High-Throughput Mechanistic Screening of Epigenetic Compounds for the Potential Treatment of Meningiomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Identification, Demographics, and Tumor Collection
2.2. Tissue Culture
2.3. Epigenetic Compound Panel Screening
2.4. Cell Viability
2.5. Statistics
3. Results
3.1. Patient Demographics and Tumor Information
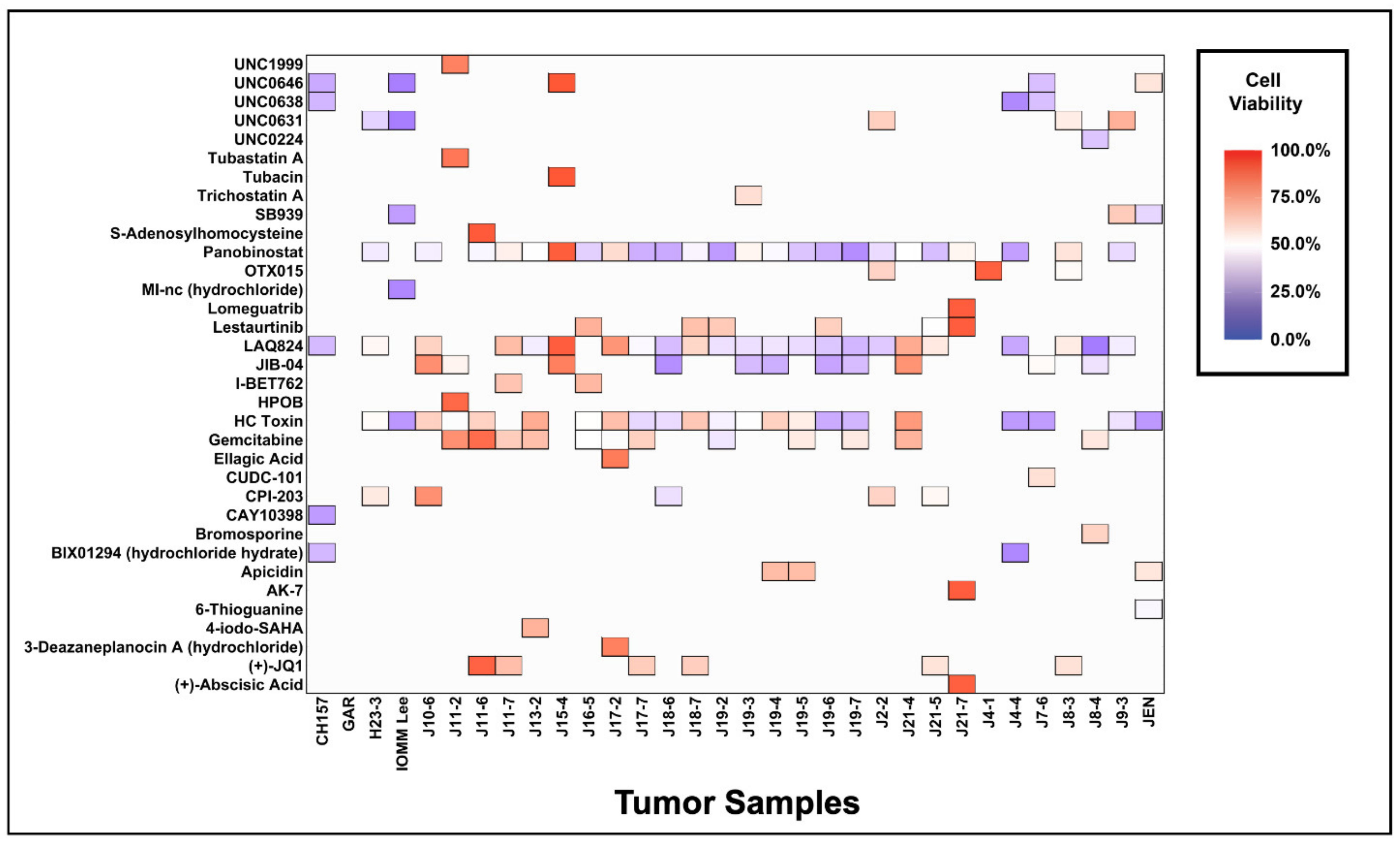
3.2. Meningiomas Are Broadly Sensitive to Epigenetic Inhibition and Have Unique Drug Sensitivity Profiles
3.3. Influence of Grade on the Use of Epigenetic Compounds for the Treatment of Meningiomas
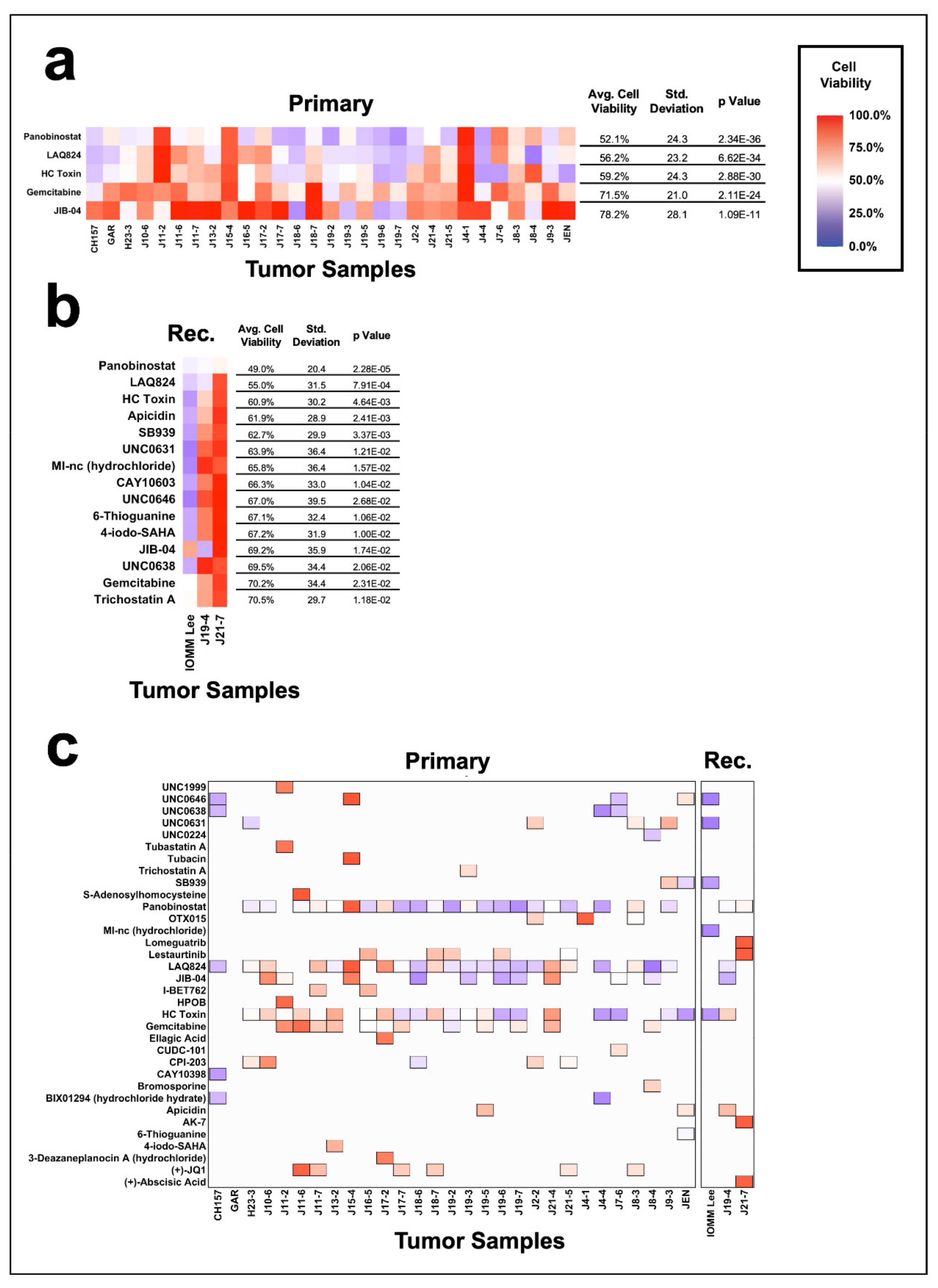
3.4. Efficacy of Epigenetic Compounds on Primary vs. Recurrent Meningiomas
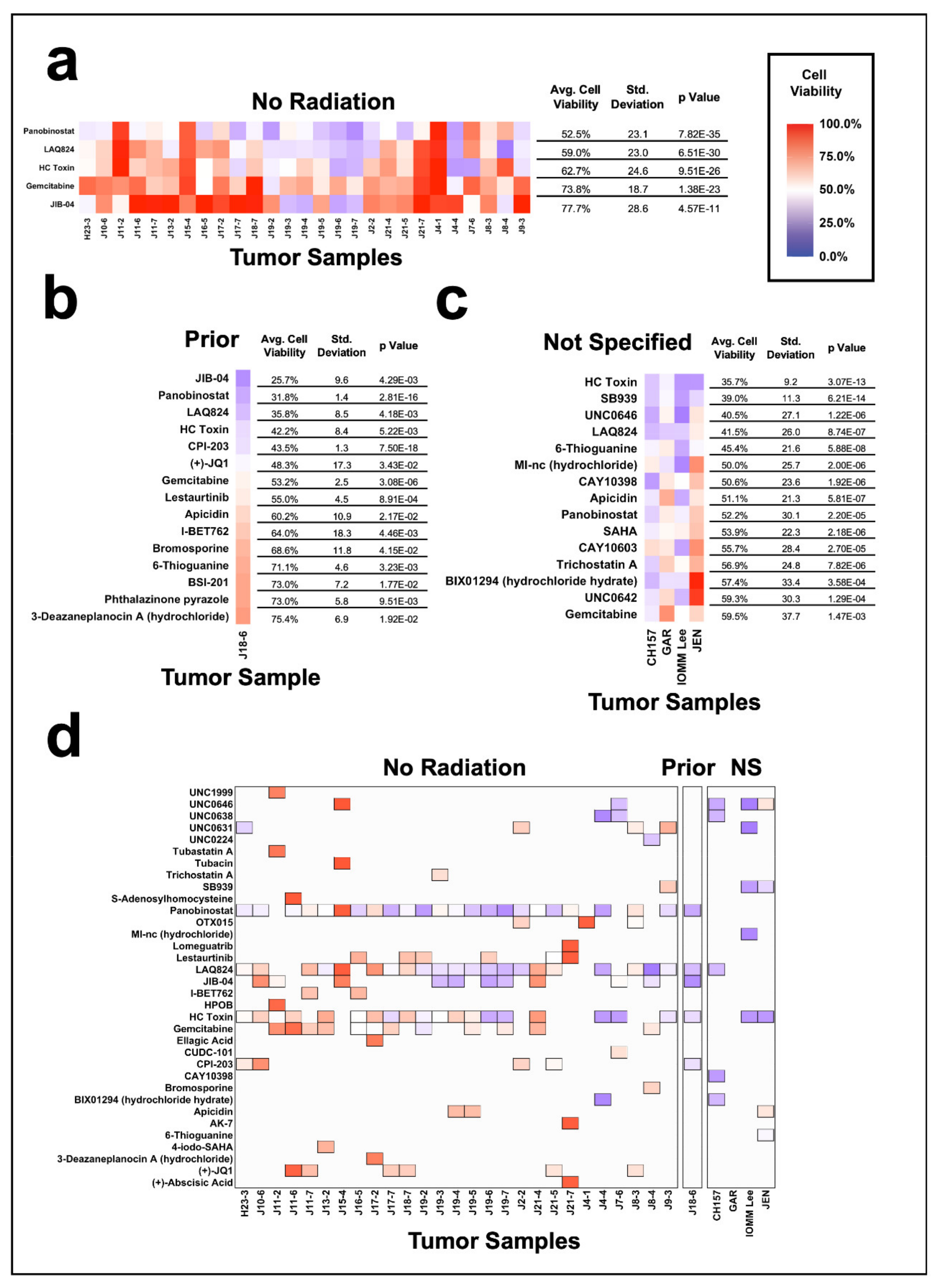
3.5. Meningioma Sensitivity to Epigenetic Inhibition Based on Radiation History
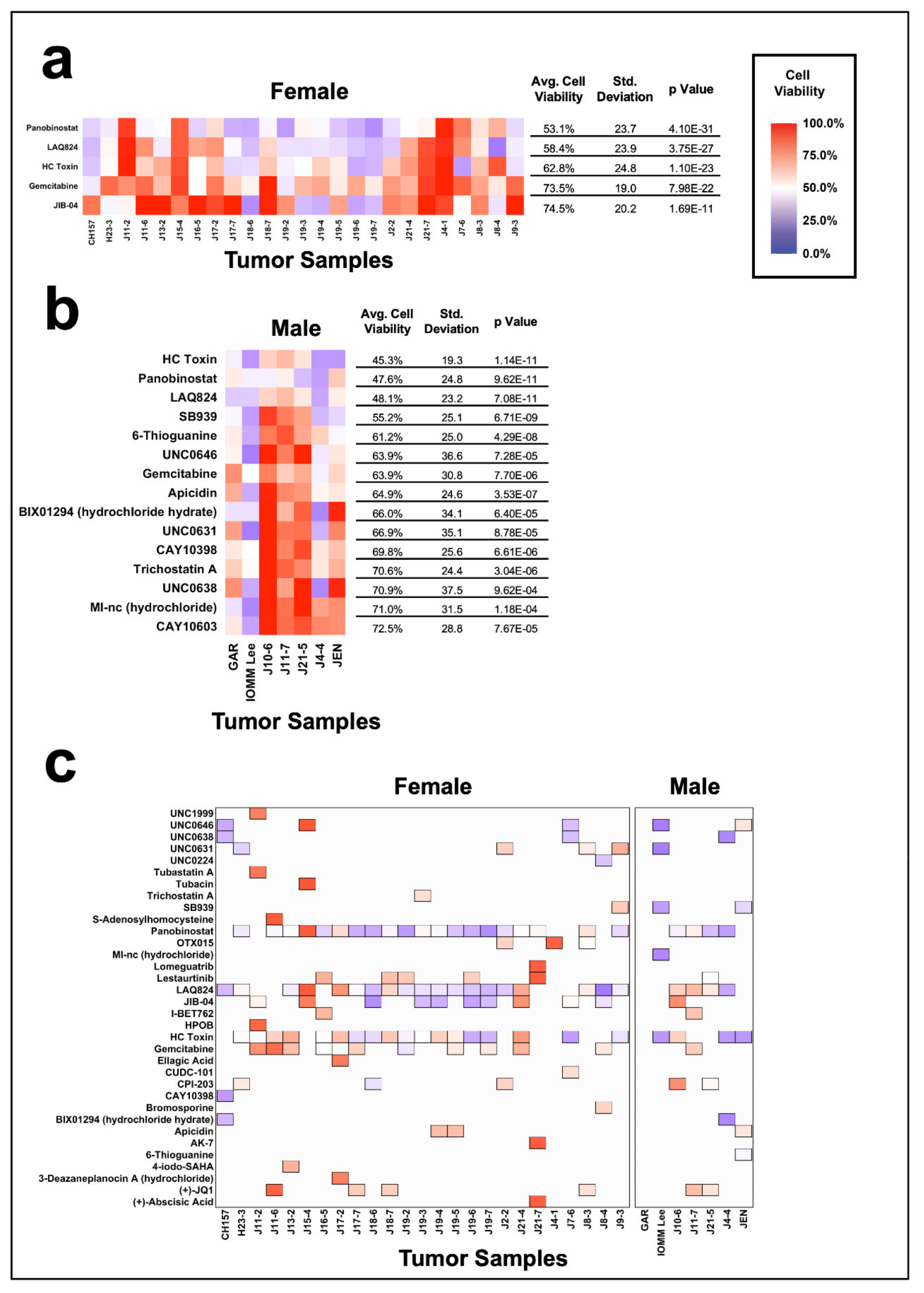
3.6. Impact of Gender on Epigenetic Inhibition of Meningiomas
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. Cbtrus statistical report: Primary brain and other central nervous system tumors diagnosed in the united states in 2011–2015. Neuro-Oncology 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [Green Version]
- Choy, W.; Kim, W.; Nagasawa, D.; Stramotas, S.; Yew, A.; Gopen, Q.; Parsa, A.T.; Yang, I. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg. Focus 2011, 30, E6. [Google Scholar] [CrossRef]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.J.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A Rano Review. J. Neurosurg. 2015, 122, 4–23. [Google Scholar] [CrossRef] [Green Version]
- Aghi, M.K.; Carter, B.S.; Cosgrove, G.R.; Ojemann, R.G.; Amin-Hanjani, S.; Martuza, R.L.; Curry, W.T., Jr.; Barker, F.G., 2nd. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 2009, 64, 56–60, discussion 60. [Google Scholar] [CrossRef]
