Axial Psoriatic Disease: Clinical and Imaging Assessment of an Underdiagnosed Condition
Abstract
:1. Introduction
2. Symptoms, Changes and Outcome Measure
3. Imaging Changes
3.1. Radiographic Changes
3.2. Magnetic Resonance Imaging Changes
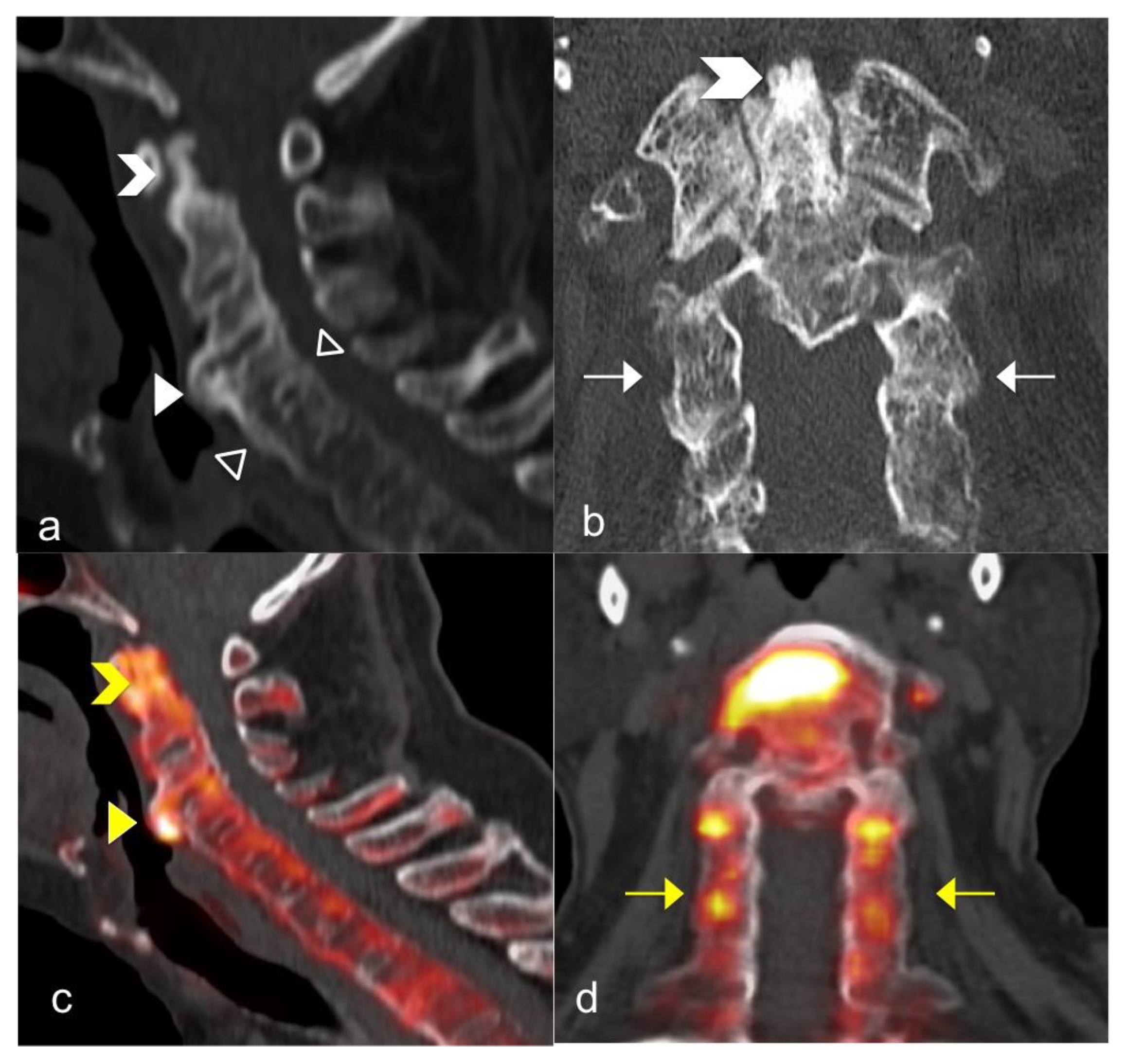
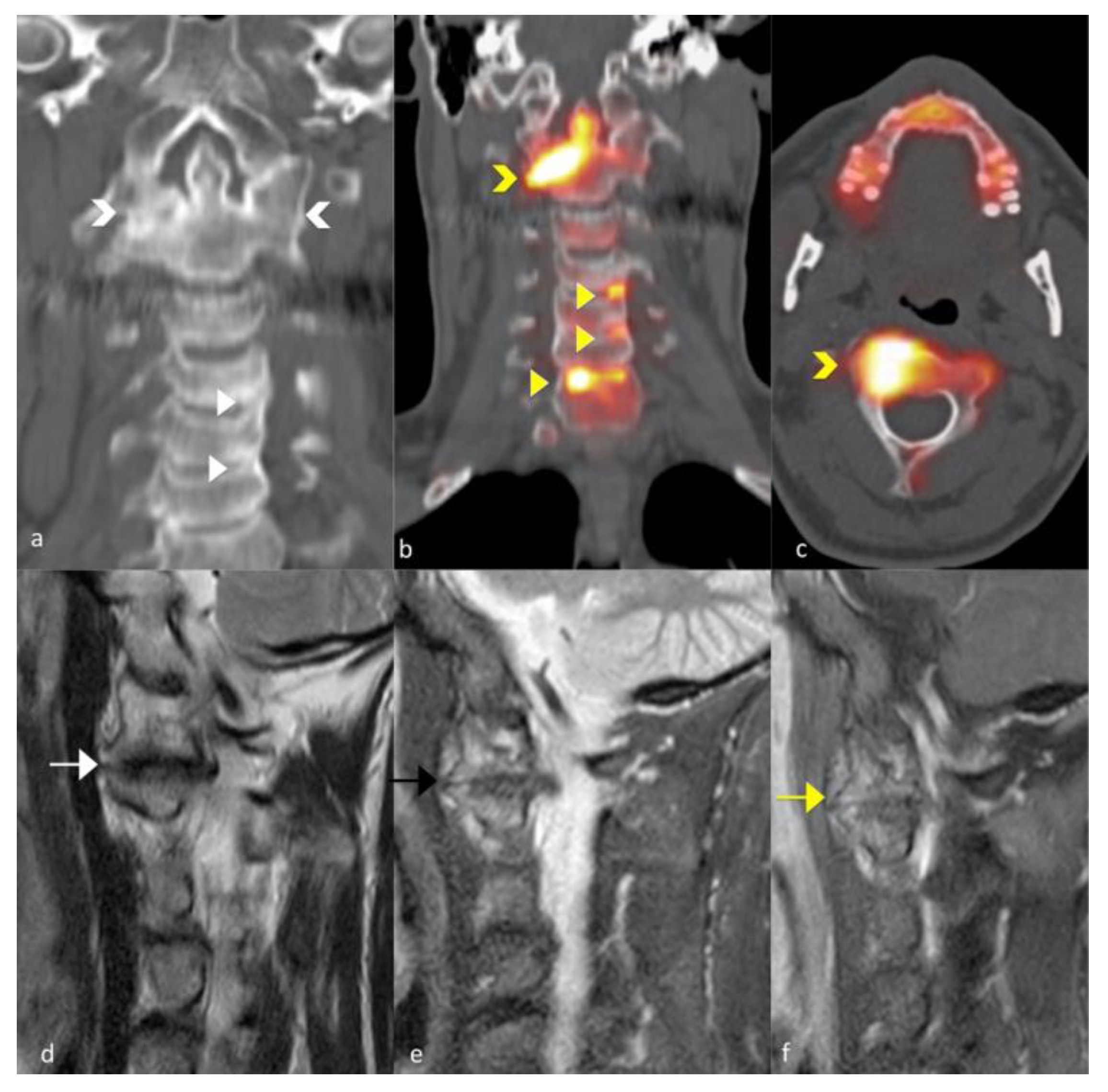
3.3. FNa 18 PET CT
4. Conclusions
5. Research Agenda
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gladman, D.D.; Antoni, C.; Mease, P.; Clegg, D.O.; Nash, P. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann. Rheum. Dis. 2005, 64 (Suppl. 2), ii14–ii17. [Google Scholar] [CrossRef] [PubMed]
- Alinaghi, F.; Calov, M.; Kristensen, L.E.; Gladman, D.D.; Coates, L.C.; Jullien, D.; Gottlieb, A.B.; Gisondi, P.; Wu, J.J.; Thyssen, J.P.; et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J. Am. Acad. Dermatol. 2019, 80, 251–265.e19. [Google Scholar] [CrossRef] [Green Version]
- Ritchlin, C.T.; Kavanaugh, A.; Gladman, D.D.; Mease, P.J.; Helliwell, P.; Boehncke, W.-H.; de Vlam, K.; Fiorentino, D.; Fitzgerald, O.; Gottlieb, A.B.; et al. Treatment recommendations for psoriatic arthritis. Ann. Rheum. Dis. 2009, 68, 1387–1394. [Google Scholar] [CrossRef]
- Coates, L.C.; Helliwell, P.S. Psoriatic arthritis: State of the art review. Clin. Med. 2017, 17, 65–70. [Google Scholar] [CrossRef]
- Moll, J.M.; Wright, V. Psoriatic arthritis. Semin. Arthritis Rheum. 1973, 3, 55–78. [Google Scholar] [CrossRef]
- Kane, D.; Stafford, L.; Bresnihan, B.; FitzGerald, O. A prospective, clinical and radiological study of early psoriatic arthritis: An early synovitis clinic experience. Rheumatology 2003, 42, 1460–1468. [Google Scholar] [CrossRef] [Green Version]
- Chandran, V.; Tolusso, D.C.; Cook, R.J.; Gladman, D.D. Risk factors for axial inflammatory arthritis in patients with psoriatic arthritis. J. Rheumatol. 2010, 37, 809–815. [Google Scholar] [CrossRef]
- Taylor, W.J.; Zmierczak, H.-G.; Helliwell, P.S. Problems with the definition of axial and peripheral disease patterns in psoriatic arthritis. J. Rheumatol. 2005, 32, 974–977. [Google Scholar] [PubMed]
- Benavent, D.; Plasencia-Rodríguez, C.; Franco-Gómez, K.; Nieto, R.; Monjo-Henry, I.; Peiteado, D.; Balsa, A.; Navarro-Compán, V. Axial spondyloarthritis and axial psoriatic arthritis: Similar or different disease spectrum? Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20971889. [Google Scholar] [CrossRef] [PubMed]
- Wright, V. Psoriatic arthritis. A comparative radiographic study of rheumatoid arthritis and arthritis associated with psoriasis. Ann. Rheum. Dis. 1961, 20, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladman, D.D. Axial disease in psoriatic arthritis. Curr. Rheumatol. Rep. 2007, 9, 455–460. [Google Scholar] [CrossRef]
