The Association between Sex and Risk of Alzheimer’s Disease in Adults with Down Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Assessments of AD-Dementia Status
2.3. Classification of AD-Dementia
2.4. Apolipoprotein E Genotypes
2.5. Statistical Analysis
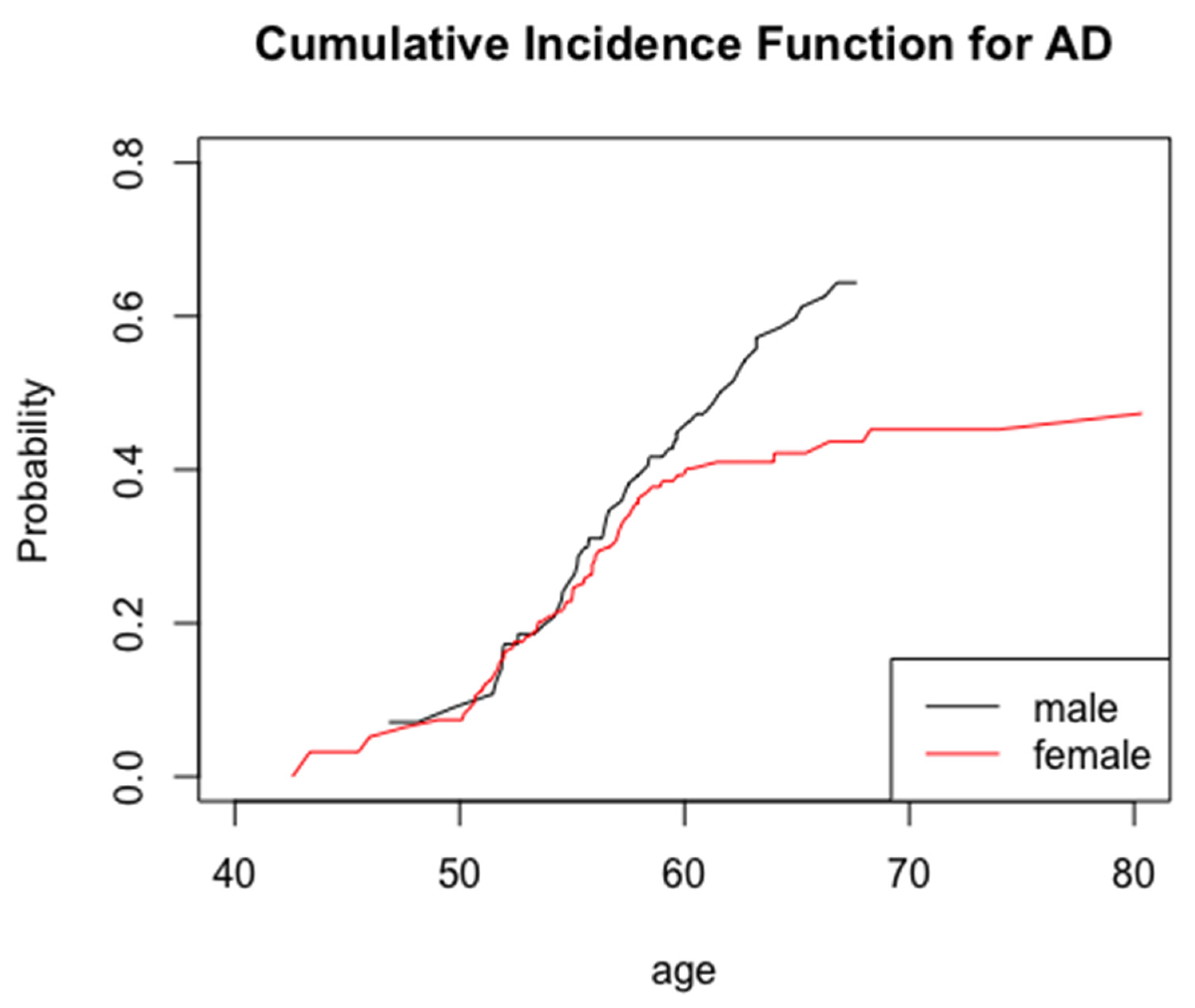
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wisniewski, H.M.; Silverman, W.; Wegiel, J. Ageing, Alzheimer disease and mental retardation. J. Intellect. Disabil. Res. 1994, 38, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Silverman, W.; Krinsky-McHale, S. Alzheimer’s risk and quality of life: History of Down syndrome as a case in point. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12171. [Google Scholar] [CrossRef]
- Bittles, A.H.; Glasson, E.J. Clinical, social, and ethical implications of changing life expectancy in Down syndrome. Dev. Med. Child. Neurol. 2004, 46, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Penrose, L.S. The incidence of mongolism in the general population. J. Ment. Sci. 1949, 95, 685–688. [Google Scholar] [CrossRef] [Green Version]
- Silverman, W.P.; Zigman, W.B.; Krinsky-Mchale, S.J.; Ryan, R.; Schupf, N. Intellectual disability, mild cognitive impairment, and risk for dementia. J. Policy Pract. Intellect. Disabil. 2013, 10, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachman, D.L.; Wolf, P.A.; Linn, R.; Knoefel, J.E.; CobbS, J.; Belanger, A.; D’Agostino, R.B.; White, L.R. Prevalence of dementia and probable senile dementia of the alzheimer type in the framingham study. Neurology 1992, 42, 115–119. [Google Scholar] [CrossRef]
- Bachman, D.L.; Wolf, P.; Linn, R.T.; Knoefel, J.E.; Cobb, J.L.; Belanger, A.J.; White, L.R.; D’Agostino, R.B. Incidence of dementia and probable alzheimer’s disease in a general population: The framingham study. Neurology 1993, 43, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef]
- Rumble, B.; Retallack, R.; Hilbich, C.; Simms, G.; Multhaup, G.; Martins, R.; Hockey, A.; Montgomery, P.; Beyreuther, K.; Masters, C.L. Amyloid A4 Protein and Its Precursor in Down’s Syndrome and Alzheimer’s Disease. N. Engl. J. Med. 1989, 320, 1446–1452. [Google Scholar] [CrossRef]
- Wiseman, F.; Pulford, L.J.; Barkus, C.; Liao, F.; Portelius, E.; Webb, R.; Chávez-Gutiérrez, L.; Cleverley, K.; Noy, S.; Sheppard, O.; et al. Trisomy of human chromosome 21 enhances amyloid-b deposition independently of an extra copy of APP. Brain 2018, 141, 2457–2474. [Google Scholar] [CrossRef] [Green Version]