- Jaaskelainen, J. Seemingly complete removal of histologically benign intracranial meningioma: Late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg. Neurol. 1986, 26, 461–469. [Google Scholar] [CrossRef]
- Van Alkemade, H.; de Leau, M.; Dieleman, E.M.; Kardaun, J.W.; van Os, R.; Vandertop, W.P.; van Furth, W.R.; Stalpers, L.J. Impaired survival and long-term neurological problems in benign meningioma. Neuro-Oncology 2012, 14, 658–666. [Google Scholar] [CrossRef]
- Nigim, F.; Wakimoto, H.; Kasper, E.M.; Ackermans, L.; Temel, Y. Emerging medical treatments for meningioma in the molecular era. Biomedicines 2018, 6, 86. [Google Scholar] [CrossRef] [Green Version]
- Preusser, M.; Brastianos, P.K.; Mawrin, C. Advances in meningioma genetics: Novel therapeutic opportunities. Nat. Rev. Neurol. 2018, 14, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, A.M.; Fernandez-Valle, C. Role of merlin/nf2 inactivation in tumor biology. Oncogene 2016, 35, 537–548. [Google Scholar] [CrossRef] [Green Version]
- Riemenschneider, M.J.; Perry, A.; Reifenberger, G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006, 5, 1045–1054. [Google Scholar] [CrossRef]
- Clark, V.E.; Erson-Omay, E.Z.; Serin, A.; Yin, J.; Cotney, J.; Ozduman, K.; Avsar, T.; Li, J.; Murray, P.B.; Henegariu, O.; et al. Genomic analysis of non-nf2 meningiomas reveals mutations in traf7, klf4, akt1, and smo. Science 2013, 339, 1077–1080. [Google Scholar] [CrossRef] [Green Version]
- Brastianos, P.K.; Horowitz, P.M.; Santagata, S.; Jones, R.T.; McKenna, A.; Getz, G.; Ligon, K.L.; Palescandolo, E.; Van Hummelen, P.; Ducar, M.D.; et al. Genomic sequencing of meningiomas identifies oncogenic smo and akt1 mutations. Nat. Genet. 2013, 45, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.E.; Harmanci, A.S.; Bai, H.; Youngblood, M.W.; Lee, T.I.; Baranoski, J.F.; Ercan-Sencicek, A.G.; Abraham, B.J.; Weintraub, A.S.; Hnisz, D.; et al. Recurrent somatic mutations in polr2a define a distinct subset of meningiomas. Nat. Genet. 2016, 48, 1253–1259. [Google Scholar] [CrossRef] [Green Version]
