A Prospective Multicenter Study of “Inside Stents” for Biliary Stricture: Multicenter Evolving Inside Stent Registry (MEISteR)
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Placement and Removal of Inside Stents
2.3. Clinical Outcomes
2.4. Definitions
2.5. Statistical Analysis
3. Results
3.1. Patients
3.2. Stent Placement
3.3. RBO, Adverse Events, and Stricture Resolution
3.4. Stent Removal
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soehendra, N.; Reynders-Frederix, V. Palliative Bile Duct Drainage - A New Endoscopic Method of Introducing a Transpapillary Drain. Endoscopy 1980, 12, 8–11. [Google Scholar] [CrossRef]
- Speer, A.G.; Cotton, P.B.; Rode, J.; Seddon, A.M.; Neal, C.R.; Holton, J.; Costerton, J.W. Biliary Stent Blockage with Bacterial Biofilm. Ann. Intern. Med. 1988, 108, 546–553. [Google Scholar] [CrossRef]
- Sung, J.J.Y.; Sollano, J.D.; Lai, C.W.; Ismael, A.; Yung, M.Y.R.N.; Tumala, I.; Chung, S.S.C. Long-Term Ciprofloxacin Treatment for The Prevention of Biliary Stent Blockage: A Prospective Randomized Study. Am. J. Gastroenterol. 1999, 94, 3197–3201. [Google Scholar] [CrossRef]
- Barrioz, T.; Besson, I.; de Ledinghen, V.; Silvain, C.; Beauchant, M.; Ingrand, P. Randomised trial of prevention of biliary stent occlusion by ursodeoxycholic acid plus norfloxacin. Lancet 1994, 344, 581–582. [Google Scholar] [CrossRef]
- Davids, P.; Groen, A.; Rauws, E.; Tytgat, G.; Huibregtse, K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet 1992, 340, 1488–1492. [Google Scholar] [CrossRef]
- Jang, S.I.; Lee, K.T.; Choi, J.S.; Jeong, S.; Lee, D.H.; Kim, Y.T.; Lee, S.H.; Yu, J.-S.; Lee, N.K. Efficacy of a paclitaxel-eluting biliary metal stent with sodium caprate in malignant biliary obstruction: A prospective randomized comparative study. Endoscopy 2018, 51, 843–851. [Google Scholar] [CrossRef]
- Hamada, T.; Isayama, H.; Nakai, Y.; Iwashita, T.; Ito, Y.; Mukai, T.; Yagioka, H.; Saito, T.; Togawa, O.; Ryozawa, S.; et al. Antireflux covered metal stent for nonresectable distal malignant biliary obstruction: Multicenter randomized controlled trial. Dig. Endosc. 2019, 31, 566–574. [Google Scholar] [CrossRef] [Green Version]
- Hamada, T.; Nakai, Y.; Isayama, H.; Koike, K. Antireflux metal stent for biliary obstruction: Any benefits? Dig. Endosc. 2021, 33, 310–320. [Google Scholar] [CrossRef]
- Kuwatani, M.; Kawakubo, K.; Sakamoto, N. Possible reasons for the regrettable results of patency of an inside stent in endoscopic transpapillary biliary stenting. Dig. Endosc. 2021. [Google Scholar] [CrossRef]