- Viña, J.; Lloret, A. Why women have more Alzheimer’s disease than men: Gender and mitochondrial toxicity of amyloid-β peptide. J. Alzheimers Dis. 2010, 20 (Suppl. 2). [Google Scholar] [CrossRef] [Green Version]
- Mosconi, L.; Berti, V.; Quinn, C.; McHugh, P.; Petrongolo, G.; Varsavsky, I.; Osorio, R.; Pupi, A.; Vallabhajosula, S.; Isaacson, R.S.; et al. Sex differences in Alzheimer risk. Neurology 2017, 89, 1382–1390. [Google Scholar] [CrossRef]
- Pike, C.J. Sex and the development of Alzheimer’s disease. J. Neurosci. Res. 2017, 95, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Snyder, H.M.; Asthana, S.; Bain, L.; Brinton, R.; Craft, S.; Dubal, D.B.; Espeland, M.A.; Gatz, M.; Mielke, M.; Raber, J.; et al. Sex biology contributions to vulnerability to Alzheimer’s disease: A think tank convened by the Women’s Alzheimer’s Research Initiative. Alzheimers Dement. 2016, 12, 1186–1196. [Google Scholar] [CrossRef]
- Lipnicki, D.M.; Crawford, J.D.; Dutta, R.; Thalamuthu, A.; Kochan, N.A.; Andrews, G.; Lima-Costa, M.F.; Costa, E.D.C.E.; Brayne, C.; Matthews, F.E.; et al. Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: A collaborative cohort study. PLoS Med. 2017, 14, e1002261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nebel, R.A.; Aggarwal, N.T.; Barnes, L.L.; Gallagher, A.; Goldstein, J.M.; Kantarci, K.; Mallampalli, M.P.; Mormino, E.C.; Scott, L.; Yu, W.H.; et al. Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement. 2018, 14, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Fiest, K.M.; Roberts, J.I.; Maxwell, C.J.; Hogan, D.; Smith, E.E.; Frolkis, A.; Cohen, A.; Kirk, A.; Pearson, D.; Pringsheim, T.; et al. The prevalence and incidence of dementia due to Alzheimer’s disease: A systematic review and meta-analysis. Can. J. Neurol. Sci. 2016, 43 (Suppl. 1), S51–S82. [Google Scholar] [CrossRef]
- Matthews, F.; Cognitive Function and Ageing Studies (CFAS) Collaboration; Stephan, B.C.M.; Robinson, L.; Jagger, C.; Barnes, L.E.; Arthur, A.; Brayne, C.E. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seshadri, S.; Wolf, P.A.; Beiser, A.; Au, R.; McNulty, K.; White, R.; D’Agostino, R.B. Lifetime risk of dementia and Alzheimer’s disease: The impact of mortality on risk estimates in the Framingham Study. Neurology 1997, 49, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Toran-Allerand, C.D.; Miranda, R.C.; Bentham, W.D.; Sohrabji, F.; Brown, T.; Hochberg, R.B.; MacLusky, N. Estrogen receptors colocalize with low-affinity nerve growth factor receptors in cholinergic neurons of the basal forebrain. Proc. Natl. Acad. Sci. USA 1992, 89, 4668–4672. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, A.B.; Toran-Allerand, C.D.; Greengard, P.; Gandy, S.E. Estrogen regulates metabolism of Alzheimer amyloid β precursor protein. J. Biol. Chem. 1994, 269, 13065–13068. [Google Scholar] [CrossRef]
- Goodman, Y.; Bruce, A.J.; Cheng, B.; Mattson, M.P. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid β-peptide toxicity in hippocampal neurons. J. Neurochem. 1996, 66, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Petanceska, S.S.; Nagy, V.; Frail, D.; Gandy, S. Ovariectomy and 17β-estradiol modulate the levels of Alzheimer’s amyloid β peptides in brain. Exp. Gerontol. 2000, 35, 1317–1325. [Google Scholar] [CrossRef]
- Murray, M.E.; Aziz, A.; Ross, O.A.; Duara, R.; Dickson, D.W.; Graff-Radford, N.R. Alzheimer’s Disease May Not be More Common in Women; Men May be More Commonly Misdiagnosed. Alzheimers Dement. 2016, 12, P292. [Google Scholar] [CrossRef]
- Raghavan, R.; Khin-Nu, C.; Brown, A.G.; Day, K.A.; Tyrer, S.P.; Ince, P.G.; Perry, E.K.; Perry, R.H. Gender differences in the phenotypic expression of alzheimer’s disease in down’s syndrome (Trisomy 21). Neuroreport 1994, 5, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Kammann, E.; Rebeck, G.W.; Anderson, A.; Chen, Y.; Nixon, R.A. APOE genotype and gender effects on Alzheimer disease in 100 adults with Down syndrome. Neurology 1999, 53, 331–336. [Google Scholar] [CrossRef]
- Schupf, N.; Kapell, D.; Nightingale, B.; Rodriguez, A.; Tycko, B.; Mayeux, R. Earlier onset of Alzheimer’s disease in men with Down syndrome. Neurology 1998, 50, 991–995. [Google Scholar] [CrossRef]
- Visser, F.E.; Aldenkamp, A.P.; Van Huffelen, A.C.; Kuilman, M.; Overweg, J.; Van Wijk, J. Prospective study of the prevalence of Alzheimer-type dementia in institutionalized individuals with Down syndrome. Am. J. Ment. Retard. 1997, 101, 400–412. Available online: https://pubmed.ncbi.nlm.nih.gov/9017086/ (accessed on 24 May 2021).