- Feld, J.; Chandran, V.; Gladman, D.D. What is axial psoriatic arthritis? J. Rheumatol. 2018, 45, 1611–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feld, J.; Chandran, V.; Haroon, N.; Inman, R.; Gladman, D. Axial disease in psoriatic arthritis and ankylosing spondylitis: A critical comparison. Nat. Rev. Rheumatol. 2018, 14, 363–371. [Google Scholar] [CrossRef]
- Salvarani, C.; Macchioni, P.; Cremonesi, T.; Mantovani, W.; Battistel, B.; Rossi, F.; Capozzoli, N.; Baricchi, R.; Portioli, I. The cervical spine in patients with psoriatic arthritis: A clinical, radiological and immunogenetic study. Ann. Rheum. Dis. 1992, 51, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Queiro, R.; Sarasqueta, C.; Torre, J.C.; Tinture, T.; López-Lagunas, I. Prevalence and predictors of cervical involvement in psoriatic spondyloarthropathy. J. Clin. Rheumatol. 2002, 8, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Sallés, M.; Clavaguera, T.; Mínguez, S.; Valencoso, O.; Lopez de Recalde, M.; Tuneu, R. Atlantoaxial rotatory dislocation in a patient with psoriatic spondyloarthritis. Jt. Bone Spine 2019, 86, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.; Yassine, Z.A.; Majdi, B.H.; Nader, N. C1–C2 instability in psoriatic arthritis. Pan. Afr. Med. J. 2020, 36. [Google Scholar] [CrossRef]
- Battistone, M.J.; Manaster, B.J.; Reda, D.J.; Clegg, D.O. The prevalence of sacroilitis in psoriatic arthritis: New perspectives from a large, multicenter cohort. A department of veterans affairs cooperative study. Skelet. Radiol. 1999, 28, 196–201. [Google Scholar] [CrossRef]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef]
- Lubrano, E.; Parsons, W.J.; Marchesoni, A.; Olivieri, I.; D’Angelo, S.; Cauli, A.; Caso, F.; Costa, L.; Scarpa, R.; Brunese, L. The definition and measurement of axial psoriatic arthritis. J. Rheumatol. Suppl. 2015, 93, 40–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queiro, R.; Belzunegui, J.; González, C.; De, D.J.R.; Sarasqueta, C.; Torre, J.C.; Figueroa, M. Clinically asymptomatic axial disease in psoriatic spondyloarthropathy. A retrospective study. Clin. Rheumatol. 2002, 21, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Queiro, R.; Sarasqueta, C.; Belzunegui, J.; Gonzalez, C.; Figueroa, M.; Torre-Alonso, J.C. Psoriatic spondyloarthropathy: A comparative study between HLA-B27 positive and HLA-B27 negative disease. Semin. Arthritis Rheum. 2002, 31, 413–418. [Google Scholar] [CrossRef]
