Sex Hormones Related Ocular Dryness in Breast Cancer Women
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Pathogenesis
3.1.1. Role of Sex Hormone Balance on the Ocular Surface Fitness
3.1.2. Estrogens
3.1.3. Androgens
3.1.4. Breast Cancer Treatment and Dry Eye Syndrome
3.1.5. The Role of Systemic Hormones Therapy on Ocular Surface
3.2. Functional Hyperandrogenism on the Ocular Surface
3.2.1. PCOS
3.2.2. Menopause
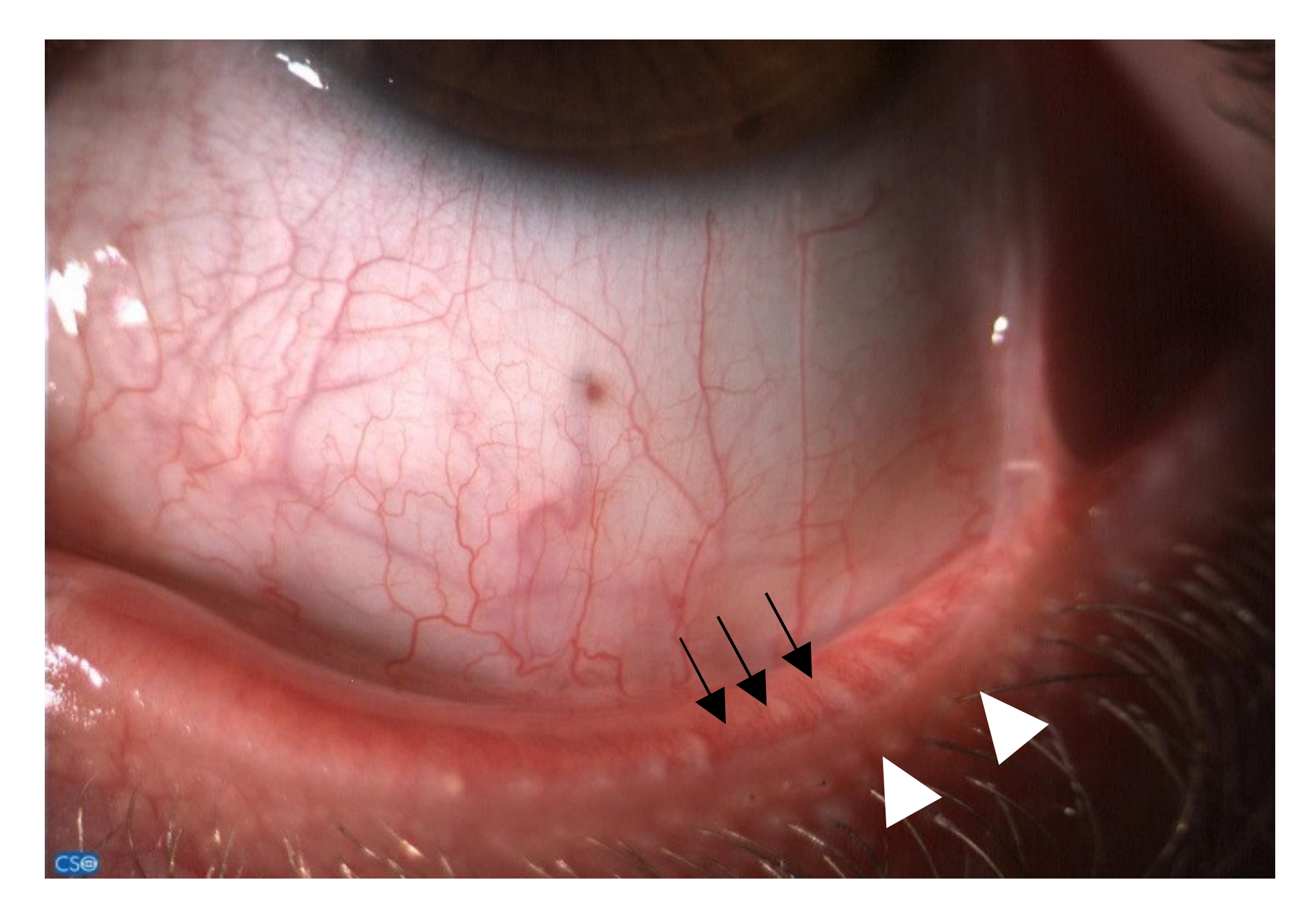
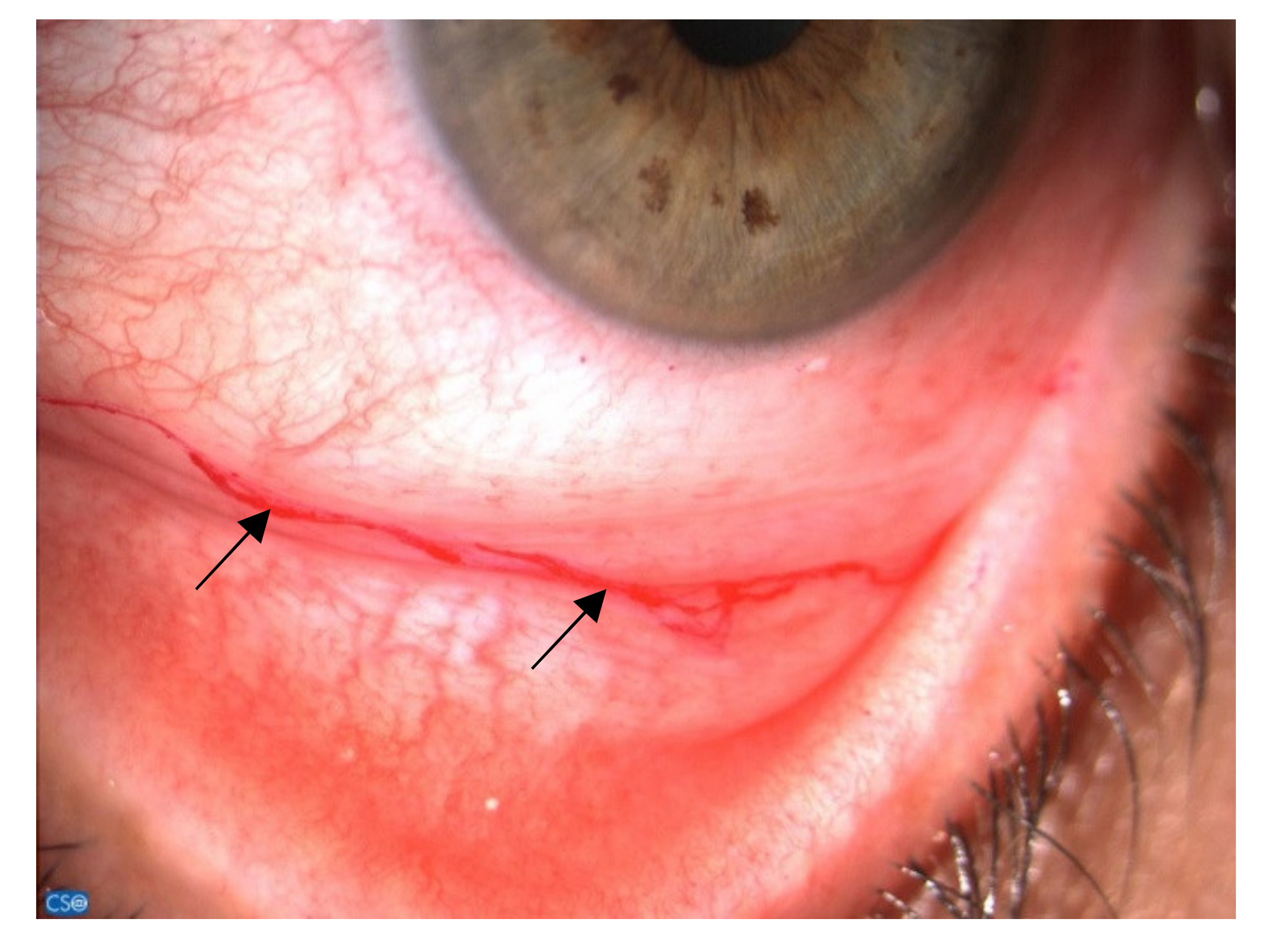
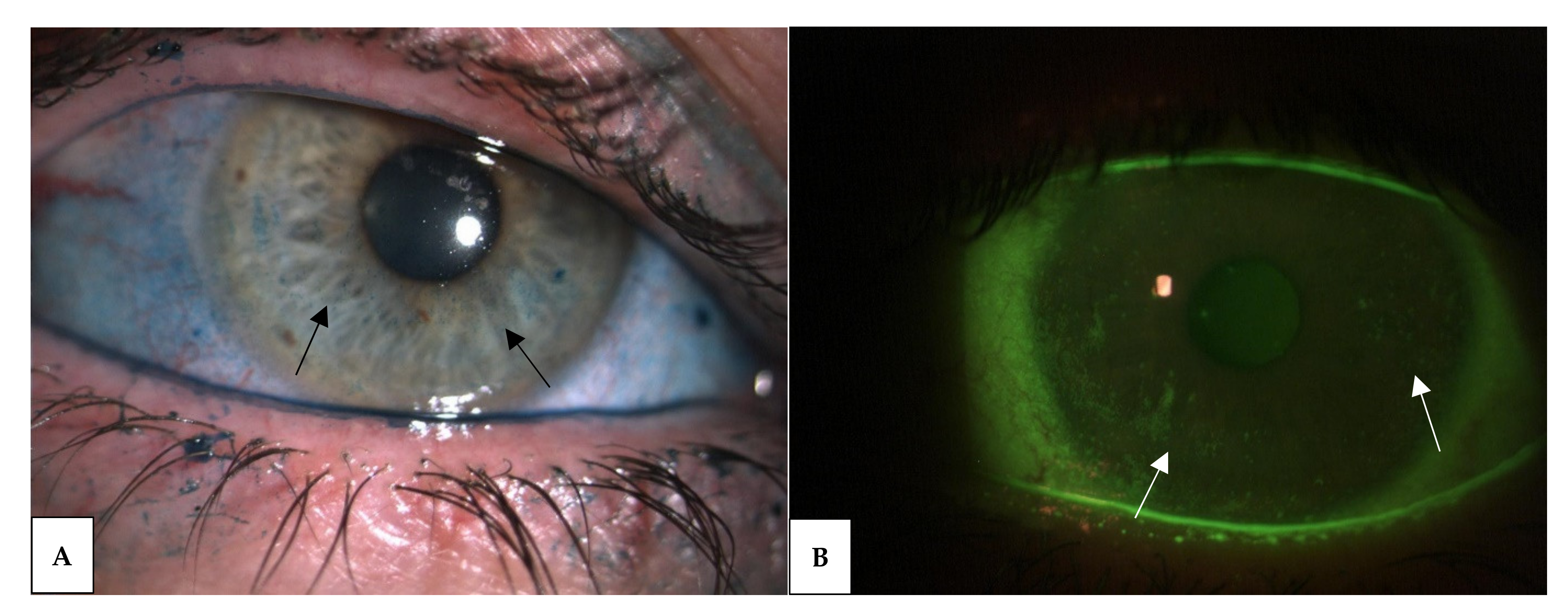
3.3. Diagnosis and Clinical Aspects
3.4. Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Gao, J.; Lambert, R.W.; Wickham, L.A.; Banting, G.; Sullivan, D.A. Androgen control of secretory component mRNA levels in the rat lacrimal gland. J. Steroid Biochem. Mol. Biol. 1995, 52, 239–249. [Google Scholar] [CrossRef]
- Gollub, E.G.; Waksman, H.; Goswami, S.; Marom, Z. Mucin genes are regulated by estrogen and dexamethasone. Biochem. Biophys. Res. Commun. 1995, 217, 1006–1014. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Yamagami, H.; Liu, M.; Steagall, R.J.; Schirra, F.; Suzuki, T.; Krenzer, K.L.; Cermak, J.M.; Sullivan, R.M.; Richards, S.M.; et al. Sex steroids, the meibomian gland and evaporative dry eye. Adv. Exp. Med. Biol. 2002, 506, 389–399. [Google Scholar] [CrossRef]
- Azzarolo, A.M.; Eihausen, H.; Schechter, J. Estrogen prevention of lacrimal gland cell death and lymphocytic infiltration. Exp. Eye Res. 2003, 77, 347–354. [Google Scholar] [CrossRef]
- Mantelli, F.; Moretti, C.; Micera, A.; Bonini, S. Conjunctival mucin deficiency in complete androgen insensitivity syndrome (CAIS). Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Esmaeli, B.; Harvey, J.T.; Hewlett, B. Immunohistochemical evidence for estrogen receptors in meibomian glands. Ophthalmology 2000, 107, 180–184. [Google Scholar] [CrossRef]
- Bergman, M.D.; Karelus, K.; Felicio, L.S.; Nelson, J.F. Tissue differences in estrogen receptor dynamics: Nuclear retention, rate of replenishment, and transient receptor loss vary in hypothalamus, pituitary, and uterus of C57BL/6J mice. Endocrinology 1987, 121, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Saceda, M.; Lippman, M.E.; Lindsey, R.K.; Puente, M.; Martin, M.B. Role of an estrogen receptor-dependent mechanism in the regulation of estrogen receptor mRNA in MCF-7 cells. Mol. Endocrinol. 1989, 3, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Asaithambi, A.; Mukherjee, S.; Thakur, M.K. Expression of 112-kDa estrogen receptor in mouse brain cortex and its autoregulation with age. Biochem. Biophys. Res. Commun. 1997, 231, 683–685. [Google Scholar] [CrossRef]
