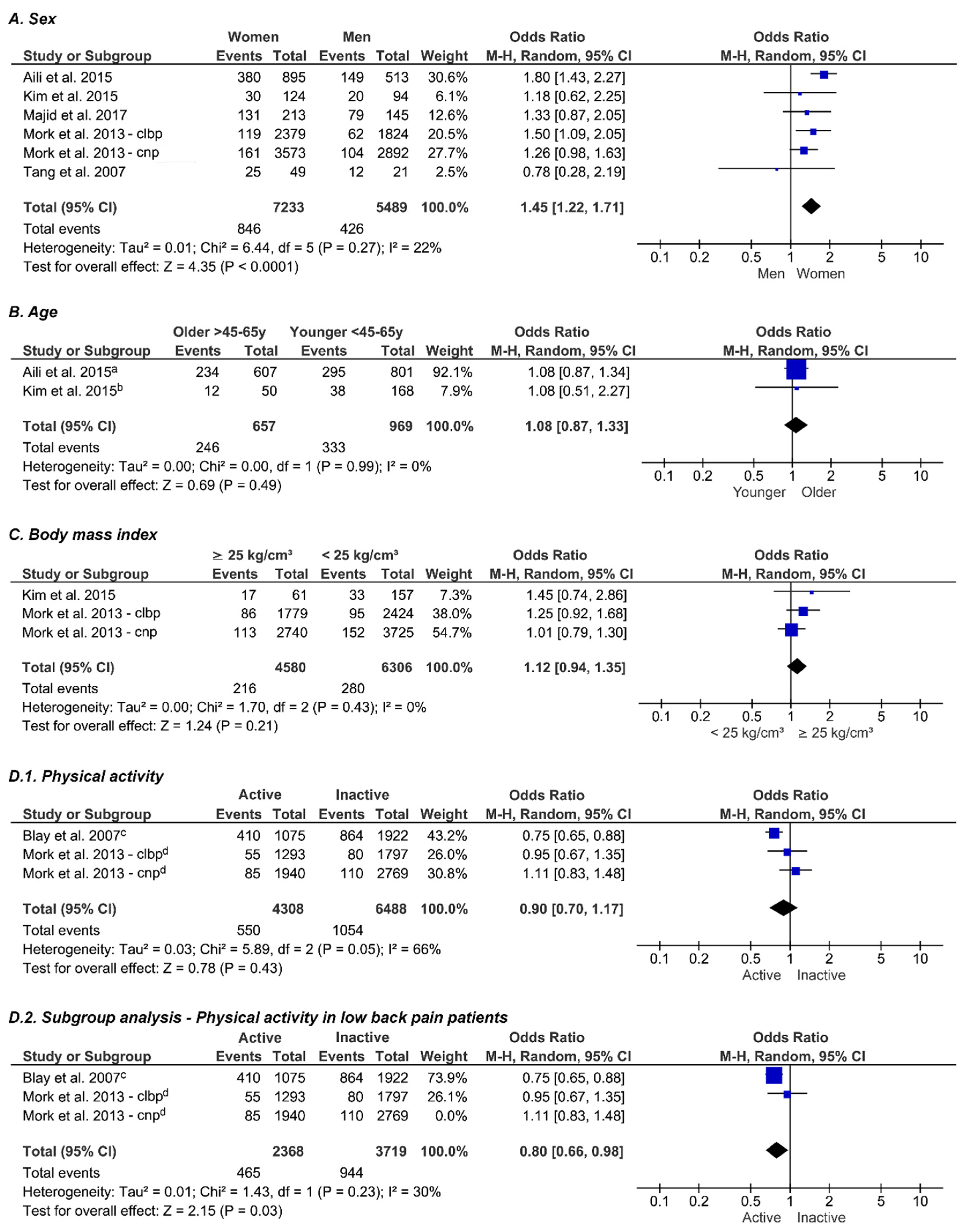
3.5. Sex
3.6. Age
3.7. Body Mass Index
3.8. Physical Activity
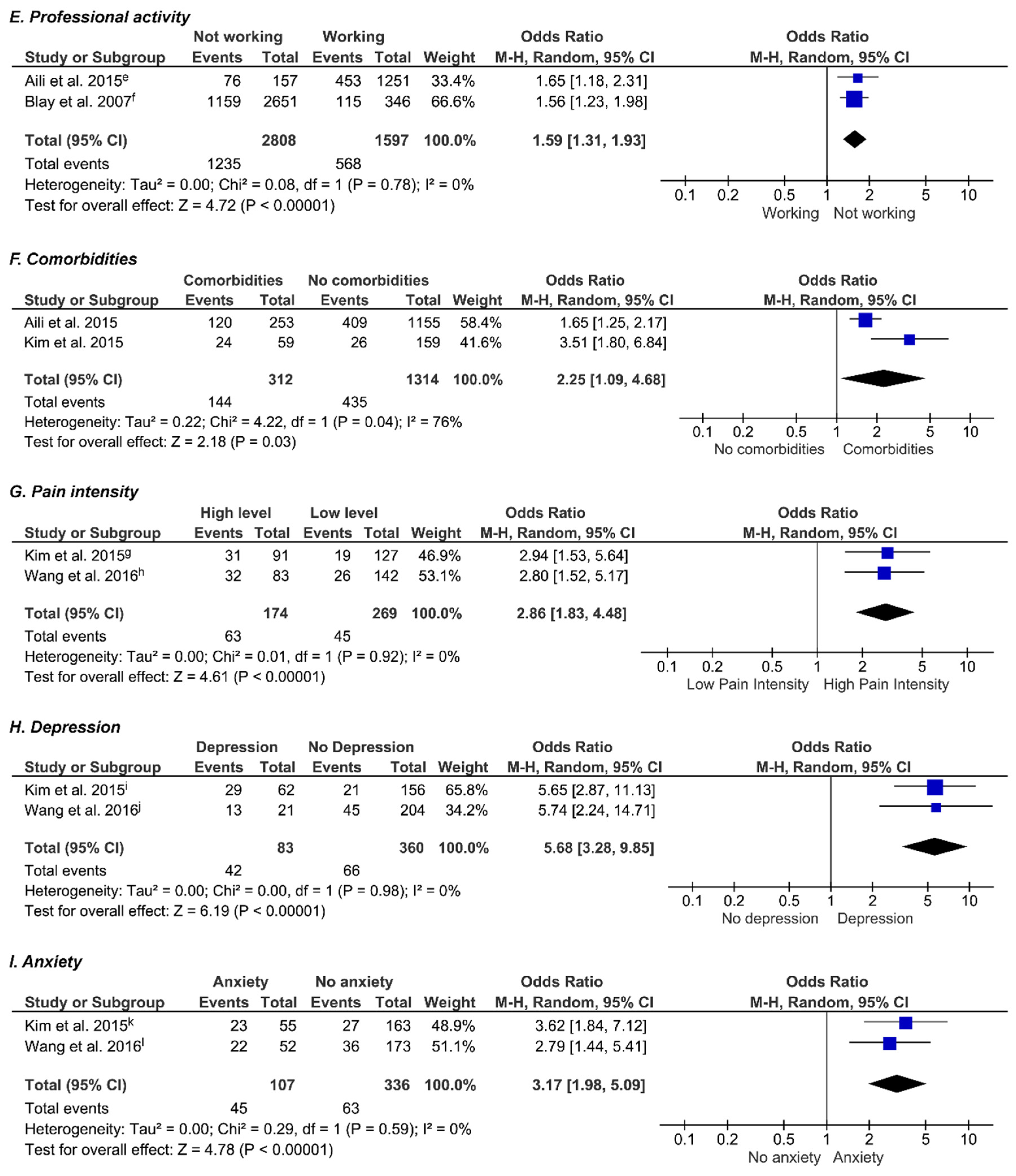
3.9. Professional Activity
3.10. Comorbidities
3.11. Pain Intensity
3.12. Depression
3.13. Anxiety
3.14. Other
4. Discussion
4.1. Strengths and Limitations
4.2. Clinical Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Balague, F.; Mannion, A.F.; Pellise, F.; Cedraschi, C. Non-specific low back pain. Lancet 2012, 379, 482–491. [Google Scholar] [CrossRef]
- Gore, M.; Tai, K.S.; Sadosky, A.; Leslie, D.; Stacey, B.R. Use and costs of prescription medications and alternative treatments in patients with osteoarthritis and chronic low back pain in community-based settings. Pain Pract. Off. J. World Inst. Pain 2012, 12, 550–560. [Google Scholar] [CrossRef]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef]
- Fejer, R.; Kyvik, K.O.; Hartvigsen, J. The prevalence of neck pain in the world population: A systematic critical review of the literature. Eur. Spine J. 2006, 15, 834–848. [Google Scholar] [CrossRef]
- Von Korff, M.; Crane, P.; Lane, M.; Miglioretti, D.L.; Simon, G.; Saunders, K.; Stang, P.; Brandenburg, N.; Kessler, R. Chronic spinal pain and physical-mental comorbidity in the United States: Results from the national comorbidity survey replication. Pain 2005, 113, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Natvig, B.; Ferreira, M. Is it all about a pain in the back? Best Pract. Res. Clin. Rheumatol. 2013, 27, 613–623. [Google Scholar] [CrossRef]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Alsaadi, S.M.; McAuley, J.H.; Hush, J.M.; Maher, C.G. Prevalence of sleep disturbance in patients with low back pain. Eur. Spine J. 2011, 20, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Bahouq, H.; Allali, F.; Rkain, H.; Hmamouchi, I.; Hajjaj-Hassouni, N. Prevalence and severity of insomnia in chronic low back pain patients. Rheumatol. Int. 2013, 33, 1277–1281. [Google Scholar] [CrossRef]
- Tang, N.K.; Wright, K.J.; Salkovskis, P.M. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J. Sleep Res. 2007, 16, 85–95. [Google Scholar] [CrossRef]
- Marin, R.; Cyhan, T.; Miklos, W. Sleep disturbance in patients with chronic low back pain. Am. J. Phys. Med. Rehabil. 2006, 85, 430–435. [Google Scholar] [CrossRef]
- Institute of Medicine Committee on Sleep Medicine and Research. The National Academies Collection: Reports funded by National Institutes of Health. In Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem; Colten, H.R., Altevogt, B.M., Eds.; National Academies Press (US), National Academy of Sciences: Washington, DC, USA, 2006. [Google Scholar]
- Sayar, K.; Arikan, M.; Yontem, T. Sleep quality in chronic pain patients. Can. J. Psychiatry 2002, 47, 844–848. [Google Scholar] [CrossRef]
- Daley, M.; Morin, C.M.; LeBlanc, M.; Gregoire, J.P.; Savard, J. The economic burden of insomnia: Direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep 2009, 32, 55–64. [Google Scholar]
- Haack, M.; Simpson, N.; Sethna, N.; Kaur, S.; Mullington, J. Sleep deficiency and chronic pain: Potential underlying mechanisms and clinical implications. Neuropsychopharmacology 2020, 45, 205–216. [Google Scholar] [CrossRef]