- Baraliakos, X.; Coates, L.C.; Braun, J. The involvement of the spine in psoriatic arthritis. Clin. Exp. Rheumatol. 2015, 33, S31–S35. [Google Scholar] [PubMed]
- Chandran, V.; Barrett, J.; Schentag, C.T.; Farewell, V.T.; Gladman, D.D. Axial psoriatic arthritis: Update on a longterm prospective study. J. Rheumatol. 2009, 36, 2744–2750. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; Landewé, R.; van der Heijde, D.; Listing, J.; Brandt, J.; Braun, J.; Burgos-Vargas, R.; Collantes-Estevez, E.; Davis, J.; Dijkmans, B.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): Classification of paper patients by expert opinion including uncertainty appraisal. Ann. Rheum. Dis. 2009, 68, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H. CASPAR Study Group Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Braun, A.; Saracbasi, E.; Grifka, J.; Schnitker, J.; Braun, J. Identifying patients with axial spondyloarthritis in primary care: How useful are items indicative of inflammatory back pain? Ann. Rheum. Dis. 2011, 70, 1782–1787. [Google Scholar] [CrossRef]
- Bonifati, C.; Elia, F.; Francesconi, F.; Ceralli, F.; Izzi, S.; Solivetti, F.M.; De Mutiis, C. The diagnosis of early psoriatic arthritis in an outpatient dermatological centre for psoriasis. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 627–633. [Google Scholar] [CrossRef]
- Queiro, R.; Alperi, M.; Lopez, A.; Sarasqueta, C.; Riestra, J.L.; Ballina, J. Clinical expression, but not disease outcome, may vary according to age at disease onset in psoriatic spondylitis. Jt. Bone Spine 2008, 75, 544–547. [Google Scholar] [CrossRef]
- Yap, K.S.; Ye, J.Y.; Li, S.; Gladman, D.D.; Chandran, V. Back pain in psoriatic arthritis: Defining prevalence, characteristics and performance of inflammatory back pain criteria in psoriatic arthritis. Ann. Rheum. Dis. 2018, 77, 1573–1577. [Google Scholar] [CrossRef]
- Michelena, X.; Poddubnyy, D.; Marzo-Ortega, H. Axial psoriatic arthritis: A distinct clinical entity in search of a definition. Rheum. Dis. Clin. North. Am. 2020, 46, 327–341. [Google Scholar] [CrossRef]
- Mease, P.J.; Palmer, J.B.; Liu, M.; Kavanaugh, A.; Pandurengan, R.; Ritchlin, C.T.; Karki, C.; Greenberg, J.D. Influence of Axial involvement on clinical characteristics of psoriatic arthritis: Analysis from the corrona psoriatic Arthritis/Spondyloarthritis registry. J. Rheumatol. 2018, 45, 1389–1396. [Google Scholar] [CrossRef] [Green Version]
- Gladman, D.D.; Inman, R.D.; Cook, R.J.; van der Heijde, D.; Landewé, R.B.M.; Braun, J.; Davis, J.C.; Mease, P.; Brandt, J.; Vargas, R.B.; et al. International spondyloarthritis interobserver reliability exercise—The INSPIRE study: I. Assessment of spinal measures. J. Rheumatol. 2007, 34, 1733–1739. [Google Scholar]