- Blanchere, M.; Berthaut, I.; Portois, M.C.; Mestayer, C.; Mowszowicz, I. Hormonal regulation of the androgen receptor expression in human prostatic cells in culture. J. Steroid Biochem. Mol. Biol. 1998, 66, 319–326. [Google Scholar] [CrossRef]
- Bonini, S.; Mantelli, F.; Moretti, C.; Lambiase, A.; Bonini, S.; Micera, A. Itchy-dry eye associated with polycystic ovary syndrome. Am. J. Ophthalmol. 2007, 143, 763–771. [Google Scholar] [CrossRef]
- Argüeso, P.; Guzman-Aranguez, A.; Mantelli, F.; Cao, Z.; Ricciuto, J.; Panjwani, N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J. Biol. Chem. 2009, 284, 23037–23045. [Google Scholar] [CrossRef]
- Mantelli, F.; Schaffer, L.; Dana, R.; Head, S.R.; Argüeso, P. Glycogene expression in conjunctiva of patients with dry eye: Downregulation of Notch signaling. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2666–2672. [Google Scholar] [CrossRef] [PubMed]
- Mantelli, F.; Argüeso, P. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, M.; Ricciuto, J.; Tisdale, A.; Gipson, I.K.; Mantelli, F.; Argüeso, P. Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Inglis, H.; Boyle, F.M.; Friedlander, M.L.; Watson, S.L. Dry eyes and AIs: If you don’t ask you won’t find out. Breast 2015, 24, 694–698. [Google Scholar] [CrossRef]
- Dowsett, M. Clinical development of aromatase inhibitors for the treatment of breast and prostate cancer. J. Steroid Biochem. Mol. Biol. 1990, 37, 1037–1041. [Google Scholar] [CrossRef]
- Smith, I.E.; Fitzharris, B.M.; McKinna, J.A.; Fahmy, D.R.; Nash, A.G.; Neville, A.M.; Gazet, J.C.; Ford, H.T.; Powles, T.J. Aminoglutethimide in treatment of metastatic breast carcinoma. Lancet 1978, 2, 646–649. [Google Scholar] [CrossRef]
- Cutolo, M.; Wilder, R.L. Different roles for androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum. Dis. Clin. N. Am. 2000, 26, 825–839. [Google Scholar] [CrossRef]
- Narita, S.; Goldblum, R.M.; Watson, C.S.; Brooks, E.G.; Estes, D.M.; Curran, E.M.; Midoro-Horiuti, T. Environmental estrogens induce mast cell degranulation and enhance IgE-mediated release of allergic mediators. Environ. Health Perspect. 2007, 115, 48–52. [Google Scholar] [CrossRef]
- Coksuer, H.; Ozcura, F.; Oghan, F.; Haliloglu, B.; Karatas, S. Effects of hyperandrogenism on tear function and tear drainage in patients with polycystic ovary syndrome. J. Reprod. Med. 2011, 56, 65–70. [Google Scholar]
- Cao, J.; Li, Q.; Shen, X.; Yao, Y.; Li, L.; Ma, H. Dehydroepiandrosterone attenuates LPS-induced inflammatory responses via activation of Nrf2 in RAW264.7 macrophages. Mol. Immunol. 2021, 131, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, A.; Bonini, S.; Fernandes, M. Adult vernal keratoconjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Giannaccare, G.; Campos, E.C. Sex-steroid imbalance in females and dry eye. Curr. Eye Res. 2015, 40, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Foulks, G.N.; Nichols, K.K.; Bron, A.J.; Holland, E.J.; McDonald, M.B.; Nelson, J.D. Improving awareness, identification, and management of meibomian gland dysfunction. Ophthalmology 2012, 119, S1–S12. [Google Scholar] [CrossRef]