- Lai, F.; Williams, R.S. A Prospective Study of Alzheimer Disease in Down Syndrome. Arch. Neurol. 1989, 46, 849–853. [Google Scholar] [CrossRef]
- Hasen, J.; Boyar, R.M.; Shapiro, L.R. Gonadal function in trisomy 21. Horm. Res. 1980, 12, 345–350. [Google Scholar] [CrossRef]
- Campbell, W.A.; Lowther, J.; McKenzie, I.; Price, W.H. Serum gonadotrophins in Down’s syndrome. J. Med. Genet. 1982, 19, 98–99. [Google Scholar] [CrossRef] [Green Version]
- Hsiang, Y.H.H.; Berkovitz, G.D.; Bland, G.L.; Migeon, C.J.; Warren, A.C. Gonadal function in patients with Down syndrome. Am. J. Med. Genet. 1987, 27, 449–458. [Google Scholar] [CrossRef]
- Hestnes, A.; Stovner, L.J.; HusØy FØlling, I.; Fougner, K.J.; Sjaastad, O. Hormonal and biochemical disturbances in Down’s syndrome. J. Intellect. Disabil Res. 1991, 35, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.; Laurin, D.; Verreault, R.; Hébert, R.; Helliwell, B.; Hill, G.B.; McDowell, I. Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, R.A. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein e and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Altmann, A.; Tian, L.; Henderson, V.W.; Greicius, M.D. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 2014, 75, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Neu, S.C.; Pa, J.; Kukull, W.; Beekly, D.; Kuzma, A.; Gangadharan, P.; Wang, L.-S.; Romero, K.; Arneric, S.P.; Redolfi, A.; et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol. 2017, 74, 1178–1189. [Google Scholar] [CrossRef]
- Hobel, Z.; Isenberg, A.L.; Raghupathy, D.; Mack, W.; Pa, J.; Alzheimer’s Disease Neuroimaging Initiative. APOE ϵ4 Gene Dose and Sex Effects on Alzheimer’s Disease MRI Biomarkers in Older Adults with Mild Cognitive Impairment. J. Alzheimers Dis. 2019, 71, 647–658. [Google Scholar] [CrossRef] [Green Version]
- Schupf, N. Genetic and host factors for dementia in Down’s syndrome. Br. J. Psychiatry 2002, 180, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Hithersay, R.; Startin, C.M.; Hamburg, S.; Mok, K.Y.; Hardy, J.; Fisher, E.M.C.; Tybulewicz, V.L.J.; Nizetic, D.; Strydom, A. Association of Dementia with Mortality among Adults with Down Syndrome Older Than 35 Years. JAMA Neurol. 2019, 76, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Rees, S.D.; Kelly, M.A.; Bain, S.C.; Barnett, A.H.; Thalitaya, D.; Prasher, V.P. Association of variants within APOE, SORL1, RUNX1, BACE1 and ALDH18A1 with dementia in Alzheimer’s disease in subjects with Down syndrome. Neurosci. Lett. 2011, 487, 144–148. [Google Scholar] [CrossRef]
- Deb, S.; Braganza, J.; Norton, N.; Williams, H.; Kehoe, P.G.; Williams, J.; Owen, M.J. APOE ε4 influences the manifestation of Alzheimer’s disease in adults with Down’s syndrome. Br. J. Psychiatry 2000, 176, 468–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasher, V.P.; Sajith, S.G.; Rees, S.D.; Patel, A.; Tewari, S.; Schupf, N.; Zigman, W.B. Significant effect of APOE epsilon 4 genotype on the risk of dementia in Alzheimer’s disease and mortality in persons with Down syndrome. Int. J. Geriatr. Psychiatry 2008, 23, 1134–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gool, W.A.; Evenhuis, H.M.; van Duijn, C.M. A case—Control study of apolipoprotein E genotypes in Alzheimer’s disease associated with Down’s syndrome. Ann. Neurol. 1995, 38, 225–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, J.C.; Pérez-Tur, J.; Dupire, M.J.; Delacourte, A.; Frigard, B.; Chartier-Harlin, M.C. Analysis of the APOE alleles impact in Down’s syndrome. Neurosci. Lett. 1996, 220, 57–60. [Google Scholar] [CrossRef]
- Prasher, V.; Chowdhury, T.; Rowe, B.; Bain, S. ApoE genotype and Alzheimer’s disease in adults with Down syndrome: Meta-analysis. Am. J. Ment. Retard. 1997, 102, 103–110. [Google Scholar] [CrossRef]