- Moon, J.H.; Choi, H.J.; Koo, H.C.; Han, S.H.; Lee, T.H.; Cho, Y.D.; Park, S.-H.; Kim, S.-J. Feasibility of placing a modified fully covered self-expandable metal stent above the papilla to minimize stent-induced bile duct injury in patients with refractory benign biliary strictures (with videos). Gastrointest. Endosc. 2012, 75, 1080–1085. [Google Scholar] [CrossRef]
- Sato, T.; Kogure, H.; Nakai, Y.; Ishigaki, K.; Hakuta, R.; Saito, K.; Saito, T.; Takahara, N.; Hamada, T.; Mizuno, S.; et al. A prospective study of fully covered metal stents for different types of refractory benign biliary strictures. Endoscopy 2020, 52, 368–376. [Google Scholar] [CrossRef]
- Jang, S.I.; Chung, T.R.; Cho, J.H.; Lee, K.; Joo, S.; Choi, J.H.; Kim, S.I.; Lee, D.K. Short fully covered self-expandable metal stent for treatment of proximal anastomotic benign biliary stricture after living-donor liver transplantation. Dig. Endosc. 2020. [Google Scholar] [CrossRef]
- Sato, T.; Kogure, H.; Nakai, Y.; Kanai, S.; Ishigaki, K.; Hakuta, R.; Saito, K.; Saito, T.; Takahara, N.; Hamada, T.; et al. Endoscopic treatment of hepaticojejunostomy anastomotic strictures using fully-covered metal stents. Dig. Endosc. 2021, 33, 451–457. [Google Scholar] [CrossRef]
- Liu, Q.; Khay, G.; Cotton, P.B. Feasibility of Stent Placement above the Sphincter of Oddi (“Inside-Stent”) for Patients with Malignant Biliary Obstruction. Endoscopy 1998, 30, 687–690. [Google Scholar] [CrossRef]
- Hisatsune, H.; Yazumi, S.; Egawa, H.; Asada, M.; Hasegawa, K.; Kodama, Y.; Okazaki, K.; Itoh, K.; Takakuwa, H.; Tanaka, K.; et al. Endoscopic management of biliary strictures after duct-to-duct biliary reconstruction in right-lobe living-donor liver transplantation. Transplantation 2003, 76, 810–815. [Google Scholar] [CrossRef]
- Tsujino, T.; Isayama, H.; Sugawara, Y.; Sasaki, T.; Kogure, H.; Nakai, Y.; Yamamoto, N.; Sasahira, N.; Yamashiki, N.; Tada, M.; et al. Endoscopic Management of Biliary Complications after Adult Living Donor Liver Transplantation. Am. J. Gastroenterol. 2006, 101, 2230–2236. [Google Scholar] [CrossRef]
- Inatomi, O.; Bamba, S.; Shioya, M.; Mochizuki, Y.; Ban, H.; Tsujikawa, T.; Saito, Y.; Andoh, A.; Fujiyama, Y. Threaded biliary inside stents are a safe and effective therapeutic option in cases of malignant hilar obstruction. BMC Gastroenterol. 2013, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Kurita, A.; Kodama, Y.; Minami, R.; Sakuma, Y.; Kuriyama, K.; Tanabe, W.; Ohta, Y.; Maruno, T.; Shiokawa, M.; Sawai, Y.; et al. Endoscopic stent placement above the intact sphincter of Oddi for biliary strictures after living donor liver transplantation. J. Gastroenterol. 2013, 48, 1097–1104. [Google Scholar] [CrossRef]