- Helliwell, P.S. Assessment of disease activity in psoriatic arthritis. Clin. Exp. Rheumatol. 2015, 33, S44–S47. [Google Scholar] [PubMed]
- Baraliakos, X.; Listing, J.; von der Recke, A.; Braun, J. The natural course of radiographic progression in ankylosing spondylitis--evidence for major individual variations in a large proportion of patients. J. Rheumatol. 2009, 36, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, L.; Nannini, C.; Cassarà, E.; Kaloudi, O.; Susini, M.; Lenzetti, I.; Cantini, F. Frequency of iridocyclitis in patients with early psoriatic arthritis: A prospective, follow up study. Int. J. Rheum. Dis. 2012, 15, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Jadon, D.R.; Sengupta, R.; Nightingale, A.; Lindsay, M.; Korendowych, E.; Robinson, G.; Jobling, A.; Shaddick, G.; Bi, J.; Winchester, R.; et al. Axial disease in psoriatic Arthritis study: Defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann. Rheum. Dis. 2017, 76, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Helliwell, P.; Hickling, P.; Wright, V. Do the radiological changes of classic ankylosing spondylitis differ from the changes found in the spondylitis associated with inflammatory bowel disease, psoriasis, and reactive arthritis? Ann. Rheum. Dis. 1998, 57, 135–140. [Google Scholar] [CrossRef]
- Lubrano, E.; Marchesoni, A.; Olivieri, I.; D’Angelo, S.; Spadaro, A.; Parsons, W.J.; Cauli, A.; Salvarani, C.; Mathieu, A.; Porter, G.; et al. Psoriatic arthritis spondylitis radiology index: A modified index for radiologic assessment of axial involvement in psoriatic arthritis. J. Rheumatol. 2009, 36, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Gladman, D.D.; Thavaneswaran, A.; Eder, L.; Helliwell, P.; Cook, R.J.; Chandran, V. Sensitivity and specificity of radiographic scoring instruments for detecting change in axial psoriatic arthritis. Arthritis Care Res. 2017, 69, 1700–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQueen, F.; Lassere, M.; Østergaard, M. Magnetic resonance imaging in psoriatic arthritis: A review of the literature. Arthritis Res. Ther. 2006, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Tehranzadeh, J.; Ashikyan, O.; Anavim, A.; Shin, J. Detailed analysis of contrast-enhanced MRI of hands and wrists in patients with psoriatic arthritis. Skelet. Radiol. 2008, 37, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Poggenborg, R.P.; Sørensen, I.J.; Pedersen, S.J.; Østergaard, M. Magnetic resonance imaging for diagnosing, monitoring and prognostication in psoriatic arthritis. Clin. Exp. Rheumatol. 2015, 33, S66–S69. [Google Scholar]