- Lai, F.; Mhatre, P.G.; Yang, Y.; Wang, M.C.; Schupf, N.; Rosas, H.D. Sex differences in risk of Alzheimer’s disease in adults with Down syndrome. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Silverman, W.; Schupf, N.; Zigman, W.; Devenny, D.; Miezejeski, C.; Schubert, R.; Ryan, R. Dementia in Adults, with Mental Retardation: Assessment at a Single Point in Time. Am. J. Ment. Retard. 2004, 109, 111–125. [Google Scholar] [CrossRef]
- Hixson, J.E.; Vernier, D.T. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J. Lipid Res. 1990, 31, 545–548. [Google Scholar] [CrossRef]
- Santabárbara, J.; Lopez-Anton, R.; De La Cámara, C.; Lobo, E.; Gracia-García, P.; Villagrasa, B.; Bueno-Notivol, J.; Marcos, G.; Lobo, A. Clinically significant anxiety as a risk factor for dementia in the elderly community. Acta Psychiatr. Scand. 2019, 139, 6–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santabárbara, J.; Villagrasa, B.; López-Antón, R.; Olaya, B.; Bueno-Notivol, J.; de la Cámara, C.; Gracia-García, P.; Lobo, E.; Lobo, A. Clinically relevant anxiety and risk of Alzheimer’s disease in an elderly community sample: 4.5 years of follow-up. J. Affect. Disord. 2019, 250, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Lee, D.S.; Fine, J.P. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation 2016, 133, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-C.; Brookmeyer, R.; Jewell, N.P. Statistical Models for Prevalent Cohort Data. Biometrics 1993, 49, 1. [Google Scholar] [CrossRef]
- Cortese, G.; Andersen, P.K. Competing Risks and Time-Dependent Covariates. Biom. J. 2010, 52, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.A.; Potashkin, J.A. The Impact of Disease Comorbidities in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 631770. [Google Scholar] [CrossRef] [PubMed]
- Crous-Bou, M.; Minguillón, C.; Gramunt, N.; Molinuevo, J.L. Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimers Res. Ther. 2017, 9. [Google Scholar] [CrossRef]
- Wisniewski, K.; Howe, J.; Williams, D.G.; Wisniewski, H.M. Precocious aging and dementia in patients with Down’s syndrome. Biol. Psychiatry 1978, 13, 619–627. [Google Scholar]
- Head, E.; Silverman, W.; Patterson, D.; Lott, I.T. Aging and down syndrome. Curr. Gerontol. Geriatr. Res. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Bayen, E.; Possin, K.L.; Chen, Y.; Cleret De Langavant, L.; Yaffe, K. Prevalence of Aging, Dementia, and Multimorbidity in Older Adults with Down Syndrome. JAMA Neurol. 2018, 75, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Houlden, H.; Crook, R.; Backhovens, H.; Prihar, G.; Baker, M.; Hutton, M.; Rossor, M.; Martin, J.J.; Van Broeckhoven, C.; Hardy, J. ApoE genotype is a risk factor in nonpresenilin early-onset Alzheimer’s disease families. Am. J. Med. Genet. 1998, 81, 117–121. [Google Scholar] [CrossRef]
- Tanzi, R.E.; Kovacs, D.M.; Kim, T.W.; Moir, R.D.; Guenette, S.Y.; Wasco, W. The gene defects responsible for familial Alzheimer’s disease. Neurobiol. Dis. 1996, 3, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Belloy, M.E.; Napolioni, V.; Greicius, M.D. A Quarter Century of APOE and Alzheimer’s Disease: Progress to Date and the Path Forward. Neuron 2019, 101, 820–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Total Group (408) | Men (141) | Women (267) | ||||
|---|---|---|---|---|---|---|
| Characteristic | Cognitively Stable | Incident Dementia | Cognitively Stable | Incident Dementia | Cognitively Stable | Incident Dementia |
| Age at First Visit (mean ± S.D.) | 49.2 (6.5) | 53.5 (5.2) * | 51.8 (4.8) | 54.4 (4.7) * | 48.0 (6.8) | 52.7 (5.5) * |