- Kaneko, T.; Sugimori, K.; Shimizu, Y.; Miwa, H.; Kameta, E.; Koh, R.; Numata, K.; Tanaka, K.; Maeda, S. Efficacy of plastic stent placement inside bile ducts for the treatment of unresectable malignant hilar obstruction (with videos). J. Hepato-Biliary-Pancreat. Sci. 2013, 21, 349–355. [Google Scholar] [CrossRef]
- Sato, T.; Kogure, H.; Nakai, Y.; Hamada, T.; Takahara, N.; Mizuno, S.; Kawaguchi, Y.; Akamatsu, N.; Kaneko, J.; Hasegawa, K.; et al. Long-term outcomes of endoscopic treatment for duct-to-duct anastomotic strictures after living donor liver transplantation. Liver Int. 2019, 39, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Ishiwatari, H.; Hayashi, T.; Ono, M.; Sato, T.; Kato, J. Newly designed plastic stent for endoscopic placement above the sphincter of Oddi in patients with malignant hilar biliary obstruction. Dig. Endosc. 2013, 25, 94–99. [Google Scholar] [CrossRef]
- Isayama, H.; Hamada, T.; Yasuda, I.; Itoi, T.; Ryozawa, S.; Nakai, Y.; Kogure, H.; Koike, K. TOKYO criteria 2014 for transpapillary biliary stenting. Dig. Endosc. 2015, 27, 259–264. [Google Scholar] [CrossRef]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A., Jr.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Moon, J.H.; Park, S. Biliary stenting for hilar malignant biliary obstruction. Dig. Endosc. 2020, 32, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; Kim, T.H.; Moon, J.H.; Lee, S.H.; Choi, H.J.; Hwangbo, Y.; Hyun, J.J.; Choi, J.-H.; Jeong, S.; Kim, J.H.; et al. Bilateral versus unilateral placement of metal stents for inoperable high-grade malignant hilar biliary strictures: A multicenter, prospective, randomized study (with video). Gastrointest. Endosc. 2017, 86, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Kogure, H.; Isayama, H.; Nakai, Y.; Tsujino, T.; Matsubara, S.; Yashima, Y.; Ito, Y.; Hamada, T.; Takahara, N.; Miyabayashi, K.; et al. High single-session success rate of endoscopic bilateral stent-in-stent placement with modified large cell N iti- S stents for malignant hilar biliary obstruction. Dig. Endosc. 2013, 26, 93–99. [Google Scholar] [CrossRef]
- Lee, T.H.; Moon, J.H.; Choi, J.-H.; Lee, S.H.; Lee, Y.N.; Paik, W.H.; Jang, D.K.; Cho, B.W.; Yang, J.K.; Hwangbo, Y.; et al. Prospective comparison of endoscopic bilateral stent-in-stent versus stent-by-stent deployment for inoperable advanced malignant hilar biliary stricture. Gastrointest. Endosc. 2019, 90, 222–230. [Google Scholar] [CrossRef]
- Ishigaki, K.; Hamada, T.; Nakai, Y.; Isayama, H.; Sato, T.; Hakuta, R.; Saito, K.; Saito, T.; Takahara, N.; Mizuno, S.; et al. Retrospective Comparative Study of Side-by-Side and Stent-in-Stent Metal Stent Placement for Hilar Malignant Biliary Obstruction. Dig. Dis. Sci. 2020, 65, 3710–3718. [Google Scholar] [CrossRef]
