Comparison between Multiple Doses and Single-Dose Steroids in Preventing the Incidence of Reintubation after Extubation among Critically Ill Patients: A Network Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Inclusion and Exclusion Criteria
2.4. Groups of Comparison: Multiple Doses vs. Single Dose vs. Placebo
2.5. Data Extraction
2.6. Quality Assessment
2.7. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Quality of Evidence
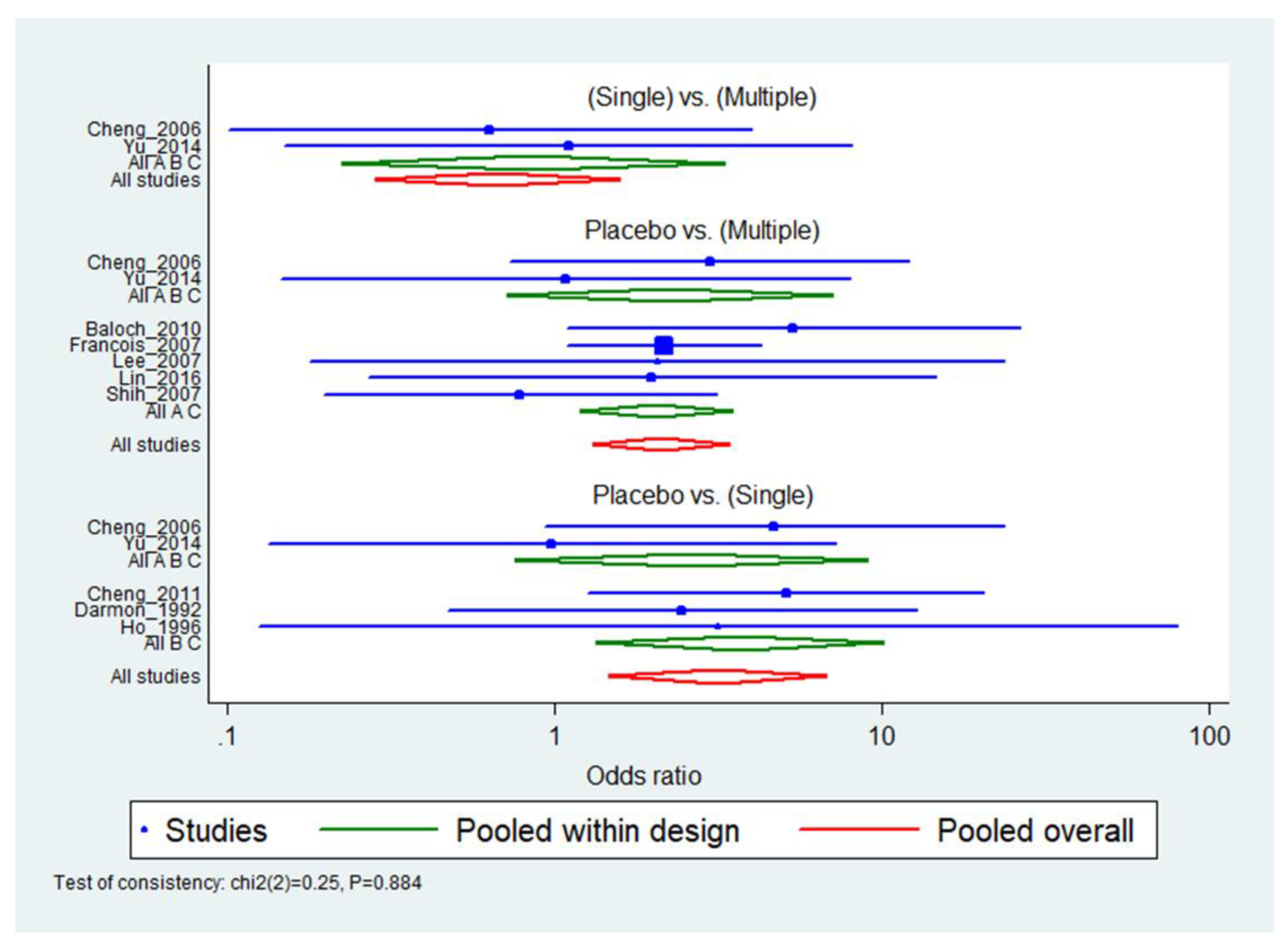
3.5. Main Analysis: Pairwise Meta-Analysis
3.6. Main Analysis: Network Meta-Analysis
3.7. Publication Bias
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frutos-Vivar, F.; Esteban, A.; Apezteguia, C.; González, M.; Arabi, Y.; Restrepo, M.I.; Gordo, F.; Santos, C.; Alhashemi, J.A.; Pérez, F.; et al. Outcome of reintubated patients after scheduled extubation. J. Crit. Care 2011, 26, 502–509. [Google Scholar] [CrossRef]
- Peterson, G.N.; Domino, K.B.; Caplan, R.A.; Posner, K.L.; Lee, L.A.; Cheney, F.W. Management of the difficult airway: A closed claims analysis. Anesthesiology 2005, 103, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Epstein, S.K. Extubation failure: An outcome to be avoided. Crit. Care 2004, 8, 310–312. [Google Scholar] [CrossRef] [Green Version]
- Difficult Airway Society Extubation Guidelines Group. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia 2012, 67, 318–340. [Google Scholar] [CrossRef]
- Higgs, A.; McGrath, B.A.; Goddard, C.; Rangasami, J.; Suntharalingam, G.; Gale, R.; Cook, T.M. Guidelines for the management of tracheal intubation in critically ill adults. Br. J. Anaesth. 2018, 120, 323–352. [Google Scholar] [CrossRef] [Green Version]
- Boles, J.-M.; Bion, J.; Connors, A.; Herridge, M.; Marsh, B.; Melot, C.; Pearl, R.; Silverman, H.; Stanchina, M.; Vieillard-Baron, A.; et al. Weaning from mechanical ventilation. Eur. Respir. J. 2007, 29, 1033–1056. [Google Scholar] [CrossRef]
- Schmidt, G.A.; Girard, T.D.; Kress, J.P.; Morris, P.E.; Ouellette, D.R.; Alhazzani, W.; Burns, S.M.; Epstein, S.K.; Esteban, A.; Fan, E.; et al. Official Executive Summary of an American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: Liberation from Mechanical Ventilation in Critically Ill Adults. Am. J. Respir. Crit. Care Med. 2017, 195, 115–119. [Google Scholar] [CrossRef]
- Kuriyama, A.; Umakoshi, N.; Sun, R. Prophylactic corticosteroids for prevention of postextubation stridor and reintubation in adults: A systematic review and meta-analysis. Chest 2017, 151, 1002–1010. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Cheng, K.-H.; Kou, L.-K.; Lee, C.-H. Comparison of high-and low-dose dexamethasone for preventing postextubation airway obstruction in adults: A prospective, randomized, double blind, placebo-controlled study. Int. J. Gerontol. 