- Whibley, D.; AlKandari, N.; Kristensen, K.; Barnish, M.; Rzewuska, M.; Druce, K.L.; Tang, N.K.Y. Sleep and Pain: A Systematic Review of Studies of Mediation. Clin. J. Pain 2019, 35, 544–558. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Schutte-Rodin, S.; Broch, L.; Buysse, D.; Dorsey, C.; Sateia, M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J. Clin. Sleep Med. 2008, 4, 487–504. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Elmagarmid, A.; Fedorowicz, Z.; Hammady, H.; Ilyas, I.; Khabsa, M.; Ouzzani, M. Rayyan: A systematic reviews web app for exploring and filtering searches for eligible studies for Cochrane Reviews. In Proceedings of the 22nd Cochrane Colloquium, Evidence-informed public health: Opportunities and challenges, Hyderabad, India, 21–25 September 2014; pp. 21–26. [Google Scholar]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Modesti, P.A.; Reboldi, G.; Cappuccio, F.P.; Agyemang, C.; Remuzzi, G.; Rapi, S.; Perruolo, E.; Parati, G. Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0147601. [Google Scholar] [CrossRef]
- McPheeters, M.L.; Kripalani, S.; Peterson, N.B.; Idowu, R.T.; Jerome, R.N.; Potter, S.A.; Andrews, J.C. Closing the quality gap: Revisiting the state of the science (vol. 3: Quality improvement interventions to address health disparities). Evid. Rep. Technol. Assess. (Full Rep.) 2012, 3, 1–475. [Google Scholar]
- Fincham, J.E. Response rates and responsiveness for surveys, standards, and the Journal. Am. J. Pharm. Educ. 2008, 72, 43. [Google Scholar] [CrossRef]
- Reyner, L.A.; Horne, J.A.; Reyner, A. Gender- and age-related differences in sleep determined by home-recorded sleep logs and actimetry from 400 adults. Sleep 1995, 18, 127–134. [Google Scholar]
- Suh, S.; Cho, N.; Zhang, J. Sex Differences in Insomnia: From Epidemiology and Etiology to Intervention. Curr. Psychiatry Rep. 2018, 20, 69. [Google Scholar] [CrossRef]
- Zhang, B.; Wing, Y.K. Sex differences in insomnia: A meta-analysis. Sleep 2006, 29, 85–93. [Google Scholar] [CrossRef]
- Van Eycken, S.; Neu, D.; Newell, J.; Kornreich, C.; Mairesse, O. Sex-Related Differences in Sleep-Related PSG Parameters and Daytime Complaints in a Clinical Population. Nat. Sci. Sleep 2020, 12, 161–171. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G.; Group, C.S.M. Analysing data and undertaking meta-analyses. Cochrane Handb. Syst. Rev. Interv. 2019, 241–284. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 4. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Huguet, A.; Hayden, J.A.; Stinson, J.; McGrath, P.J.; Chambers, C.T.; Tougas, M.E.; Wozney, L. Judging the quality of evidence in reviews of prognostic factor research: Adapting the GRADE framework. Syst. Rev. 2013, 2, 71. [Google Scholar] [CrossRef]
- Sterne, J.A.; Gavaghan, D.; Egger, M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 2000, 53, 1119–1129. [Google Scholar] [CrossRef]
- Blay, S.L.; Andreoli, S.B.; Gastal, F.L. Chronic painful physical conditions, disturbed sleep and psychiatric morbidity: Results from an elderly survey. Ann. Clin. Psychiatry 2007, 19, 169–174. [Google Scholar] [CrossRef]
- DiMarco, L.A.; Ramger, B.C.; Howell, G.P.; Serrani, A.M.; Givens, D.L.; Rhon, D.I.; Cook, C.E. Differences in Characteristics and Downstream Drug Use Among Opioid-Naive and Prior Opioid Users with Low Back Pain. Pain Pract. Off. J. World Inst. Pain 2019, 19, 149–157. [Google Scholar] [CrossRef]
- Ho, K.K.N.; Simic, M.; Cvancarova Smastuen, M.; de Barros Pinheiro, M.; Ferreira, P.H.; Bakke Johnsen, M.; Heuch, I.; Grotle, M.; Zwart, J.A.; Nilsen, K.B. The association between insomnia, c-reactive protein, and chronic low back pain: Cross-sectional analysis of the HUNT study, Norway. Scand. J. Pain 2019. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, D.H.; Yoon, K.B.; An, J.R.; Yoon, D.M. Factors Associated with Increased Risk for Clinical Insomnia in Patients with Chronic Neck Pain. Pain Physician 2015, 18, 593–598. [Google Scholar] [PubMed]