- Nagino, M.; Hirano, S.; Yoshitomi, H.; Aoki, T.; Uesaka, K.; Unno, M.; Ebata, T.; Konishi, M.; Sano, K.; Shimada, K.; et al. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J. Hepato-Biliary-Pancreat. Sci. 2021, 28, 26–54. [Google Scholar] [CrossRef]
- Mukai, T.; Yasuda, I.; Nakashima, M.; Doi, S.; Iwashita, T.; Iwata, K.; Kato, T.; Tomita, E.; Moriwaki, H. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: A randomized controlled trial. J. Hepato-Biliary-Pancreat. Sci. 2012, 20, 214–222. [Google Scholar] [CrossRef]
- Kawakami, H.; Kuwatani, M.; Onodera, M.; Haba, S.; Eto, K.; Ehira, N.; Yamato, H.; Kudo, T.; Tanaka, E.; Hirano, S.; et al. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J. Gastroenterol. 2010, 46, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, N.; Watanabe, S.; Hosono, K.; Kubota, K.; Nakajima, A.; Kaneko, T.; Sugimori, K.; Tokuhisa, M.; Goto, A.; Mori, R.; et al. Endoscopic inside stent placement is suitable as a bridging treatment for preoperative biliary tract cancer. BMC Gastroenterol. 2015, 15, 8. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Sasahira, N.; Sasaki, T.; Inoue, Y.; Mise, Y.; Sato, T.; Ono, Y.; Oba, A.; Saiura, A.; Ito, H. The role of stent placement above the papilla (inside-stent) as a bridging therapy for perihilar biliary malignancy: An initial experience. Surg. Today 2021, 1–10. [Google Scholar] [CrossRef]
- Sung, J.Y.; Leung, J.W.C.; Shaffer, E.A.; Lam, K.; Olson, M.E.; Costerton, J.W. Ascending infection of the biliary tract after surgical sphincterotomy and biliary stenting. J. Gastroenterol. Hepatol. 1992, 7, 240–245. [Google Scholar] [CrossRef]
- Van Berkel, A.M.; Van Marle, J.; Groen, A.K.; Bruno, M.J. Mechanisms of Biliary Stent Clogging: Confocal Laser Scanning and Scanning Electron Microscopy. Endoscopy 2005, 37, 729–734. [Google Scholar] [CrossRef]

| Benign (n = 51) | Malignant (n = 81) | |||
|---|---|---|---|---|
| Age | 63 (54–71) | 73 (65–79) | ||
| Sex, Male | 30 (59%) | 41 (51%) | ||
| ASA PS 1/2/3 | 30/20/1 | 44/37/0 | ||
| Etiology | Liver transplantation | 31 (61%) | Bile duct cancer | 36 (44%) |
| Post-surgical stricture | 7 (14%) | Gallbladder cancer | 16 (20%) | |
| Primary sclerosing cholangitis | 5 (10%) | Hepatocellular carcinoma | 16 (20%) | |
| Others | 8 (16%) | Others | 13 (16%) | |
| Concomitant treatment for malignancy | - | Surgical resection | 10 (12%) | |
| - | Chemotherapy/radiation/TACE | 40/4/12 | ||
| Stricture site | Intrahepatic | 15 (29%) | 4 (5%) | |
| Hilar | 27 (53%) | 64 (79%) | ||
| Common bile duct | 9 (18%) | 13 (16%) | ||
| Ampullary intervention | ES/EPBD/None | 17 (33%)/9 (18%)/25 (49%) | 40 (49%)/0/41 (51%) | |