2016, 10, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Shimmer, B.P.; Funder, J.W. ACTH, adrenal steroids, and pharmacology of the adrenal cortex. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 12th ed.; McGraw-Hill Education: New York, NY, USA, 2011. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Donegan, S.; Williamson, P.; D’Alessandro, U.; Smith, C.T. Assessing key assumptions of network meta-analysis: A review of methods. Res. Synth. Methods 2013, 4, 291–323. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Jackson, D.; Barrett, J.K.; Lu, G.; Ades, A.E.; White, I.R. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth. Methods 2012, 3, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2010, 64, 163–171. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Bonner, A.; Alexander, P.E.; Siemieniuk, R.A.; Furukawa, T.A.; Rochwerg, B.; Hazlewood, G.S.; Alhazzani, W.; Mustafa, R.A.; Murad, M.H.; et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J. Clin. Epidemiol. 2018, 93, 36–44. [Google Scholar] [CrossRef]
- Baloch, R.; Jakhrani, N.; Lal, A.; Mehmood, N. Role of dexamethasone for prevention of post-extubation airway obstruction in critically ill adult patients. J. Surg. Pak. 2010, 15, 3–8. [Google Scholar]
- Yu, Y.; Zhu, C.; Mao, E. Use of dexamethasone for preventing postextubation airway obstruction in adults: A prospective randomized study. J. Intern. Med. Concepts Pract. 2014, 9, 134–137. [Google Scholar]
- Darmon, J.Y.; Rauss, A.; Dreyfuss, D.; Bleichner, G.; Elkharrat, D.; Schlemmer, B.; Tenaillon, A.; Brun-Buisson, C.; Huet, Y. Evaluation of risk factors for laryngeal edema after tracheal extubation in adults and its prevention by dexamethasone. A placebo-controlled, double-blind, multicenter study. Anesthesiology 1992, 77, 245–251. [Google Scholar] [CrossRef]
- Ho, L.I.; Harn, H.J.; Lien, T.C.; Hu, P.Y.; Wang, J.H. Postextubation laryngeal edema in adults. Risk factor evaluation and prevention by hydrocortisone. Intensive Care Med. 1996, 22, 933–936. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Hou, C.-C.; Huang, H.-C.; Lin, S.-C.; Zhang, H. Intravenous injection of methylprednisolone reduces the incidence of postextubation stridor in intensive care unit patients. Crit. Care Med. 2006, 34, 1345–1350. [Google Scholar] [CrossRef]
- François, B.; Bellissant, E.; Gissot, V.; Desachy, A.; Normand, S.; Boulain, T.; Brenet, O.; Preux, P.; Vignon, P. Association des Réanimateurs du Centre-Ouest (ARCO). 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: A randomised double-blind trial. Lancet 2007, 369, 1083–1089. [Google Scholar] [CrossRef]
