Risk Factors for Stent Migration into the Abdominal Cavity after Endoscopic Ultrasound-Guided Hepaticogastrostomy
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. The Stents and the Procedures of EUS-HGS
2.3. Study Outcomes
2.4. Definitions
2.5. Statistical Analyses
3. Results
3.1. Patients
3.2. Technical and Clinical Success, and Adverse Events
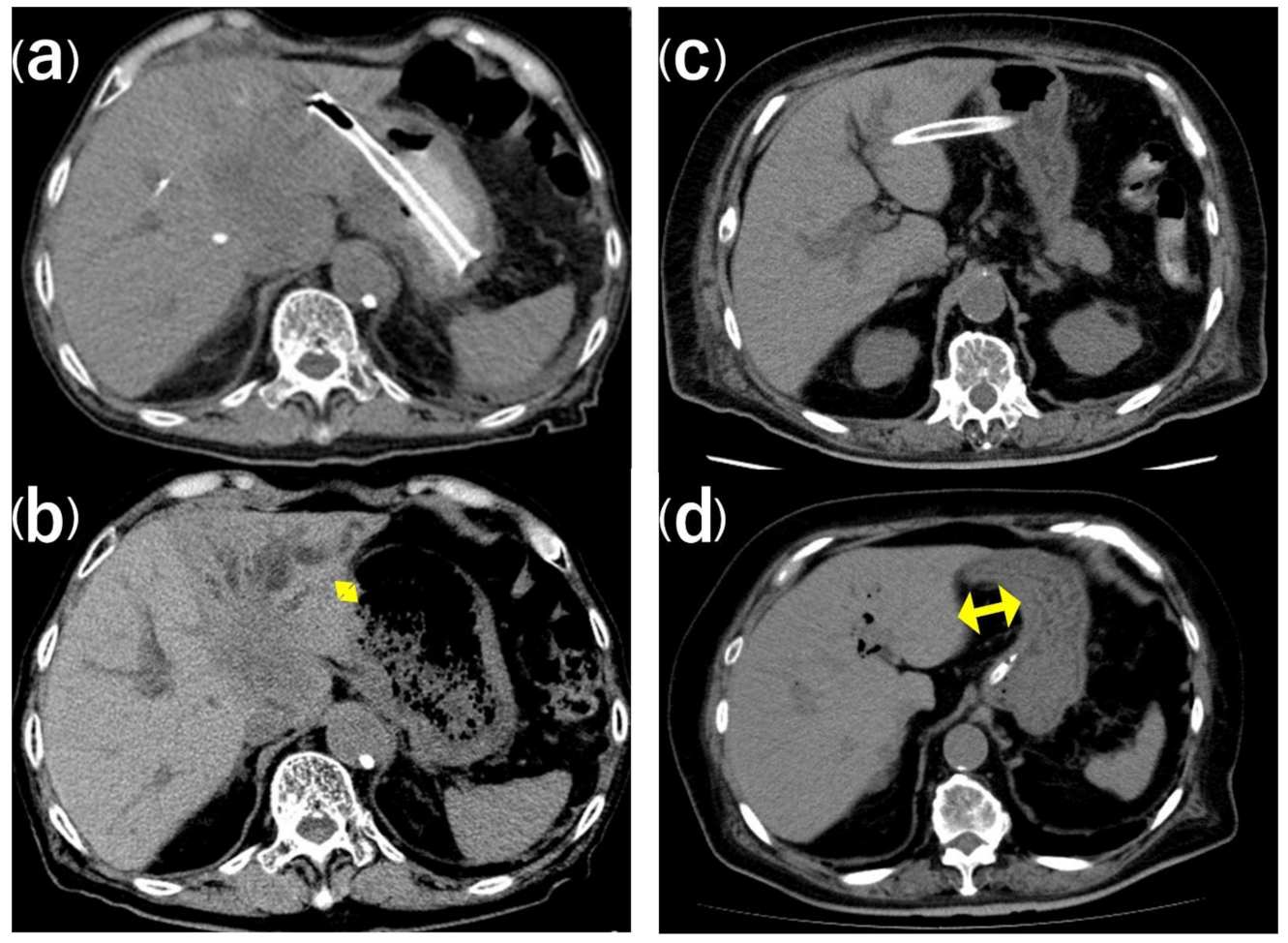
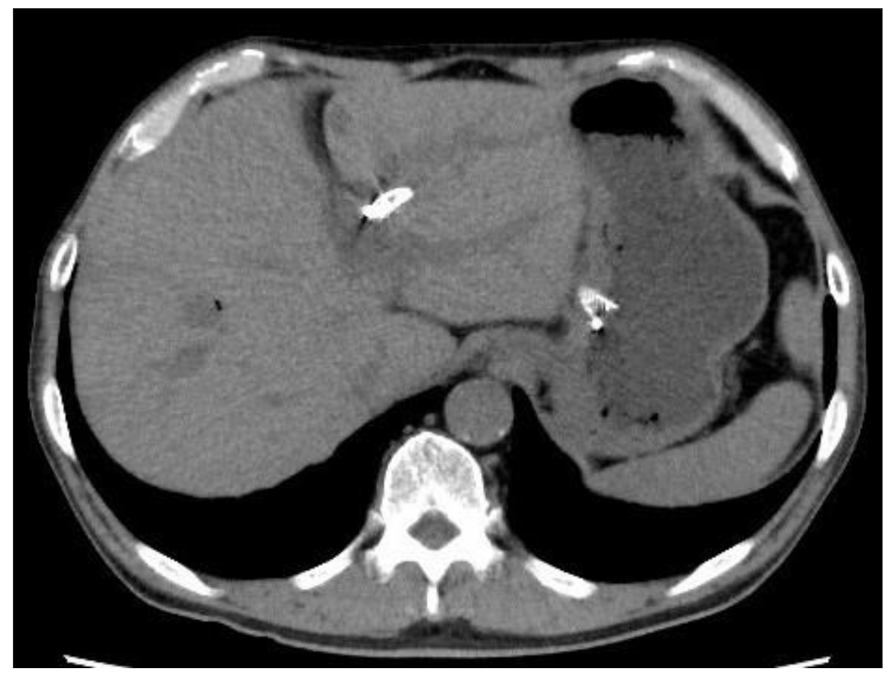
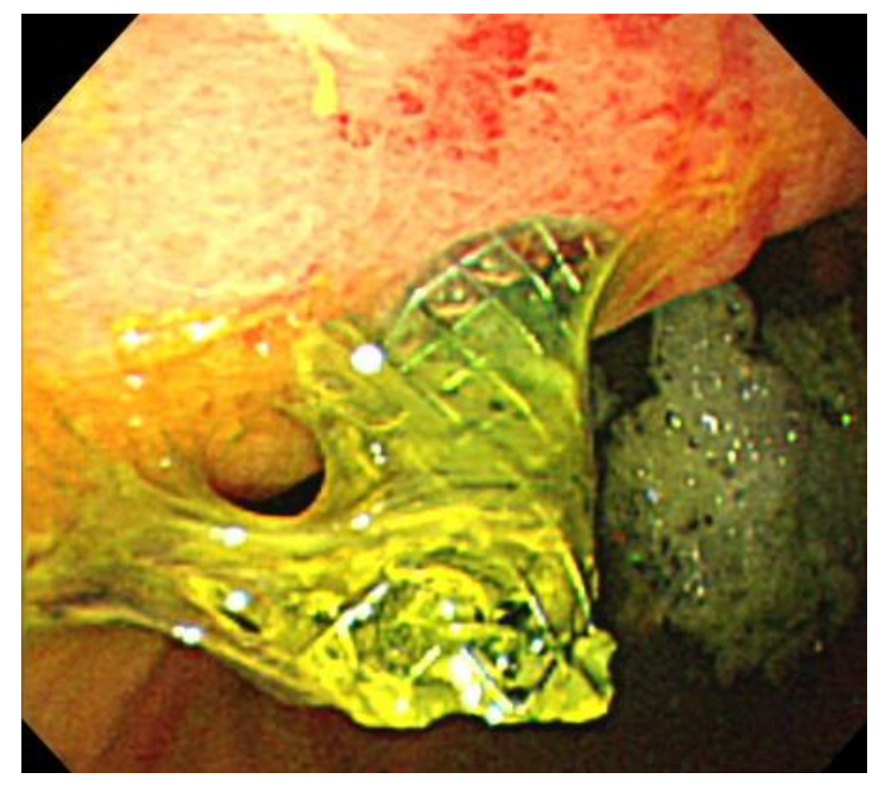
3.3. Stent Migration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakai, Y.; Isayama, H.; Yamamoto, N.; Matsubara, S.; Kogure, H.; Mizuno, S.; Hamada, T.; Takahara, N.; Uchino, R.; Akiyama, D.; et al. Indications for endoscopic ultrasonography (EUS)-guided biliary intervention: Does EUS always come after failed endoscopic retrograde cholangiopancreatography? Dig. Endosc. 2017, 29, 218–225. [Google Scholar] [CrossRef]
- Hamada, T.; Isayama, H.; Nakai, Y.; Kogure, H.; Yamamoto, N.; Kawakubo, K.; Takahara, N.; Uchino, R.; Mizuno, S.; Sasaki, T.; et al. Transmural Biliary Drainage Can Be an Alternative to Transpapillary Drainage in Patients with an Indwelling Duodenal Stent. Dig. Dis. Sci. 2014, 59, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Paik, W.H.; Lee, N.K.; Oh, D.; Park, D.H.; Nakai, Y.; Isayama, H.; Song, T.J.; Seo, D.-W.; Kim, M.-H. Conversion of external percutaneous transhepatic biliary drainage to endoscopic ultrasound-guided hepaticogastrostomy after failed standard internal stenting for malignant biliary obstruction. Endoscopy 2017, 49, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.M.; Teoh, A.Y.B. Endoscopic Ultrasound-Guided Biliary Drainage: A Review. Curr. Treat. Options Gastroenterol. 2015, 13, 171–184. [Google Scholar] [CrossRef]