- Majid, B.; Arif, M.A.; Saeed, R.; Ahmad, A.; Fatima, M. Frequency and severity of insomnia in chronic low back pain. Rawal Med. J. 2017, 42, 528–530. [Google Scholar]
- Mork, P.J.; Vik, K.L.; Moe, B.; Lier, R.; Bardal, E.M.; Nilsen, T.I. Sleep problems, exercise and obesity and risk of chronic musculoskeletal pain: The Norwegian HUNT study. Eur. J. Public Health 2014, 24, 924–929. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, R.; Yoon, D.M.; Yoon, K.B.; Kim, K.; Kim, S.H. Factors associated with increased risk for pain catastrophizing in patients with chronic neck pain: A retrospective cross-sectional study. Med. (Baltim.) 2016, 95, e4698. [Google Scholar] [CrossRef]
- Ris, I.; Juul-Kristensen, B.; Boyle, E.; Kongsted, A.; Manniche, C.; Sogaard, K. Chronic neck pain patients with traumatic or non-traumatic onset: Differences in characteristics. A cross-sectional study. Scand. J. Pain 2017, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shmagel, A.; Foley, R.; Ibrahim, H. Epidemiology of Chronic Low Back Pain in US Adults: Data From the 2009-2010 National Health and Nutrition Examination Survey. Arthritis Care Res. (Hoboken) 2016, 68, 1688–1694. [Google Scholar] [CrossRef]
- Wang, H.Y.; Fu, T.S.; Hsu, S.C.; Hung, C.I. Association of depression with sleep quality might be greater than that of pain intensity among outpatients with chronic low back pain. Neuropsychiatr. Dis. Treat. 2016, 12, 1993–1998. [Google Scholar] [CrossRef][Green Version]
- Aili, K.; Nyman, T.; Hillert, L.; Svartengren, M. Sleep disturbances predict future sickness absence among individuals with lower back or neck-shoulder pain: A 5-year prospective study. Scand. J. Public Health 2015, 43, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Roth, T. Insomnia: Definition, prevalence, etiology, and consequences. J. Clin. Sleep Med. 2007, 3, S7–S10. [Google Scholar] [CrossRef]
- Scullin, M.K.; Bliwise, D.L. Sleep, cognition, and normal aging: Integrating a half century of multidisciplinary research. Perspect. Psychol. Sci. 2015, 10, 97–137. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Patel, D.; Steinberg, J.; Patel, P. Insomnia in the Elderly: A Review. J. Clin. Sleep Med. 2018, 14, 1017–1024. [Google Scholar] [CrossRef]
- Ancoli-Israel, S. Sleep and its disorders in aging populations. Sleep Med. 2009, 10 (Suppl. 1), S7–S11. [Google Scholar] [CrossRef]
- Smagula, S.F.; Stone, K.L.; Fabio, A.; Cauley, J.A. Risk factors for sleep disturbances in older adults: Evidence from prospective studies. Sleep Med. Rev. 2016, 25, 21–30. [Google Scholar] [CrossRef]
- Husak, A.J.; Bair, M.J. Chronic Pain and Sleep Disturbances: A Pragmatic Review of Their Relationships, Comorbidities, and Treatments. Pain Med. 2020. [Google Scholar] [CrossRef]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The association of sleep and pain: An update and a path forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef]
- Cheatle, M.D.; Foster, S.; Pinkett, A.; Lesneski, M.; Qu, D.; Dhingra, L. Assessing and Managing Sleep Disturbance in Patients with Chronic Pain. Anesthesiol. Clin. 2016, 34, 379–393. [Google Scholar] [CrossRef]
- Nijs, J.; Mairesse, O.; Neu, D.; Leysen, L.; Danneels, L.; Cagnie, B.; Meeus, M.; Moens, M.; Ickmans, K.; Goubert, D. Sleep Disturbances in Chronic Pain: Neurobiology, Assessment, and Treatment in Physical Therapist Practice. Phys. Ther. 2018, 98, 325–335. [Google Scholar] [CrossRef]