- Williamson, L.; Dockerty, J.L.; Dalbeth, N.; McNally, E.; Ostlere, S.; Wordsworth, B.P. Clinical assessment of sacroiliitis and HLA-B27 are poor predictors of sacroiliitis diagnosed by magnetic resonance imaging in psoriatic arthritis. Rheumatology 2004, 43, 85–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado-Ficco, H.; Sheane, B.J.; Thavaneswaran, A.; Chandran, V.; Gladman, D.D. Magnetic resonance imaging in psoriatic arthritis: A descriptive study of indications, features and effect on treatment change. J. Clin. Rheumatol. 2017, 23, 243–245. [Google Scholar] [CrossRef]
- Castillo-Gallego, C.; Aydin, S.Z.; Emery, P.; McGonagle, D.G.; Marzo-Ortega, H. Magnetic resonance imaging assessment of axial psoriatic arthritis: Extent of disease relates to HLA-B27. Arthritis Rheum. 2013, 65, 2274–2278. [Google Scholar] [CrossRef]
- Hermann, K.-G.A.; Baraliakos, X.; van der Heijde, D.M.F.M.; Jurik, A.-G.; Landewé, R.; Marzo-Ortega, H.; Østergaard, M.; Rudwaleit, M.; Sieper, J.; Braun, J.; et al. Descriptions of spinal MRI lesions and definition of a positive MRI of the spine in axial spondyloarthritis: A consensual approach by the ASAS/OMERACT MRI study group. Ann. Rheum. Dis. 2012, 71, 1278–1288. [Google Scholar] [CrossRef] [Green Version]
- Lambert, R.; Pedersen, S.; Maksymowych, W.; Chiowchanwisawakit, P.; Ostergaard, M. Active inflammatory lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis—Definitions, assessment system, and reference image set. J. Rheumatol. Suppl. 2009, 84, 3–17. [Google Scholar] [CrossRef]
- Krabbe, S.; Sørensen, I.J.; Jensen, B.; Møller, J.M.; Balding, L.; Madsen, O.R.; Lambert, R.G.W.; Maksymowych, W.P.; Pedersen, S.J.; Østergaard, M. Inflammatory and structural changes in vertebral bodies and posterior elements of the spine in axial spondyloarthritis: Construct validity, responsiveness and discriminatory ability of the anatomy-based CANDEN scoring system in a randomised placebo-controlled trial. RMD Open 2018, 4, e000624. [Google Scholar]
- Krabbe, S.; Østergaard, M.; Pedersen, S.; Weber, U.; Kröber, G.; Makysmowych, W.; Lambert, R. Canada-Denmark MRI scoring system of the spine in patients with axial spondyloarthritis: Updated definitions, scoring rules and inter-reader reliability in a multiple reader setting. RMD Open 2019, 5, e001057. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lee, J.Y.; Hwang, J.H.; Shin, J.H.; Kim, T.-H.; Kim, S.