- Chhadva, P.; Goldhardt, R.; Galor, A. Meibomian Gland Disease: The Role of Gland Dysfunction in Dry Eye Disease. Ophthalmology 2017, 124, S20–S26. [Google Scholar] [CrossRef]
- Schirra, F.; Suzuki, T.; Dickinson, D.P.; Townsend, D.J.; Gipson, I.K.; Sullivan, D.A. Identification of steroidogenic enzyme mRNAs in the human lacrimal gland, meibomian gland, cornea, and conjunctiva. Cornea 2006, 25, 438–442. [Google Scholar] [CrossRef]
- Golebiowski, B.; Badarudin, N.; Eden, J.; You, J.; Hampel, U.; Stapleton, F. Does endogenous serum oestrogen play a role in meibomian gland dysfunction in postmenopausal women with dry eye? Br. J. Ophthalmol. 2017, 101, 218–222. [Google Scholar] [CrossRef]
- Ablamowicz, A.F.; Nichols, J.J.; Nichols, K.K. Association between Serum Levels of Testosterone and Estradiol with Meibomian Gland Assessments in Postmenopausal Women. Investig. Ophthalmol. Vis. Sci. 2016, 57, 295–300. [Google Scholar] [CrossRef]
- Gagliano, C.; Caruso, S.; Napolitano, G.; Malaguarnera, G.; Cicinelli, M.V.; Amato, R.; Reibaldi, M.; Incarbone, G.; Bucolo, C.; Drago, F.; et al. Low levels of 17-β-oestradiol, oestrone and testosterone correlate with severe evaporative dysfunctional tear syndrome in postmenopausal women: A case-control study. Br. J. Ophthalmol. 2014, 98, 371–376. [Google Scholar] [CrossRef]
- Suzuki, T.; Schirra, F.; Richards, S.M.; Jensen, R.V.; Sullivan, D.A. Estrogen and progesterone control of gene expression in the mouse meibomian gland. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1797–1808. [Google Scholar] [CrossRef]
- Kam, W.; Sullivan, D. Suppressive Effects of 17β-Estradiol on Immortalized Human Meibomian Gland Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4316. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2149165 (accessed on 4 June 2021).
- Truong, S.; Cole, N.; Stapleton, F.; Golebiowski, B. Sex hormones and the dry eye. Clin. Exp. Optom. 2014, 97, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.H.; Lange, C.A. Deciphering Steroid Receptor Crosstalk in Hormone-Driven Cancers. Endocrinology 2018, 159, 3897–3907. [Google Scholar] [CrossRef] [PubMed]
- Erdem, U.; Ozdegirmenci, O.; Sobaci, E.; Sobaci, G.; Göktolga, U.; Dagli, S. Dry eye in post-menopausal women using hormone replacement therapy. Maturitas 2007, 56, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sullivan, B.D.; Liu, M.; Schirra, F.; Richards, S.M.; Yamagami, H.; Sullivan, D.A. Estrogen and progesterone effects on the morphology of the mouse meibomian gland. Adv. Exp. Med. Biol. 2002, 506, 483–488. [Google Scholar] [CrossRef]