- Wall, P.D.; Melzack, R.; Bonica, J.J. Textbook of Pain; Churchill Livingstone: Edinburgh, Scotland, UK, 1999; Volume 994. [Google Scholar]
- Kim, E.J.; Dimsdale, J.E. The effect of psychosocial stress on sleep: A review of polysomnographic evidence. Behav. Sleep Med. 2007, 5, 256–278. [Google Scholar] [CrossRef] [PubMed]
- Crombez, G.; Vlaeyen, J.W.; Heuts, P.H.; Lysens, R. Pain-related fear is more disabling than pain itself: Evidence on the role of pain-related fear in chronic back pain disability. Pain 1999, 80, 329–339. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Neblett, R.; Kishino, N.; Ray, C.T. Fear-Avoidance Beliefs and Chronic Pain. J. Orthop. Sports Phys. Ther. 2016, 46, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Outcalt, S.; Krebs, E.; Bair, M.J.; Wu, J.; Chumbler, N.; Yu, Z. Association between anxiety, health-related quality of life and functional impairment in primary care patients with chronic pain. Gen. Hosp. Psychiatry 2013, 35, 359–365. [Google Scholar] [CrossRef]
- Mullington, J.M.; Simpson, N.S.; Meier-Ewert, H.K.; Haack, M. Sleep loss and inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 775–784. [Google Scholar] [CrossRef]
- Haack, M.; Lee, E.; Cohen, D.A.; Mullington, J.M. Activation of the prostaglandin system in response to sleep loss in healthy humans: Potential mediator of increased spontaneous pain. Pain 2009, 145, 136–141. [Google Scholar] [CrossRef]
- Pollmacher, T.; Haack, M.; Schuld, A.; Reichenberg, A.; Yirmiya, R. Low levels of circulating inflammatory cytokines--do they affect human brain functions? Brain Behav. Immun. 2002, 16, 525–532. [Google Scholar] [CrossRef]
- Wodarski, R.; Schuh-Hofer, S.; Yurek, D.A.; Wafford, K.A.; Gilmour, G.; Treede, R.D.; Kennedy, J.D. Development and pharmacological characterization of a model of sleep disruption-induced hypersensitivity in the rat. Eur. J. Pain 2015, 19, 554–566. [Google Scholar] [CrossRef]
- Schuh-Hofer, S.; Wodarski, R.; Pfau, D.B.; Caspani, O.; Magerl, W.; Kennedy, J.D.; Treede, R.D. One night of total sleep deprivation promotes a state of generalized hyperalgesia: A surrogate pain model to study the relationship of insomnia and pain. Pain 2013, 154, 1613–1621. [Google Scholar] [CrossRef]
- Staner, L. Comorbidity of insomnia and depression. Sleep Med. Rev. 2010, 14, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.G.; Franca, M.H.; Paiva, M.C.A.; Andrade, L.H.; Viana, M.C. Prevalence and clinical profile of chronic pain and its association with mental disorders. Rev. Saude Publica 2017, 51, 96. [Google Scholar] [CrossRef]
- Bair, M.J.; Robinson, R.L.; Katon, W.; Kroenke, K. Depression and pain comorbidity: A literature review. Arch. Intern. Med. 2003, 163, 2433–2445. [Google Scholar] [CrossRef]
- Dunietz, G.L.; Swanson, L.M.; Jansen, E.C.; Chervin, R.D.; O’Brien, L.M.; Lisabeth, L.D.; Braley, T.J. Key insomnia symptoms and incident pain in older adults: Direct and mediated pathways through depression and anxiety. Sleep 2018, 41. [Google Scholar] [CrossRef]
- Kredlow, M.A.; Capozzoli, M.C.; Hearon, B.A.; Calkins, A.W.; Otto, M.W. The effects of physical activity on sleep: A meta-analytic review. J. Behav. Med. 2015, 38, 427–449. [Google Scholar] [CrossRef]
- Quante, M.; Mariani, S.; Weng, J.; Marinac, C.R.; Kaplan, E.R.; Rueschman, M.; Mitchell, J.A.; James, P.; Hipp, J.A.; Cespedes Feliciano, E.M.; et al. Zeitgebers and their association with rest-activity patterns. Chronobiol. Int. 2019, 36, 203–213. [Google Scholar] [CrossRef]