- Lee, C.H.; Peng, M.J.; Wu, C.L. Dexamethasone to prevent postextubation airway obstruction in adults: A prospective, randomized, double-blind, placebo-controlled study. Crit. Care 2007, 11, R72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.C.; Chen, C.M.; Tan, C.K.; Chen, H.M.; Lu, C.L.; Zhang, H. Methylprednisolone reduces the rates of postextubation stridor and reintubation associated with attenuated cytokine responses in critically ill patients. Minerva Anestesiol. 2011, 77, 503–509. [Google Scholar] [PubMed]
- Yasir, M.; Goyal, A.; Bansal, P.; Sonthalia, S. Corticosteroid Adverse Effects; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Safiya, S.; Verma, H.; Yadav, N.; Jauhari, M.; Bullangowda, J. Applications of steroid in clinical practice: A review. Int. Sch. Res. Not. 2012, 2012, 11. [Google Scholar] [CrossRef] [Green Version]
- Patel, G.P.; Balk, R.A. Systemic steroids in severe sepsis and septic shock. Am. J. Respir. Crit. Care Med. 2012, 185, 133–139. [Google Scholar] [CrossRef]
- Vargas, F.; Clavel, M.; Sanchez-Verlan, P.; Garnier, S.; Boyer, A.; Bui, H.; Clouzeau, B.; Sazio, C.; Kerchache, A.; Guisset, O.; et al. Intermittent noninvasive ventilation after extubation in patients with chronic respiratory disorders: A multicenter randomized controlled trial (VHYPER). Intensive Care Med. 2017, 43, 1626–1636. [Google Scholar] [CrossRef]
- Fernandez, R.; Subira, C.; Frutos-Vivar, F.; Rialp, G.; Laborda, C.; Masclans, J.R.; Lesmes, A.; Panadero, L.; Hernandez, G. High-flow nasal cannula to prevent postextubation respiratory failure in high-risk non-hypercapnic patients: A randomized multicenter trial. Ann. Intensive Care 2017, 7, 47. [Google Scholar] [CrossRef]
- Hernández, G.; Vaquero, C.; González, P.; Subira, C.; Frutos-Vivar, F.; Rialp, G.; Laborda, C.; Colinas, L.; Cuena, R.; Fernández, R. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: A randomized clinical trial. JAMA 2016, 315, 1354–1361. [Google Scholar] [CrossRef]
- Zhou, X. Preventive use of respiratory support after scheduled extubation in critically ill medical patients-a network meta-analysis of randomized controlled trials. Crit. Care 2020, 24, 370. [Google Scholar] [CrossRef]

| Author | Year of Publication | Region | Period | Population in Meta-Analysis | Age | Steroid | Dose Frequency | Equivalent Dose of Hydrocortisone * |
|---|---|---|---|---|---|---|---|---|
| Darmon et al. [19] | 1992 | France | 1986–1987 | 664 | 53.16 | DM | Single | 200 |
| Ho et al. [20] | 1996 | Taiwan | 1990 | 77 | 62 | HC | Single | 100 |
| Cheng et al. [21] | 2006 | Taiwan | 2002–2004 | 128 | 66.12 | MP | Multiple or Single | 800 or 200 |
| Francois et al. [22] | 2007 | France | 2001–2002 | 698 | 66 | MP | Multiple | 400 |