- Shin, I.; Park, Y.W.; Ahn, S.S.; Kang, S.G.; Chang, J.H.; Kim, S.H.; Lee, S.K. Clinical and diffusion parameters may noninvasively predict tert promoter mutation status in grade ii meningiomas. J. Neuroradiol. 2021. [CrossRef]
- Barresi, V.; Simbolo, M.; Fioravanzo, A.; Piredda, M.L.; Caffo, M.; Ghimenton, C.; Pinna, G.; Longhi, M.; Nicolato, A.; Scarpa, A. Molecular profiling of 22 primary atypical meningiomas shows the prognostic significance of 18q heterozygous loss and cdkn2a/b homozygous deletion on recurrence-free survival. Cancers 2021, 13, 903. [Google Scholar] [CrossRef] [PubMed]
- Biczok, A.; Kraus, T.; Suchorska, B.; Terpolilli, N.A.; Thorsteinsdottir, J.; Giese, A.; Tonn, J.C.; Schichor, C. Tert promoter mutation is associated with worse prognosis in who grade ii and iii meningiomas. J. Neurooncol. 2018, 139, 671–678. [Google Scholar] [CrossRef]
- Juratli, T.A.; Thiede, C.; Koerner, M.V.A.; Tummala, S.S.; Daubner, D.; Shankar, G.M.; Williams, E.A.; Martinez-Lage, M.; Soucek, S.; Robel, K.; et al. Intratumoral heterogeneity and tert promoter mutations in progressive/higher-grade meningiomas. Oncotarget 2017, 8, 109228–109237. [Google Scholar] [CrossRef] [Green Version]
- Guyot, A.; Duchesne, M.; Robert, S.; Lia, A.S.; Derouault, P.; Scaon, E.; Lemnos, L.; Salle, H.; Durand, K.; Labrousse, F. Analysis of cdkn2a gene alterations in recurrent and non-recurrent meningioma. J. Neurooncol. 2019, 145, 449–459. [Google Scholar] [CrossRef]
- Hatada, I. The epigenomics of cancer. In An Omics Perspective on Cancer Research; Cho, W.C., Ed.; Springer: New York, NY, USA, 2010; pp. 51–67. [Google Scholar]
- Perdigoto, C.N. Epigenetic cancer evolution, one cell at a time. Nat. Rev. Genet. 2019, 20, 434–435. [Google Scholar] [CrossRef]