- Cermak, J.M.; Krenzer, K.L.; Sullivan, R.M.; Dana, M.R.; Sullivan, D.A. Is complete androgen insensitivity syndrome associated with alterations in the meibomian gland and ocular surface? Cornea 2003, 22, 516–521. [Google Scholar] [CrossRef]
- Jester, J.V.; Brown, D.J. Wakayama Symposium: Peroxisome proliferator-activated receptor-gamma (PPARγ) and meibomian gland dysfunction. Ocul. Surf. 2012, 10, 224–229. [Google Scholar] [CrossRef]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef]
- Schirra, F.; Richards, S.M.; Liu, M.; Suzuki, T.; Yamagami, H.; Sullivan, D.A. Androgen regulation of lipogenic pathways in the mouse meibomian gland. Exp. Eye Res. 2006, 83, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.A.; Sullivan, B.D.; Evans, J.E.; Schirra, F.; Yamagami, H.; Liu, M.; Richards, S.M.; Suzuki, T.; Schaumberg, D.A.; Sullivan, R.M.; et al. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann. N. Y. Acad. Sci. 2002, 966, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Krenzer, K.L.; Dana, M.R.; Ullman, M.D.; Cermak, J.M.; Tolls, D.B.; Evans, J.E.; Sullivan, D.A. Effect of androgen deficiency on the human meibomian gland and ocular surface. J. Clin. Endocrinol. Metab. 2000, 85, 4874–4882. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Dana, R.; Buring, J.E.; Sullivan, D.A. Prevalence of dry eye disease among US men: Estimates from the Physicians’ Health Studies. Arch. Ophthalmol. 2009, 127, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.A.; Bélanger, A.; Cermak, J.M.; Bérubé, R.; Papas, A.S.; Sullivan, R.M.; Yamagami, H.; Dana, M.R.; Labrie, F. Are women with Sjögren’s syndrome androgen-deficient? J. Rheumatol. 2003, 30, 2413–2419. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA. Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Grimm, S.L.; Hartig, S.M.; Edwards, D.P. Progesterone Receptor Signaling Mechanisms. J. Mol. Biol. 2016, 428, 3831–3849. [Google Scholar] [CrossRef]
- Maughan, K.L.; Lutterbie, M.A.; Ham, P.S. Treatment of breast cancer. Am. Fam. Physician 2010, 81, 1339–1346. [Google Scholar]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Lovelace, D.L.; McDaniel, L.R.; Golden, D. Long-Term Effects of Breast Cancer Surgery, Treatment, and Survivor Care. J. Midwifery Women Health 2019, 64, 713–724. [Google Scholar] [CrossRef]
- Barabino, S.; Aragona, P.; di Zazzo, A.; Rolando, M. Updated definition and classification of dry eye disease: Renewed proposals using the nominal group and Delphi techniques. Eur. J. Ophthalmol. 2021, 31, 42–48. [Google Scholar] [CrossRef]