- Khan, M.A.; Akbar, A.H.; Baron, T.H.; Khan, S.; Kocak, M.; Alastal, Y.; A Hammad, T.; Lee-Smith, W.; Sofi, A.; Artifon, E.L.A.; et al. Endoscopic Ultrasound-Guided Biliary Drainage: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2016, 61, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Sharaiha, R.Z.; Kumta, N.A.; Desai, A.P.; DeFilippis, E.M.; Gabr, M.; Sarkisian, A.M.; Salgado, S.; Millman, J.; Benvenuto, A.; Cohen, M.; et al. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage: Predictors of successful outcome in patients who fail endoscopic retrograde cholangiopancreatography. Surg. Endosc. 2016, 30, 5500–5505. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Lee, S.S.; Oh, D.; Song, T.J.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.-H. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos). Gastrointest. Endosc. 2017, 85, 1067–1075. [Google Scholar] [CrossRef]
- Martins, F.P.; Rossini, L.G.; Ferrari, A.P. Migration of a covered metallic stent following endoscopic ultrasound-guided hepaticogastrostomy: Fatal complication. Endoscopy 2010, 42, E126–E127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuno, N.; Hara, K.; Mizuno, N.; Hijioka, S.; Imaoka, H.; Yamao, K. Stent migration into the peritoneal cavity following endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2015, 47, E311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.H.; Jang, J.W.; Lee, S.S.; Seo, D.-W.; Lee, S.K.; Kim, M.-H. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest. Endosc. 2011, 74, 1276–1284. [Google Scholar] [CrossRef]
- Song, T.J.; Hyun, Y.S.; Lee, S.S.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.-H. Endoscopic ultrasound-guided choledochoduodenostomies with fully covered self-expandable metallic stents. World J. Gastroenterol. 2012, 18, 4435–4440. [Google Scholar] [CrossRef]
- Park, D.H.; Jeong, S.U.; Lee, B.U.; Lee, S.S.; Seo, D.-W.; Lee, S.K.; Kim, M.-H. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video). Gastrointest. Endosc. 2013, 78, 91–101. [Google Scholar] [CrossRef]