| Age at Dementia Onset (mean ± S.D.) | − | 56.4 (5.3) | − | 57.6 (4.7) * | − | 55.4 (5.5) * |
| Ethnicity (n, %) | ||||||
| Non-White | 29 (78.4) | 8 (21.6) | 5 (62.5) | 3 (37.5) | 24 (82.8) | 5 (17.2) |
| White | 277 (74.7) | 94 (25.3) | 91 (68.4) | 42 (31.6) | 186 (78.2) | 52 (21.8) |
| Level of Intellectual Disability (n, %) | ||||||
| Severe/Profound | 113 (72.0) | 44 (28.0) | 34 (65.4) | 18 (34.6) | 79 (75.2) | 26 (24.8) |
| Mild Moderate | 193 (76.9) | 58 (23.1) | 62 (69.7) | 27 (30.3) | 131 (80.9) | 31 (19.1) |
| APOE Allele (n, %) | ||||||
| ε4 Allele | 63 (69.2) | 28 (30.8) | 19 (65.6) | 10 (34.5) | 44 (71.0) | 18 (29.0) |
| No ε4 Allele | 243 (76.7) | 74 (23.3) | 77 (68.8) | 35 (31.3) | 166 (81.0) | 39 (19.0) |
| Total Group | N | Demented | Hazard Ratio | 95% CI |
|---|---|---|---|---|
| Sex-Only Model | ||||
| Sex | ||||
| Men | 141 | 45 (31.9) | 1.58 | 1.07–2.36 * |
| Women | 267 | 57 (21.3) | 1.0 | Reference |
| APOE-ε4-Only Model | ||||
| APOE | ||||
| ε4 Allele | 91 | 28 (27.5) | 1.81 | 1.16–2.82 * |
| No ε4 Allele | 317 | 74 (20.6) | 1.0 | Reference |
| Full Model | ||||
| Sex | ||||
| Men | 141 | 45 (31.9) | 1.53 | 1.03–2.29 * |
| Women | 267 | 57 (21.3) | 1 | Reference |
| APOE | ||||
| ε4 Allele | 91 | 28 (27.5) | 1.79 | 1.14–2.79 * |
| No ε4 Allele | 317 | 74 (20.6) | 1 | Reference |
| Ethnicity | ||||
| Non-White | 37 | 8 (21.6) | 0.76 | 0.36–1.59 |
| White | 371 | 94 (25.3) | 1 | Reference |
| Level of Intellectual Disability | ||||
| Severe/Profound | 157 | 44 (28.0) | 1.12 | 0.75–1.66 |
| Mild/Moderate | 251 | 58 (23.1) | 1 | Reference |
| ≤60 Years | ||||
| Sex | ||||
| Men | 102 | 31 (30.4) | 1.16 | 0.74–1.83 |
| Women | 206 | 51 (24.8) | 1 | Reference |
| APOE | ||||
| ε4 Allele | 81 | 26 (32.1) | 1.93 | 1.20–3.08 ** |
| Non-White | 29 | 7 (24.1) | 0.79 | 0.36–1.73 |
| White | 279 | 75 (26.9) | 1 | Reference |
| Level of Intellectual Disability | ||||
| Severe/Profound | 116 | 35 (30.2) | 1.11 | 0.72–1.73 |
| Mild/Moderate | 192 | 47 (24.5) | 1 | Reference |
| Over 60 Years | ||||
| Sex | ||||
| Men | 39 | 14 (35.9) | 6.32 | 2.11–18.96 *** |
| Women | 61 | 6 (9.8) | 1 | Reference |
| APOE | ||||
| ε4 Allele | 10 | 2 (20.2) | 0.73 | 0.16–3.33 |
| Ethnicity | ||||
| Non-White | 8 | 1 (12.5) | 0.78 | 0.09–6.77 |
| White | 92 | 19 (20.7) | 1 | Reference |
| Level of Intellectual Disability | ||||
| Severe/Profound | 41 | 9 (22.0) | 1.39 | 0.55–3.50 |
| Mild/Moderate | 59 | 11 (18.6) | 1 | Reference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhatre, P.G.; Lee, J.H.; Pang, D.; Zigman, W.B.; Tycko, B.; Krinsky-McHale, S.J.; Yang, Y.; Silverman, W.; Schupf, N. The Association between Sex and Risk of Alzheimer’s Disease in Adults with Down Syndrome. J. Clin. Med. 2021, 10, 2966. https://doi.org/10.3390/jcm10132966
Mhatre PG, Lee JH, Pang D, Zigman WB, Tycko B, Krinsky-McHale SJ, Yang Y, Silverman W, Schupf N. The Association between Sex and Risk of Alzheimer’s Disease in Adults with Down Syndrome. Journal of Clinical Medicine. 2021; 10(13):2966. https://doi.org/10.3390/jcm10132966
Chicago/Turabian StyleMhatre, Pooja Girish, Joseph H. Lee, Deborah Pang, Warren B. Zigman, Benjamin Tycko, Sharon J. Krinsky-McHale, Yuchen Yang, Wayne Silverman, and Nicole Schupf. 2021. "The Association between Sex and Risk of Alzheimer’s Disease in Adults with Down Syndrome" Journal of Clinical Medicine 10, no. 13: 2966. https://doi.org/10.3390/jcm10132966
APA StyleMhatre, P. G., Lee, J. H., Pang, D., Zigman, W. B., Tycko, B., Krinsky-McHale, S. J., Yang, Y., Silverman, W., & Schupf, N. (2021). The Association between Sex and Risk of Alzheimer’s Disease in Adults with Down Syndrome. Journal of Clinical Medicine, 10(13), 2966. https://doi.org/10.3390/jcm10132966