- Sabatino, F.; Di Zazzo, A.; De Simone, L.; Bonini, S. The Intriguing Role of Neuropeptides at the Ocular Surface. Ocul. Surf. 2017, 15, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Asfuroğlu, Y.; Kan, Ö.; Asfuroğlu, M.; Baser, E. Association Between Dry Eye and Polycystic Ovary Syndrome: Subclinical Inflammation May Be Part of the Process. Eye Contact Lens 2021, 47, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.A.; Higginbotham, E.J.; Guzinski, G.M.; Davis, I.L.; Ellish, N.J. A survey of ocular complaints in postmenopausal women. J. Assoc. Acad. Minor. Physicians Off. Publ. Assoc. Acad. Minor. Physicians 2000, 11, 44–49. [Google Scholar]
- Ziemanski, J.F.; Wolters, L.R.; Jones-Jordan, L.; Nichols, J.J.; Nichols, K.K. Relation Between Dietary Essential Fatty Acid Intake and Dry Eye Disease and Meibomian Gland Dysfunction in Postmenopausal Women. Am. J. Ophthalmol. 2018, 189, 29–40. [Google Scholar] [CrossRef]
- Dang, A.; Nayeni, M.; Mather, R.; Malvankar-Mehta, M.S. Hormone replacement therapy for dry eye disease patients: Systematic review and meta-analysis. Can. J. Ophthalmol. 2020, 55, 3–11. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Nichols, J.J.; Papas, E.B.; Tong, L.; Uchino, M.; Nichols, K.K. The international workshop on meibomian gland dysfunction: Report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1994–2005. [Google Scholar] [CrossRef]
- Yavas, G.F.; Ozturk, F.; Kusbeci, T.; Ermis, S.S.; Yilmazer, M.; Cevrioglu, S.; Aktepe, F.; Kose, S. Meibomian gland alterations in polycystic ovary syndrome. Curr. Eye Res. 2008, 33, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Gonen, T.; Celik, C.; Oznur, M.; Abali, R.; Gonen, K.A.; Horozoglu, F.; Aksu, E.; Keskinbora, K.H. Tear osmolarity and ocular surface changes in patient with polycystic ovary syndrome. Curr. Eye Res. 2013, 38, 621–625. [Google Scholar] [CrossRef]
- Yuksel, B.; Ozturk, I.; Seven, A.; Aktas, S.; Aktas, H.; Kucur, S.K.; Polat, M.; Kilic, S. Tear function alterations in patients with polycystic ovary syndrome. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3556–3562. [Google Scholar]
- Baser, G.; Yildiz, N.; Calan, M. Evaluation of Meibomian Gland Dysfunction in Polycystic Ovary Syndrome and Obesity. Curr. Eye Res. 2017, 42, 661–665. [Google Scholar] [CrossRef]
- Metka, M.; Enzelsberger, H.; Knogler, W.; Schurz, B.; Aichmair, H. Eye manifestations as climacteric symptom. Geburtshilfe Frauenheilkd. 1991, 51, 143–145. [Google Scholar] [CrossRef]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Executive summary. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liang, K.; Jiang, Z.; Tao, L. Sex hormone therapy’s effect on dry eye syndrome in postmenopausal women: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e12572. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Feng, G.; Peng, S.; Li, H. The effects of hormone replacement therapy on dry eye syndromes evaluated by Schirmer test depend on patient age. Cont. Lens Anterior Eye 2016, 39, 124–127. [Google Scholar] [CrossRef]