- Fujisawa, T.; Saito, H.; Isayama, H. Endoscopic removal of a metal stent that migrated into the peritoneal cavity after endoscopic ultrasound-guided hepaticogastrostomy. Dig. Endosc. 2019, 31, e74–e75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamada, T.; Nakai, Y.; Isayama, H.; Koike, K. Tandem stent placement as a rescue for stent misplacement in endoscopic ultrasonography-guided hepaticogastrostomy. Dig. Endosc. 2013, 25, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Miyano, A.; Ogura, T.; Yamamoto, K.; Okuda, A.; Nishioka, N.; Higuchi, K. Clinical Impact of the Intra-scope Channel Stent Release Technique in Preventing Stent Migration During EUS-Guided Hepaticogastrostomy. J. Gastrointest. Surg. 2018, 22, 1312–1318. [Google Scholar] [CrossRef]
- Fujisawa, T.; Isayama, H.; Ishii, S. “ClipFlap” anchoring method for endoscopic ultrasonography-guided hepaticogastrostomy with a covered self-expandable metallic stent. Dig. Endosc. 2020, 32, 628. [Google Scholar] [CrossRef]
- Shima, Y.; Isayama, H.; Ito, Y.; Hamada, T.; Nakai, Y.; Tsujino, T.; Nakata, R.; Koike, K. Crisscross anchor-stents to prevent metal stent migration during endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2014, 46, E563. [Google Scholar] [CrossRef] [Green Version]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A., Jr.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef]
- Kaneko, J.; Ishiwatari, H.; Takizawa, K.; Satoh, T.; Sato, J.; Matsubayashi, H.; Ono, H. Mediastinitis due to perforation by a metal stent after endoscopic ultrasound-guided hepaticogastrostomy: A rare complication. Endosc. 2019, 52, 519–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakai, Y.; Isayama, H.; Yamamoto, N.; Matsubara, S.; Ito, Y.; Sasahira, N.; Hakuta, R.; Umefune, G.; Takahara, N.; Hamada, T.; et al. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy 2016, 48, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Sato, T.; Hakuta, R.; Ishigaki, K.; Saito, K.; Saito, T.; Takahara, N.; Hamada, T.; Mizuno, S.; Kogure, H.; et al. Long-term outcomes of a long, partially covered metal stent for EUS-guided hepaticogastrostomy in patients with malignant biliary obstruction (with video). Gastrointest. Endosc. 2020, 92, 623–631.e1. [Google Scholar] [CrossRef] [PubMed]
| Age, years | 71 (50–93) | |
| Sex | Male | 30 (63%) |