| Lee et al. [23] | 2007 | Taiwan | 2004–2006 | 80 | 72.55 | DM | Multiple | 500 |
| Baloch et al. [17] | 2010 | Pakistan | 2006–2008 | 92 | 39.65 | DM | Multiple | 500 |
| Cheng et al. [24] | 2011 | Taiwan | 2005–2006 | 71 | 60.49 | MP | Single | 200 |
| Yu et al. [18] | 2014 | China | 2010–2013 | 162 | 67 | DM | Multiple or Single | 250 or 125 |
| Lin et al. [9] | 2016 | Taiwan | 2007–2010 | 126 | 74.09 | DM | Multiple | 1000 or 500 |
| Treatment 1 | Treatment 2 | No. of Studies | I2 | OR (95% CI) | GRADE |
|---|---|---|---|---|---|
| Multiple doses | Placebo | 6 | 0% | 0.43 (0.25–0.72) | High |
| Single dose | Placebo | 5 | 0% | 0.31 (0.14–0.69) | High |
| Multiple doses | Single dose | 2 | 0% | 1.22 (0.32–4.74) | Moderate a |
| Placebo | Multiple Doses | Single Dose | |
|---|---|---|---|
| Placebo | - | 0.42(0.25–0.69) | 0.31 (0.14–0.67) |
| Multiple doses | 2.41 (1.44–4.03) | - | 0.75 (0.31–1.79) |
| Single dose | 3.21 (1.49–6.92) | 1.33 (0.56–3.20) | - |
| Rank | 1 | 2 | 3 | Mean Rank | SUCRA |
|---|---|---|---|---|---|
| Single dose | 0.741 | 0.257 | 0.002 | 1.3 | 0.871 |
| Multiple dose | 0.259 | 0.741 | 0.001 | 1.7 | 0.628 |
| Placebo | 0.000 | 0.003 | 0.998 | 3.0 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, C.; Na, M.K.; Choi, K.-S.; Lim, T.H.; Jang, B.-H.; Kim, W.; Cho, Y.; Shin, H.; Kim, J.G.; Lee, J. Comparison between Multiple Doses and Single-Dose Steroids in Preventing the Incidence of Reintubation after Extubation among Critically Ill Patients: A Network Meta-Analysis. J. Clin. Med. 2021, 10, 2900. https://doi.org/10.3390/jcm10132900
Ahn C, Na MK, Choi K-S, Lim TH, Jang B-H, Kim W, Cho Y, Shin H, Kim JG, Lee J. Comparison between Multiple Doses and Single-Dose Steroids in Preventing the Incidence of Reintubation after Extubation among Critically Ill Patients: A Network Meta-Analysis. Journal of Clinical Medicine. 2021; 10(13):2900. https://doi.org/10.3390/jcm10132900
Chicago/Turabian StyleAhn, Chiwon, Min Kyun Na, Kyu-Sun Choi, Tae Ho Lim, Bo-Hyoung Jang, Wonhee Kim, Youngsuk Cho, Hyungoo Shin, Jae Guk Kim, and Juncheol Lee. 2021. "Comparison between Multiple Doses and Single-Dose Steroids in Preventing the Incidence of Reintubation after Extubation among Critically Ill Patients: A Network Meta-Analysis" Journal of Clinical Medicine 10, no. 13: 2900. https://doi.org/10.3390/jcm10132900
APA StyleAhn, C., Na, M. K., Choi, K.-S., Lim, T. H., Jang, B.-H., Kim, W., Cho, Y., Shin, H., Kim, J. G., & Lee, J. (2021). Comparison between Multiple Doses and Single-Dose Steroids in Preventing the Incidence of Reintubation after Extubation among Critically Ill Patients: A Network Meta-Analysis. Journal of Clinical Medicine, 10(13), 2900. https://doi.org/10.3390/jcm10132900