-K. Clinical importance of inflammatory facet joints of the spine in ankylosing spondylitis: A magnetic resonance imaging study. Scand J. Rheumatol. 2016, 45, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, M.; Eshed, I.; Althoff, C.E.; Poggenborg, R.P.; Diekhoff, T.; Krabbe, S.; Weckbach, S.; Lambert, R.G.W.; Pedersen, S.J.; Maksymowych, W.P.; et al. Whole-body magnetic resonance imaging in inflammatory arthritis: Systematic literature review and first steps toward standardization and an OMERACT scoring system. J. Rheumatol. 2017, 44, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J. Psoriatic arthritis: Update on pathophysiology, assessment and management. Ann. Rheum. Dis. 2011, 70 (Suppl. 1), i77–i84. [Google Scholar] [CrossRef]
- Poggenborg, R.P.; Pedersen, S.J.; Eshed, I.; Sørensen, I.J.; Møller, J.M.; Madsen, O.R.; Thomsen, H.S.; Østergaard, M. Head-to-toe whole-body MRI in psoriatic arthritis, axial spondyloarthritis and healthy subjects: First steps towards global inflammation and damage scores of peripheral and axial joints. Rheumatology 2015, 54, 1039–1049. [Google Scholar] [CrossRef] [Green Version]
- Poggenborg, R.P.; Eshed, I.; Østergaard, M.; Sørensen, I.J.; Møller, J.M.; Madsen, O.R.; Pedersen, S.J. Enthesitis in patients with psoriatic arthritis, axial spondyloarthritis and healthy subjects assessed by “head-to-toe” whole-body MRI and clinical examination. Ann. Rheum. Dis. 2015, 74, 823–829. [Google Scholar] [CrossRef]
- Mager, A.-K.; Althoff, C.E.; Sieper, J.; Hamm, B.; Hermann, K.-G.A. Role of whole-body magnetic resonance imaging in diagnosing early spondyloarthritis. Eur. J. Radiol. 2009, 71, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Raynal, M.; Bouderraoui, F.; Ouichka, R.; Melchior, J.; Morel, O.; Blum, A.; Chary-Valckenaere, I.; Ngueyon Sime, W.; Roch, V.; Maksymowych, W.; et al. Performance of 18F-sodium fluoride positron emission tomography with computed tomography to assess inflammatory and structural sacroiliitis on magnetic resonance imaging and computed tomography, respectively, in axial spondyloarthritis. Arthritis Res. Ther. 2019, 21, 119. [Google Scholar] [CrossRef] [Green Version]
- Glaudemans, A.W.J.M.; de Vries, E.F.J.; Galli, F.; Dierckx, R.A.J.O.; Slart, R.H.J.A.; Signore, A. The use of (18)F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin. Dev. Immunol. 2013, 2013, 623036. [Google Scholar] [CrossRef] [Green Version]