| Prior biliary drainage | 35 (59%) | 34 (42%) | ||
| Planned strategy | Planned exchange | 41 (80%) | 6 (7%) | |
| On demand exchange | 7 (14%) | 44 (54%) | ||
| Temporary placement | 1 (2%) | 19 (23%) | ||
| Unknown | 2 (4%) | 2 (2%) | ||
| White blood cell count | 5890 (4600–7500) | 6000 (4450–7740) | ||
| ALT | 28 (15–46) | 87 (55–177) | ||
| ALP | 411 (299–945) | 863 (547–1601) | ||
| Total bilirubin | 0.8 (0.6–1.2) | 4.1 (1.6–9.0) | ||
| C-reactive protein | 0.42 (0.09–1.49) | 1.59 (047–3.88) |
| Benign (n = 103) | Malignant (n = 106) | ||
|---|---|---|---|
| Balloon dilation of the stricture | 39 (38%) | 8 (8%) | |
| The number of stents | 1/2/3- | 41 (40%)/53 (51%)/9 (9%) | 64 (60%)/34 (32%)/8 (8%) |
| Stent size | 7-Fr/8.5-Fr/Unknown | 48 (47%)/54 (52%)/1 (1%) | 48 (45%)/57 (54%)/1 (1%) |
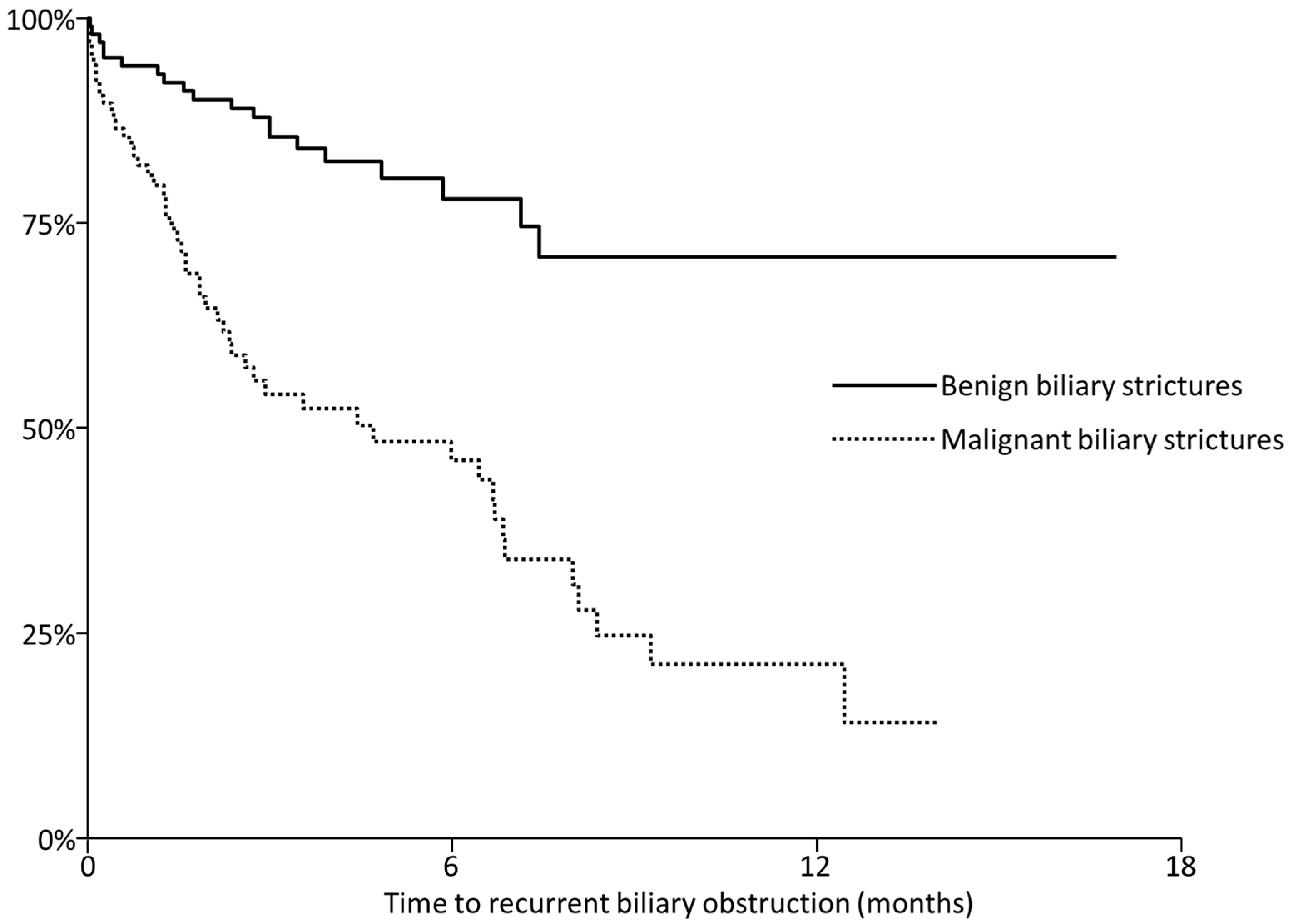
| RBO | All | 20 (19%) | 52 (49%) |
| Stent occlusion | 13 (13%) | 49 (46%) | |
| Stent migration | 7 (7%) | 3 (3%) | |
| Adverse events | All | 8 (8%) | 8 (8%) |
| Cholangitis without RBO | 4 (4%): 4 mild | 1 (1%): 1 mild | |
| Cholecystitis | 1 (1%): 1 severe | 4 (4%): 4 moderate | |
| Pancreatitis | 3 (3%): 3 mild | 3 (3%): 3 moderate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kogure, H.; Kato, H.; Kawakubo, K.; Ishiwatari, H.; Katanuma, A.; Okabe, Y.; Ueki, T.; Ban, T.; Hanada, K.; Sugimori, K.; et al. A Prospective Multicenter Study of “Inside Stents” for Biliary Stricture: Multicenter Evolving Inside Stent Registry (MEISteR). J. Clin. Med. 2021, 10, 2936. https://doi.org/10.3390/jcm10132936
Kogure H, Kato H, Kawakubo K, Ishiwatari H, Katanuma A, Okabe Y, Ueki T, Ban T, Hanada K, Sugimori K, et al. A Prospective Multicenter Study of “Inside Stents” for Biliary Stricture: Multicenter Evolving Inside Stent Registry (MEISteR). Journal of Clinical Medicine. 2021; 10(13):2936. https://doi.org/10.3390/jcm10132936
Chicago/Turabian StyleKogure, Hirofumi, Hironari Kato, Kazumichi Kawakubo, Hirotoshi Ishiwatari, Akio Katanuma, Yoshinobu Okabe, Toru Ueki, Tesshin Ban, Keiji Hanada, Kazuya Sugimori, and et al. 2021. "A Prospective Multicenter Study of “Inside Stents” for Biliary Stricture: Multicenter Evolving Inside Stent Registry (MEISteR)" Journal of Clinical Medicine 10, no. 13: 2936. https://doi.org/10.3390/jcm10132936
APA StyleKogure, H., Kato, H., Kawakubo, K., Ishiwatari, H., Katanuma, A., Okabe, Y., Ueki, T., Ban, T., Hanada, K., Sugimori, K., Nakai, Y., & Isayama, H. (2021). A Prospective Multicenter Study of “Inside Stents” for Biliary Stricture: Multicenter Evolving Inside Stent Registry (MEISteR). Journal of Clinical Medicine, 10(13), 2936. https://doi.org/10.3390/jcm10132936