| Performance status | 0/1/2/3/4 | 10(21%)/20(42%)/12(25%)/3(6%)/3(6%) |
| Primary cancer | Pancreatic cancer | 24 (50%) |
| Biliary tract cancer | 8 (17%) | |
| Gallbladder cancer | 2 (4%) | |
| Gastric cancer | 4 (8%) | |
| Hepatocellular carcinoma | 2 (4%) | |
| Other | 8 (16%) | |
| Liver metastasis | 17 (35%) | |
| Ascites | 18 (38%) | |
| Stricture location | Distal | 39 (81%) |
| Hilar | 9 (19%) | |
| Reasons for EUS-BD | GOO | 27 (56%) |
| Altered anatomy | 10 (21%) | |
| Failed ERCP | 10 (21%) | |
| High-risk ERCP | 1 (2%) | |
| Prior biliary drainage | 16 (33%) | |
| Duodenal stenting | 10 (21%) | |
| Bilirubin, mg/dL | 7.2 (0.4–23.5) | |
| Alkaline phosphatase, U/L | 1570 (94–4255) | |
| White blood cell count, µL | 7696 (2700–29,200) | |
| C-reactive protein, mg/dL | 5.06 (0.10–24.38) | |
| Albumin, g/dL | 2.7 (1.4–3.8) |
| Procedure time, min | 42 (29–55) | |
| Puncture site | B3 | 41 (85%) |
| B2 | 7 (15%) | |
| Fistular dilation | Bougie | 45 (94%) |
| Balloon | 40 (83%) | |
| Cautery | 5 (10%) | |
| Stent diameter, mm | 8 | 45 (94%) |
| 10 | 3 (6%) | |
| Stent length, cm | 10 | 42 (88%) |
| 12 | 6 (13%) | |
| Technical success (n = 48) | 48 (100%) |
| Clinical success (n = 48) | 43 (90%) |
| Adverse events (n = 48) | 8 (17%) |
| Migration (actual and imminent) | 5 (1 actual and 4 imminent, 10%) |
| Peritonitis | 3 (6%) |
| Cholangitis | 0 (0%) |
| Cholecystitis | 0 (0%) |
| Bleeding | 0 (0%) |
| Non-Migration Group (n = 43) | Migration Group (n = 5) | p-Value, Univariate | p-Value, Multivariate | |
|---|---|---|---|---|
| Age > 70 years, n (%) | 25, (58.1) | 3, (60) | 1.000 | |
| Male, n (%) | 27, (62.8) | 3, (60) | 1.000 | |
| PS > 1, n (%) | 27, (62.8) | 3, (60) | 1.000 | |
| Pancreatic cancer, n (%) | 21, (48.8) | 3, (60) | 1.000 | |
| Liver metastasis, n (%) | 14, (32.6) | 3, (60) | 0.331 | |
| Ascites, n (%) | 4, (9.3) | 1, (20) | 0.637 | |
| Distal stricture, n (%) | 34, (79) | 5, (100) | 0.322 | |
| GOO as the reason for EUS-HGS, n (%) | 23, (53.5) | 4, (80) | 0.437 | |
| Prior biliary drainage, n (%) | 13, (30.2) | 3, (60) | 0.316 | |