- Ma, J.; Pazo, E.E.; Zou, Z.; Jin, F. Prevalence of symptomatic dry eye in breast cancer patients undergoing systemic adjuvant treatment: A cross-sectional study. Breast 2020, 53, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gong, L.; Chapin, W.J.; Zhu, M. Assessment of vision-related quality of life in dry eye patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5722–5727. [Google Scholar] [CrossRef] [PubMed]
- Vigo, L.; Pellegrini, M.; Bernabei, F.; Carones, F.; Scorcia, V.; Giannaccare, G. Diagnostic Performance of a Novel Noninvasive Workup in the Setting of Dry Eye Disease. J. Ophthalmol. 2020, 2020, 5804123. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, A.; Khanal, S.; Ramaesh, K.; Diaper, C.; McFadyen, A. Tear film osmolarity: Determination of a referent for dry eye diagnosis. Investig. Ophthalmol. Vis. Sci. 2010, 47, 4309–4315. [Google Scholar] [CrossRef] [PubMed]
- Keech, A.; Senchyna, M.; Jones, L. Impact of time between collection and collection method on human tear fluid osmolarity. Curr. Eye Res. 2013, 38, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Itoh, K.; Maeda, S.; Maeda, K.; Amano, S. A newly developed noninvasive and mobile pen-shaped meibography system. Cornea 2013, 32, 242–247. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Bernabei, F.; Senni, C.; Aloi, M.; Scalzo, G.C.; Ceravolo, D.; Iovino, C.; Scorcia, V. Comparative analysis of ocular redness score evaluated automatically in glaucoma patients under different topical medications. Eur. J. Ophthalmol. 2020, 1120672120969612. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Campos, E.C. Neurotrophic keratitis: Current challenges and future prospects. Eye Brain 2018, 10, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Streicher, L.; Simon, J.A. Sexual Function Post-Breast Cancer. Cancer Treat. Res. 2018, 173, 167–189. [Google Scholar] [CrossRef]
- Baumgart, J.; Nilsson, K.; Evers, A.S.; Kallak, T.K.; Poromaa, I.S. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause 2013, 20, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Tucker, P.E.; Saunders, C.; Bulsara, M.K.; Tan, J.J.-S.; Salfinger, S.G.; Green, H.; Cohen, P.A. Sexuality and quality of life in women with a prior diagnosis of breast cancer after risk-reducing salpingo-oophorectomy. Breast 2016, 30, 26–31. [Google Scholar] [CrossRef]