- Gordon, R.; Bloxham, S. A Systematic Review of the Effects of Exercise and Physical Activity on Non-Specific Chronic Low Back Pain. Healthcare 2016, 4, 22. [Google Scholar] [CrossRef]
- Malfliet, A.; Ickmans, K.; Huysmans, E.; Coppieters, I.; Willaert, W.; Bogaert, W.V.; Rheel, E.; Bilterys, T.; Wilgen, P.V.; Nijs, J. Best Evidence Rehabilitation for Chronic Pain Part 3: Low Back Pain. J. Clin. Med. 2019, 8, 1063. [Google Scholar] [CrossRef]
- Palmlof, L.; Holm, L.W.; Alfredsson, L.; Magnusson, C.; Vingard, E.; Skillgate, E. The impact of work related physical activity and leisure physical activity on the risk and prognosis of neck pain—A population based cohort study on workers. BMC Musculoskelet. Disord. 2016, 17, 219. [Google Scholar] [CrossRef]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 4, Cd011279. [Google Scholar] [CrossRef] [PubMed]
- Quartana, P.J.; Campbell, C.M.; Edwards, R.R. Pain catastrophizing: A critical review. Expert Rev. Neurother. 2009, 9, 745–758. [Google Scholar] [CrossRef]
- Boersma, K.; Linton, S.J. Psychological processes underlying the development of a chronic pain problem: A prospective study of the relationship between profiles of psychological variables in the fear-avoidance model and disability. Clin. J. Pain 2006, 22, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Walton, D.M.; Macdermid, J.C.; Giorgianni, A.A.; Mascarenhas, J.C.; West, S.C.; Zammit, C.A. Risk factors for persistent problems following acute whiplash injury: Update of a systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2013, 43, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Koes, B.W.; van Tulder, M.W.; Thomas, S. Diagnosis and treatment of low back pain. BMJ 2006, 332, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.R.; Leake, H.B.; Chalmers, K.J.; Moseley, G.L. Evidence of Impaired Proprioception in Chronic, Idiopathic Neck Pain: Systematic Review and Meta-Analysis. Phys. Ther. 2016, 96, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Sun, J.M.; Yoon, K.B.; Moon, J.H.; An, J.R.; Yoon, D.M. Risk factors associated with clinical insomnia in chronic low back pain: A retrospective analysis in a university hospital in Korea. Korean J. Pain 2015, 28, 137–143. [Google Scholar] [CrossRef]
- Yun, S.Y.; Kim, D.H.; Do, H.Y.; Kim, S.H. Clinical insomnia and associated factors in failed back surgery syndrome: A retrospective cross-sectional study. Int. J. Med. Sci. 2017, 14, 536–542. [Google Scholar] [CrossRef]
- Jackson, D.; Turner, R. Power analysis for random-effects meta-analysis. Res. Synth. Methods 2017, 8, 290–302. [Google Scholar] [CrossRef]
| Author | Design | Sample Size (N) | Nature of the Sample | Age (Range and/or Years ± SD) | Pain Duration | Sleep Outcome | Prevalence Rates of Insomnia | Investigated Factors |
|---|---|---|---|---|---|---|---|---|
| Aili et al. 2015 | C | 1408 | Care seeking CLBP and CNP, community sample | Range: 20–59 y | ≥6 mo | Karolinska Sleep Questionnaire | NM | Sex, age, other physical illness, professional activity |
| Blay et al. 2007 | CS | 2997 | CLBP, population-based sample | Range: 60–81 y | ≥6 mo | Short Psychiatric Evaluation Schedule | 42.5% sleep disturbance | Professional activity, income, medical consultation, hospitalizations, self-rated health, physical activity |
| Dimarco et al. 2018 | CS | 709 | CLBP, sample in clinical setting | 34.9 ± 11.9 y | Opioid naïve: 26.04 ± 50.21 mo Prior opioid users: 22.64 ± 46.26 mo | Data extracted from Military Health System Data Repository | 19% insomnia | Prior opioid use |