- Esteller, M. Epigenetics in cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef]
- Gillette, T.G.; Hill, J.A. Readers, writers, and erasers: Chromatin as the whiteboard of heart disease. Circ. Res. 2015, 116, 1245–1253. [Google Scholar] [CrossRef] [Green Version]
- Al-Mefty, O.; Kadri, P.A.; Pravdenkova, S.; Sawyer, J.R.; Stangeby, C.; Husain, M. Malignant progression in meningioma: Documentation of a series and analysis of cytogenetic findings. J. Neurosurg. 2004, 101, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Barski, D.; Wolter, M.; Reifenberger, G.; Riemenschneider, M.J. Hypermethylation and transcriptional downregulation of the timp3 gene is associated with allelic loss on 22q123 and malignancy in meningiomas. Brain Pathol. 2010, 20, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.J.; Aminoso, C.; Lopez-Marin, I.; Arjona, D.; Gonzalez-Gomez, P.; Alonso, M.E.; Lomas, J.; de Campos, J.M.; Kusak, M.E.; Vaquero, J.; et al. DNA methylation of multiple promoter-associated cpg islands in meningiomas: Relationship with the allelic status at 1p and 22q. Acta Neuropathol. 2004, 108, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.P.; Weisenberger, D.J.; Aman, J.F.; Hinoue, T.; Ramjan, Z.; Liu, Y.; Noushmehr, H.; Lange, C.P.; van Dijk, C.M.; Tollenaar, R.A.; et al. Regions of focal DNA hypermethylation and long-range hypomethylation in colorectal cancer coincide with nuclear lamina-associated domains. Nat. Genet. 2011, 44, 40–46. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Pham, M.H.; Pease, M.; Zada, G.; Giannotta, S.L.; Wang, K.; Mack, W.J. A review of epigenetic and gene expression alterations associated with intracranial meningiomas. Neurosurg. Focus 2013, 35, E5. [Google Scholar] [CrossRef] [Green Version]