- Worda, C.; Nepp, J.; Huber, J.C.; Sator, M.O. Treatment of keratoconjunctivitis sicca with topical androgen. Maturitas 2001, 37, 209–212. [Google Scholar] [CrossRef]
- Connor, C.G.; Primo, E.J. A weak androgenic artificial tear solution decreases the osmolarity of dry eye patients. Investig. Ophthalmol. Vis. Sci. 2001, 42, S30. [Google Scholar]
- Safety and Efficacy Study of Testosterone Eye Drop for the Treatment of Meibomian Gland Dysfunction – Full Text View – ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00755183 (accessed on 14 June 2021).
- Connor, C. Symptomatic Relief of Dry Eye Assessed With the OSDI in Patients Using 5% Testosterone Cream. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2032. [Google Scholar]
- Sullivan, D.A.; Wickham, L.A.; Rocha, E.M.; Kelleher, R.S.; da Silveira, L.A.; Toda, I. Influence of gender, sex steroid hormones, and the hypothalamic-pituitary axis on the structure and function of the lacrimal gland. Adv. Exp. Med. Biol. 1998, 438, 11–42. [Google Scholar] [CrossRef] [PubMed]
- Gurwood, A.S.; Gurwood, I.; Gubman, D.T.; Brzezicki, L.J. Idiosyncratic ocular symptoms associated with the estradiol transdermal estrogen replacement patch system. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 1995, 72, 29–33. [Google Scholar] [CrossRef]
- Sato, E.H.; Sullivan, D.A. Comparative influence of steroid hormones and immunosuppressive agents on autoimmune expression in lacrimal glands of a female mouse model of Sjögren’s syndrome. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2632–2642. [Google Scholar]
- Thody, A.J.; Shuster, S. Control and function of sebaceous glands. Physiol. Rev. 1989, 69, 383–416. [Google Scholar] [CrossRef] [PubMed]
- Pochi, P.E.; Strauss, J.S. Endocrinologic control of the development and activity of the human sebaceous gland. J. Investig. Dermatol. 1974, 62, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, D.A.; Buring, J.E.; Sullivan, D.A.; Dana, M.R. Hormone replacement therapy and dry eye syndrome. JAMA 2001, 286, 2114–2119. [Google Scholar] [CrossRef] [PubMed]
- Chia, E.-M.; Mitchell, P.; Rochtchina, E.; Lee, A.J.; Maroun, R.; Wang, J.J. Prevalence and associations of dry eye syndrome in an older population: The Blue Mountains Eye Study. Clin. Exp. Ophthalmol. 2003, 31, 229–232. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Azar, D.T.; Baudouin, C.; Efron, N.; Hirayama, M.; Horwath-Winter, J.; Kim, T.; Mehta, J.S.; Messmer, E.M.; Pepose, J.S.; et al. TFOS DEWS II iatrogenic report. Ocul. Surf. 2017, 15, 511–538. [Google Scholar] [CrossRef]
- Paulsen, A.J.; Cruickshanks, K.J.; Fischer, M.E.; Huang, G.-H.; Klein, B.E.K.; Klein, R.; Dalton, D.S. Dry eye in the beaver dam offspring study: Prevalence, risk factors, and health-related quality of life. Am. J. Ophthalmol. 2014, 157, 799–806. [Google Scholar] [CrossRef]