| Ho et al. 2019 | CS | 6559 | CLBP, community sample | 52.2 ± 15.2 y Range: 19.1–95.9 y | ≥3 mo | Modified insomnia criteria from DSM-5 | 10.9% insomnia | High CRP level |
| Kim et al. 2015 | CS | 218 | CNP, sample in clinical setting | 52.8 ± 14.3 y Range: 20–83 y | ≥3 mo | Insomnia Severity Index | 53.7% mild to severe insomnia | Sex, age, BMI, pain duration, pain score, spine surgery history, shoulder or arm pain, neck mobility problems, myofascial pain components, anxiety, depression, headache, comorbid musculoskeletal conditions |
| Majid et al. 2017 | CS | 358 | CLBP, sample in clinical setting | NM | ≥3 mo | Insomnia Severity Index | 58.7% sleep disturbance | Sex |
| Marin et al. 2006 | CS | 268 | CLBP, sample in clinical setting | 47 y ± NM Range: 18–89 y | ≥6 mo | Pittsburgh Sleep Quality Index | 92% sleep disturbances | Sleep medication intake after pain |
| Mork et al. 2013 | CS | 10,849 | CLBP and CNP, community sample | 43.0 ± 13.9 y | ≥3 mo | Self-Reported Questionnaire | NM | Sex, physical activity, BMI |
| Park et al. 2016 | CS | 256 | CNP, sample in clinical setting | 52.8 ± 14.7 y Range: 20–84 y | ≥3 mo | Insomnia Severity Index | 24.22% clinical insomnia | Pain catastrophizing |
| Ris et al. 2017 | CS | 200 | CNP, sample in clinical setting | Traumatic: 43.5 ± 11.4 y Non-traumatic: 47.5 ± 11.3 y | ≥6 mo | Self-reported Disturbed nights/week | 19.5% sleep disturbances | Traumatic Onset |
| Shmagel et al. 2016 | CS | 700 | CLBP, community sample | Range: 20–69 y | ≥3 mo | NAHANS Questionnaires | 52.7% sleep disturbances | Healthcare Use |
| Tang et al. 2007 | CS | 70 | CLBP, sample in clinical setting | 46 ± 10.9 y Range: 18–65 y | ≥6 mo | Insomnia Severity Index | 53% with moderate or severe insomnia | Sex, race |
| Wang et al. 2016 | CS | 225 | CLBP | 40.7 ± 11.4 y | ≥3 mo | Insomnia Severity Index | 25.8% clinical insomnia | Depression, anxiety, severity of CLBP |
| Studies | Selection | Comparability | Outcome | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the Sample (Maximum 1 star) | Sample Size (Maximum 1 Star) | Non-Respondents (Maximum 1 Star) | Ascertainment of the Exposure (Factor) (Maximum 2 Stars) | Confounding Factors (Maximum 2 Stars) | Assessment of the Outcome (Maximum 2 Stars) | Statistical Test (Maximum 1 Star) | Mean = 6.23 Median = 6 | |
| Aili et al. 2015 | ☆ | ☆ | ☆☆ | ☆☆ | ☆ | ☆ | 8 | |
| Blay et al. 2007 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | |
| Dimarco et al. 2019 | ☆ | ☆ | ☆☆ | ☆☆ | ☆ | 7 | ||
| Ho et al. 2019 | ☆ | ☆ | ☆☆ | ☆☆ | ☆ | ☆ | 8 | |
| Kim et al. 2015 | ☆ | ☆☆ | ☆ | ☆ | 5 | |||
| Majid et al. 2017 | ☆ | ☆ | ☆☆ | ☆ | 5 | |||
| Marin et al. 2006 | ☆ | ☆ | ☆ | ☆ | ☆ | 5 | ||
| Mork et al. 2014 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | |
| Park et al. 2016 | ☆ | ☆ | ☆☆ | ☆ | ☆ | 6 | ||
| Ris et al. 2017 | ☆ | ☆ | ☆ | ☆ | ☆ | 5 | ||
| Shmagel et al. 2016 | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | |
| Tang et al. 2007 | ☆ | ☆☆ | ☆ | ☆ | ☆ | 6 | ||
| Wang et al. 2016 | ☆ | ☆☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Author | Factor | Number of Participants with Insomnia (n) | Number of Participants without Insomnia (n) | Number of Participants in Reference and Investigated Subgroup (n) | (Adjusted) Odds Ratio [95% CI] |
|---|---|---|---|---|---|
| Aili et al. 2015 | Sex | 529 | 879 | ||
| - Women | 380 | 515 | 895 | 1.80 [1.43–2.27] | |