- Bush, M.L.; Oblinger, J.; Brendel, V.; Santarelli, G.; Huang, J.; Akhmametyeva, E.M.; Burns, S.S.; Wheeler, J.; Davis, J.; Yates, C.W.; et al. Ar42, a novel histone deacetylase inhibitor, as a potential therapy for vestibular schwannomas and meningiomas. Neuro-Oncology 2011, 13, 983–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.S.; Weng, S.C.; Tseng, P.H.; Lin, H.P.; Chen, C.S. Histone acetylation-independent effect of histone deacetylase inhibitors on akt through the reshuffling of protein phosphatase 1 complexes. J. Biol. Chem. 2005, 280, 38879–38887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, R.; Tang, X.; Gutmann, D.H.; Ye, K. Neurofibromatosis 2 (nf2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to pike-l. Proc. Natl. Acad. Sci. USA 2004, 101, 18200–18205. [Google Scholar] [CrossRef] [Green Version]
- DeQuach, J.A.; Yuan, S.H.; Goldstein, L.S.; Christman, K.L. Decellularized porcine brain matrix for cell culture and tissue engineering scaffolds. Tissue Eng. Part A 2011, 17, 2583–2592. [Google Scholar] [CrossRef] [Green Version]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell viability assays. In Assay Guidance Manual; Markossian, S., Sittampalam, G.S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Caaveiro, J.M.M., Chung, T.D.Y., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar] [PubMed]
- Thomas, S.; Miller, A.; Thurn, K.T.; Munster, P. Chapter 37—clinical applications of histone deacetylase inhibitors. In Handbook of Epigenetics; Tollefsbol, T., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 597–615. [Google Scholar]
- Jin, J.X.; Lee, S.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. The hdac inhibitor laq824 enhances epigenetic reprogramming and in vitro development of porcine scnt embryos. Cell Physiol. Biochem. 2017, 41, 1255–1266. [Google Scholar] [CrossRef]