- Chen, S.P.; Massaro-Giordano, G.; Pistilli, M.; Schreiber, C.A.; Bunya, V.Y. Tear osmolarity and dry eye symptoms in women using oral contraception and contact lenses. Cornea 2013, 32, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, A.; Pearce, E.I.; Simmons, P.A.; Blades, K. Effect of oral contraceptives on tear physiology. Ophthalmic Physiol. Opt. 2001, 21, 9–16. [Google Scholar] [CrossRef]
- Frankel, S.H.; Ellis, P.P. Effect of oral contraceptives on tear production. Ann. Ophthalmol. 1978, 10, 1585–1588. [Google Scholar] [PubMed]
- Sriprasert, I.; Warren, D.W.; Mircheff, A.K.; Stanczyk, F.Z. Dry eye in postmenopausal women: A hormonal disorder. Menopause 2016, 23, 343–351. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Micera, A.; Coassin, M.; Varacalli, G.; Foulsham, W.; De Piano, M.; Bonini, S. Inflammaging at ocular surface: Clinical and biomolecular analyses in healthy volunteers. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, A.; Coassin, M.; Micera, A.; Mori, T.; De Piano, M.; Scartozzi, L.; Sgrulletta, R.; Bonini, S. Ocular surface diabetic disease: A neurogenic condition? Ocul. Surf. 2021, 19, 218–223. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Gaudenzi, D.; Yin, J.; Coassin, M.; Fernandes, M.; Dana, R.; Bonini, S. Corneal angiogenic privilege and its failure. Exp. Eye Res. 2021, 204, 108457. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grasso, A.; Di Zazzo, A.; Giannaccare, G.; Sung, J.; Inomata, T.; Shih, K.C.; Micera, A.; Gaudenzi, D.; Spelta, S.; Romeo, M.A.; et al. Sex Hormones Related Ocular Dryness in Breast Cancer Women. J. Clin. Med. 2021, 10, 2620. https://doi.org/10.3390/jcm10122620
Grasso A, Di Zazzo A, Giannaccare G, Sung J, Inomata T, Shih KC, Micera A, Gaudenzi D, Spelta S, Romeo MA, et al. Sex Hormones Related Ocular Dryness in Breast Cancer Women. Journal of Clinical Medicine. 2021; 10(12):2620. https://doi.org/10.3390/jcm10122620
Chicago/Turabian StyleGrasso, Antonella, Antonio Di Zazzo, Giuseppe Giannaccare, Jaemyoung Sung, Takenori Inomata, Kendrick Co Shih, Alessandra Micera, Daniele Gaudenzi, Sara Spelta, Maria Angela Romeo, and et al. 2021. "Sex Hormones Related Ocular Dryness in Breast Cancer Women" Journal of Clinical Medicine 10, no. 12: 2620. https://doi.org/10.3390/jcm10122620
APA StyleGrasso, A., Di Zazzo, A., Giannaccare, G., Sung, J., Inomata, T., Shih, K. C., Micera, A., Gaudenzi, D., Spelta, S., Romeo, M. A., Orsaria, P., Coassin, M., & Altomare, V. (2021). Sex Hormones Related Ocular Dryness in Breast Cancer Women. Journal of Clinical Medicine, 10(12), 2620. https://doi.org/10.3390/jcm10122620