| - Men | 149 | 364 | 513 | 1.0 | |
| Age | 529 | 879 | |||
| - ≥45 years | 234 | 373 | 607 | 1.08 [0.87–1.34] | |
| - <45 years | 295 | 506 | 801 | 1.0 | |
| Other physical illness | 529 | 879 | |||
| - Yes | 120 | 133 | 253 | 1.65 [1.25–2.17] | |
| - No | 409 | 746 | 1155 | 1.0 | |
| Professional activity | 529 | 879 | |||
| - Not working | 76 | 81 | 157 | 1.65 [1.18–2.31] | |
| - Working | 453 | 798 | 1251 | 1.0 | |
| Blay et al. 2007 | Professional activity | 1274 | 1723 | ||
| - Yes | 115 | 231 | 346 | 1.0 | |
| - No | 1159 | 1492 | 2651 | 1.56 [1.23–1.98] | |
| Income | 1274 | 1723 | |||
| - High | 312 | 631 | 943 | 0.56 [0.48–0.66] | |
| - Low | 962 | 1092 | 2054 | 1.0 | |
| Medical Consultation | 1274 | 1723 | |||
| - Yes | 1041 | 1299 | 2340 | 1.46 [1.22–1.74] | |
| - No | 233 | 424 | 657 | 1.0 | |
| Hospitalizations | 1274 | 1723 | |||
| - >1 | 359 | 323 | 682 | 1.70 [1.43–2.02] | |
| - ≤1 | 915 | 1400 | 2315 | 1.0 | |
| Self-rated health | 1274 | 1723 | |||
| - Impaired | 1117 | 1170 | 2287 | 3.36 [2.77–4.09] | |
| - Not impaired | 157 | 553 | 710 | 1.0 | |
| Physical activity | 1274 | 1723 | |||
| - Yes | 410 | 665 | 1075 | 0.75 [0.65–0.88] | |
| - No | 864 | 1058 | 1922 | 1.0 | |
| Dimarco et al. 2018 | Opioid user | 112 | 592 | ||
| - Yes | 93 | 391 | 484 | 2.52 [1.49–4.24] | |
| - No | 19 | 201 | 220 | 1.0 | |
| Ho et al. 2019 | CRP Level | 719 | 5840 | ||
| - Elevated or very high | 205 | 1390 | 1595 | 1.27 [1.07–1.52] | |
| - Very high | 37 | 256 | 296 | 1.25 [0.88–1.79] | |
| - Elevated | 168 | 1134 | 1302 | 1.28 [1.06–1.54] | |
| - Normal | 514 | 4450 | 4964 | 1.0 | |
| Kim et al. 2015 | Sex | 50 | 168 | ||
| - Women | 30 | 94 | 124 | 1.18 [0.62–2.25] | |
| - Men | 20 | 74 | 94 | 1.0 | |
| Age | 50 | 168 | |||
| - ≥65 years | 12 | 38 | 50 | 1.08 [0.51–2.27] | |
| - <65 years | 38 | 130 | 168 | 1.0 | |
| BMI | 50 | 168 | |||
| - ≥25 kg/m2 | 17 | 44 | 61 | 1.45 [0.74–2.86] | |
| - <25 kg/m2 | 33 | 124 | 157 | 1.0 | |
| Pain duration | 50 | 168 | |||
| - ≥1 year | 28 | 78 | 106 | 1.47 [0.78–2.77] | |
| - <1 year | 22 | 90 | 112 | 1.0 | |
| Pain score | 50 | 168 | |||
| - ≥7 NRS | 31 | 60 | 91 | 2.94 [1.53–5.64]; Adj. 2.46 [1.12–5.40] | |
| - <7 NRS | 19 | 108 | 127 | 1.0 | |
| History of spine surgery | 50 | 168 | |||
| - Yes | 7 | 15 | 22 | 1.74 [0.50–6.04] | |
| - No | 43 | 153 | 196 | 1.0 | |
| Shoulder or arm pain | 50 | 168 | |||
| - Yes | 31 | 99 | 130 | 1.14 [0.60–2.18] | |
| - No | 19 | 69 | 88 | 1.0 | |
| Neck mobility problems | 50 | 168 | |||
| - Yes | 13 | 43 | 56 | 1.02 [0.50–2.10] | |
| - No | 37 | 125 | 162 | 1.0 | |
| Comorbid musculoskeletal pain conditions | 50 | 168 | |||
| - Yes | 24 | 35 | 59 | 3.51 [1.80–6.84]; Adj. 2.82 [1.22–6.54] | |
| - No | 26 | 133 | 159 | 1.0 | |
| Comorbid neuropathic pain component | 50 | 168 | |||
| - Yes | 16 | 24 | 40 | 2.824 [1.354–5.887] | |
| - No | 34 | 144 | 178 | 1.0 | |
| Myofascial pain components | 50 | 168 | |||
| - Yes | 20 | 50 | 70 | 1.57 [0.82–3.03] | |
| - No | 30 | 118 | 148 | 1.0 | |
| Anxiety | 50 | 168 | |||
| - HADS-A ≥ 8 | 23 | 32 | 55 | 3.62 [1.84–7.12]; Adj. 1.42 [0.58–3.48] | |
| - HADS-A < 8 | 27 | 136 | 163 | 1.0 | |
| Depression | 50 | 168 | |||
| - HADS-D ≥ 8 | 29 | 33 | 62 | 5.65 [2.87–11.13]; Adj. 3.69 [1.57–8.67] | |
| - HADS-D < 8 | 21 | 135 | 156 | 1.0 | |
| Headache | 50 | 168 | |||
| - Yes | 13 | 35 | 48 | 1.34 [0.64–2.78] | |
| - No | 37 | 133 | 170 | 1.0 | |
| Majid et al. 2017 | Sex | 210 | 148 | ||
| - Women | 131 | 82 | 213 | 1.33 [0.87–2.05] | |