- Brosch, G.; Ransom, R.; Lechner, T.; Walton, J.D.; Loidl, P. Inhibition of maize histone deacetylases by hc toxin, the host-selective toxin of cochliobolus carbonum. Plant Cell 1995, 7, 1941–1950. [Google Scholar]
- Darkin-Rattray, S.J.; Gurnett, A.M.; Myers, R.W.; Dulski, P.M.; Crumley, T.M.; Allocco, J.J.; Cannova, C.; Meinke, P.T.; Colletti, S.L.; Bednarek, M.A.; et al. Apicidin: A novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 1996, 93, 13143–13147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goutagny, S.; Kalamarides, M. Meningiomas and neurofibromatosis. J. Neurooncol. 2010, 99, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.S.; Akhmametyeva, E.M.; Oblinger, J.L.; Bush, M.L.; Huang, J.; Senner, V.; Chen, C.S.; Jacob, A.; Welling, D.B.; Chang, L.S. Histone deacetylase inhibitor ar-42 differentially affects cell-cycle transit in meningeal and meningioma cells, potently inhibiting nf2-deficient meningioma growth. Cancer Res. 2013, 73, 792–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Qi, L.; Du, Y.; Huang, L.F.; Braun, F.K.; Kogiso, M.; Zhao, Y.; Li, C.; Lindsay, H.; Zhao, S.; et al. Patient-derived orthotopic xenograft (pdox) mouse models of primary and recurrent meningioma. Cancers 2020, 12, 1478. [Google Scholar] [CrossRef]
- Liston, D.R.; Davis, M. Clinically relevant concentrations of anticancer drugs: A guide for nonclinical studies. Clin. Cancer Res. 2017, 23, 3489–3498. [Google Scholar] [CrossRef] [Green Version]
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An overview of meningiomas. Future Oncol. 2018, 14, 2161–2177. [Google Scholar] [CrossRef]
- Ogasawara, C.; Philbrick, B.D.; Adamson, D.C. Meningioma: A review of epidemiology, pathology, diagnosis, treatment, and future directions. Biomedicines 2021, 9, 319. [Google Scholar] [CrossRef]


| No. | Sample | Age | Gender | Grade | Histologic Subtype | Primary/Recurrent | Radiation | Chemotherapy |