| Duodenal stenting, n (%) | 8, (18.6) | 2, (40) | 0.276 | |
| Initial distance between the puncture site and the stomach (mm) | 20.1 ± 12.8 | 47.0 ± 11.3 | <0.001 | 0.012 |
| Initial distance from the cardia to the stent (mm) | 41.9 ± 14.8 | 54.0 ± 16.8 | 0.097 | 0.099 |
| Distance between the Stomach and Liver at the Puncture Site | |||
|---|---|---|---|
| Before EUS-HGS | After EUS-HGS | p-value | |
| Non-migration group (n = 43) | 19.1 ± 12.8 mm | 17.7 ± 9.8 mm | 0.291 |
| Migration group (n = 5, 1 actual and 4 imminent) | 47.0 ± 11.3 mm | 44.8 ± 10.7 mm | 0.691 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochiai, K.; Fujisawa, T.; Ishii, S.; Suzuki, A.; Saito, H.; Takasaki, Y.; Ushio, M.; Takahashi, S.; Yamagata, W.; Tomishima, K.; et al. Risk Factors for Stent Migration into the Abdominal Cavity after Endoscopic Ultrasound-Guided Hepaticogastrostomy. J. Clin. Med. 2021, 10, 3111. https://doi.org/10.3390/jcm10143111
Ochiai K, Fujisawa T, Ishii S, Suzuki A, Saito H, Takasaki Y, Ushio M, Takahashi S, Yamagata W, Tomishima K, et al. Risk Factors for Stent Migration into the Abdominal Cavity after Endoscopic Ultrasound-Guided Hepaticogastrostomy. Journal of Clinical Medicine. 2021; 10(14):3111. https://doi.org/10.3390/jcm10143111
Chicago/Turabian StyleOchiai, Kazushige, Toshio Fujisawa, Shigeto Ishii, Akinori Suzuki, Hiroaki Saito, Yusuke Takasaki, Mako Ushio, Sho Takahashi, Wataru Yamagata, Ko Tomishima, and et al. 2021. "Risk Factors for Stent Migration into the Abdominal Cavity after Endoscopic Ultrasound-Guided Hepaticogastrostomy" Journal of Clinical Medicine 10, no. 14: 3111. https://doi.org/10.3390/jcm10143111
APA StyleOchiai, K., Fujisawa, T., Ishii, S., Suzuki, A., Saito, H., Takasaki, Y., Ushio, M., Takahashi, S., Yamagata, W., Tomishima, K., Hisamatsu, T., & Isayama, H. (2021). Risk Factors for Stent Migration into the Abdominal Cavity after Endoscopic Ultrasound-Guided Hepaticogastrostomy. Journal of Clinical Medicine, 10(14), 3111. https://doi.org/10.3390/jcm10143111