| - Men | 79 | 66 | 145 | 1.0 | |
| Marin et al. 2006 | Sleep medication intake after pain | 230 | 18 | ||
| - Yes | 130 | 4 | 134 | 4.55 [1.45–14.25] | |
| - No | 100 | 14 | 114 | 1.0 | |
| Mork et al. 2013 | Sex | ||||
| Low back pain | 181 | 4203 | |||
| - Women | 119 | 2260 | 2379 | 1.50 [1.09–2.05] | |
| - Men | 62 | 1762 | 1824 | 1.0 | |
| Neck pain | 265 | 6200 | |||
| - Women | 161 | 3412 | 3573 | 1.26 [0.98–1.63] | |
| - Men | 104 | 2788 | 2892 | 1.0 | |
| Activity Level: leisure time physical exercise | |||||
| Low back pain | 135 | 2955 | |||
| - Inactive | 80 | 1717 | 1797 | 1.0 | |
| - Active | 55 | 1238 | 1293 | 0.95 [0.67–1.35] | |
| Neck pain | 195 | 4514 | |||
| - Inactive | 110 | 2659 | 2769 | 1.0 | |
| - Active | 85 | 1855 | 1940 | 1.11 [0.83–1.48] | |
| BMI | |||||
| Low back pain | 181 | 4022 | |||
| - ≥25 kg/cm3 | 86 | 1693 | 1779 | 1.25 [0.92–1.68] | |
| - <25 kg/cm3 | 95 | 2329 | 2424 | 1.0 | |
| Neck pain | 265 | 6200 | |||
| - ≥25 kg/cm3 | 113 | 2627 | 2740 | 1.01 [0.79–1.30] | |
| - <25 kg/cm3 | 152 | 3573 | 3725 | 1.0 | |
| Park et al. 2016 | Pain catastrophizing | 62 | 194 | ||
| - High | 42 | 44 | 86 | 7.16 [3.81–13.43] | |
| - Low | 20 | 150 | 170 | 1.0 | |
| Ris et al. 2017 | Traumatic onset | 39 | 161 | ||
| - Yes | 19 | 101 | 120 | 0.56 [0.28–1.14] | |
| - No | 20 | 60 | 80 | 1.0 | |
| Shmagel et al. 2016 | Healthcare use | 172 | 528 | ||
| - ≥10 healthcare visits/year | 124 | 246 | 370 | 2.96 [2.03–4.31] | |
| - <10 visits/year | 48 | 282 | 330 | 1.0 | |
| Tang et al. 2007 | Sex | 37 | 33 | ||
| - Women | 25 | 24 | 49 | 0.78 [0.28–2.19] | |
| - Men | 12 | 9 | 21 | 1.0 | |
| Race | 37 | 33 | |||
| - Caucasian | 26 | 20 | 46 | 1.54 [0.57–4.14] | |
| - Non-Caucasian | 11 | 13 | 24 | 1.0 | |
| Wang et al. 2016 | Depression (Diagnosis of major depressive episode) | 58 | 167 | ||
| - Yes | 13 | 8 | 21 | 5.74 [2.24–14.71] | |
| - No | 45 | 159 | 204 | 1.0 | |
| Anxiety (Diagnosis of an anxiety disorder) | 58 | 167 | |||
| - Yes | 22 | 30 | 52 | 2.79 [1.44–5.41] | |
| - No | 36 | 137 | 173 | 1.0 | |
| Pain score | 58 | 167 | |||
| - VAS ≥ 7 | 32 | 51 | 83 | 2.80 [1.52–5.17] | |
| - VAS < 7 | 26 | 116 | 142 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilterys, T.; Siffain, C.; De Maeyer, I.; Van Looveren, E.; Mairesse, O.; Nijs, J.; Meeus, M.; Ickmans, K.; Cagnie, B.; Goubert, D.; et al. Associates of Insomnia in People with Chronic Spinal Pain: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3175. https://doi.org/10.3390/jcm10143175
Bilterys T, Siffain C, De Maeyer I, Van Looveren E, Mairesse O, Nijs J, Meeus M, Ickmans K, Cagnie B, Goubert D, et al. Associates of Insomnia in People with Chronic Spinal Pain: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(14):3175. https://doi.org/10.3390/jcm10143175
Chicago/Turabian StyleBilterys, Thomas, Carolie Siffain, Ina De Maeyer, Eveline Van Looveren, Olivier Mairesse, Jo Nijs, Mira Meeus, Kelly Ickmans, Barbara Cagnie, Dorien Goubert, and et al. 2021. "Associates of Insomnia in People with Chronic Spinal Pain: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 14: 3175. https://doi.org/10.3390/jcm10143175
APA StyleBilterys, T., Siffain, C., De Maeyer, I., Van Looveren, E., Mairesse, O., Nijs, J., Meeus, M., Ickmans, K., Cagnie, B., Goubert, D., Danneels, L., Moens, M., & Malfliet, A. (2021). Associates of Insomnia in People with Chronic Spinal Pain: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(14), 3175. https://doi.org/10.3390/jcm10143175