|---|---|---|---|---|---|---|---|---|
| 1 | *IOMM Lee | 61 | Male | 3 | Anaplastic | Recurrent | Unknown | Unknown |
| 2 | *CH157 | 59 | Female | 2 | Unknown | Primary | Unknown | Unknown |
| 3 | J8-4 | 48 | Female | 2 | Atypical | Primary | No | No |
| 4 | J13-2 | 54 | Female | 2 | Atypical | Primary | No | No |
| 5 | J17-2 | 68 | Female | 2 | Atypical | Primary | No | No |
| 6 | J19-3 | 95 | Female | 2 | Atypical | Primary | No | No |
| 7 | J19-4 | 49 | Female | 2 | Atypical | Recurrent | No | No |
| 8 | *GAR | 71 | Male | 1 | Unknown | Primary | Unknown | No |
| 9 | H23-3 | 83 | Female | 1 | Small cell with psammoma bodies | Primary | No | No |
| 10 | J10-6 | 54 | Male | 1 | Meningothelial | Primary | No | No |
| 11 | J11-2 | 57 | Female | 1 | Not Specified | Primary | No | No |
| 12 | J11-6 | 56 | Female | 1 | Not Specified | Primary | No | No |
| 13 | J11-7 | 58 | Male | 1 | Meningothelial | Primary | No | No |
| 14 | J15-4 | 40 | Female | 1 | Myxoid | Primary | No | No |
| 15 | J16-5 | 49 | Female | 1 | Psammomatous | Primary | No | No |
| 16 | J17-7 | 49 | Female | 1 | Not Specified | Primary | No | No |
| 17 | J18-6 | 64 | Female | 1 | Meningothelial | Primary | Gamma Knife 36Gy | 6 Cycles of TAC Chemotherapy |
| 18 | J18-7 | 41 | Female | 1 | Meningothelial | Primary | No | No |
| 19 | J19-2 | 44 | Female | 1 | Psammomatous | Primary | No | No |
| 20 | J19-5 | 39 | Female | 1 | Meningothelial | Primary | No | No |
| 21 | J19-6 | 44 | Female | 1 | Transitional | Primary | No | No |
| 22 | J19-7 | 57 | Female | 1 | Meningothelial | Primary | No | No |
| 23 | J2-2 | 65 | Female | 1 | Meningothelial | Primary | No | No |
| 24 | J21-4 | 55 | Female | 1 | Rhabdoid Morphology Present | Primary | No | No |
| 25 | J21-5 | 65 | Male | 1 | Secretory | Primary | No | No |
| 26 | J21-7 | 51 | Female | 1 | Fibrous | Recurrent | No | No |
| 27 | J4-1 | 63 | Female | 1 | Fibrous | Primary | No | No |
| 28 | J4-4 | 67 | Male | 1 | Fibrous | Primary | No | No |
| 29 | J7-6 | 57 | Female | 1 | Transitional | Primary | No | No |
| 30 | J8-3 | 38 | Female | 1 | Meningothelial | Primary | No | No |
| 31 | J9-3 | 70 | Female | 1 | Psammomatous | Primary | No | No |
| 32 | *JEN | 55 | Male | 1 | Psammomatous | Primary | Unknown | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatman, P.D.; Wroblewski, T.H.; Fringuello, A.R.; Scherer, S.R.; Foreman, W.B.; Damek, D.M.; Lillehei, K.; Youssef, A.S.; Jensen, R.L.; Graner, M.W.; et al. High-Throughput Mechanistic Screening of Epigenetic Compounds for the Potential Treatment of Meningiomas. J. Clin. Med. 2021, 10, 3150. https://doi.org/10.3390/jcm10143150
Tatman PD, Wroblewski TH, Fringuello AR, Scherer SR, Foreman WB, Damek DM, Lillehei K, Youssef AS, Jensen RL, Graner MW, et al. High-Throughput Mechanistic Screening of Epigenetic Compounds for the Potential Treatment of Meningiomas. Journal of Clinical Medicine. 2021; 10(14):3150. https://doi.org/10.3390/jcm10143150
Chicago/Turabian StyleTatman, Philip D., Tadeusz H. Wroblewski, Anthony R. Fringuello, Samuel R. Scherer, William B. Foreman, Denise M. Damek, Kevin Lillehei, A. Samy Youssef, Randy L. Jensen, Michael W. Graner, and et al. 2021. "High-Throughput Mechanistic Screening of Epigenetic Compounds for the Potential Treatment of Meningiomas" Journal of Clinical Medicine 10, no. 14: 3150. https://doi.org/10.3390/jcm10143150
APA StyleTatman, P. D., Wroblewski, T. H., Fringuello, A. R., Scherer, S. R., Foreman, W. B., Damek, D. M., Lillehei, K., Youssef, A. S., Jensen, R. L., Graner, M. W., & Ormond, D. R. (2021). High-Throughput Mechanistic Screening of Epigenetic Compounds for the Potential Treatment of Meningiomas. Journal of Clinical Medicine, 10(14), 3150. https://doi.org/10.3390/jcm10143150