- Son, S.M.; Kim, K.; Pak, K.; Kim, S.-J.; Goh, T.S.; Lee, J.S. Evaluation of the diagnostic performance of 18F-NaF positron emission tomography/computed tomography in patients with suspected ankylosing spondylitis according to the Assessment of SpondyloArthritis International Society criteria. Spine J. 2020, 20, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.Z.; Kucuksahin, O.; Kilic, L.; Dogru, A.; Bayindir, O.; Ozisler, C.; Omma, A.; Tarhan, E.F.; Erden, A.; Kimyon, G.; et al. Axial psoriatic arthritis: The impact of underdiagnosed disease on outcomes in real life. Clin. Rheumatol. 2018, 37, 3443–3448. [Google Scholar] [CrossRef]
- Carvalho, P.D.; Savy, F.; Moragues, C.; Juanola, X.; Rodriguez-Moreno, J. Axial involvement according to ASAS criteria in an observational psoriatic arthritis cohort. Acta Reumatol. Port. 2017, 42, 176–182. [Google Scholar]
- Gossec, L.; Baraliakos, X.; Kerschbaumer, A.; de Wit, M.; McInnes, I.; Dougados, M.; Primdahl, J.; McGonagle, D.G.; Aletaha, D.; Balanescu, A.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 2020, 79, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Landewé, R.; van Tubergen, A. Clinical tools to assess and monitor spondyloarthritis. Curr. Rheumatol. Rep. 2015, 17, 47. [Google Scholar] [CrossRef] [Green Version]
- Baeten, D.; Østergaard, M.; Wei, J.C.-C.; Sieper, J.; Järvinen, P.; Tam, L.-S.; Salvarani, C.; Kim, T.-H.; Solinger, A.; Datsenko, Y.; et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: Results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis. 2018, 77, 1295–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deodhar, A.; Gensler, L.S.; Sieper, J.; Clark, M.; Calderon, C.; Wang, Y.; Zhou, Y.; Leu, J.H.; Campbell, K.; Sweet, K.; et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol. 2019, 71, 258–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helliwell, P.S.; Gladman, D.D.; Chakravarty, S.D.; Kafka, S.; Karyekar, C.S.; You, Y.; Campbell, K.; Sweet, K.; Kavanaugh, A.; Gensler, L.S. Effects of ustekinumab on spondylitis-associated endpoints in TNFi-naïve active psoriatic arthritis patients with physician-reported spondylitis: Pooled results from two phase 3, randomised, controlled trials. RMD Open 2020, 6. [Google Scholar] [CrossRef] [Green Version]
- Mease, P. Ustekinumab fails to show efficacy in a Phase III Axial spondyloarthritis program: The importance of negative results. Arthritis Rheumatol. 2019, 71, 179–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van derHeijde, D.; Baraliakos, X.; Gensler, L.S.; Maksymowych, W.P.; Tseluyko, V.; Nadashkevich, O.; Abi-Saab, W.; Tasset, C.; Meuleners, L.; Besuyen, R.; et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): Results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018, 392, 2378–2387. [Google Scholar] [CrossRef] [Green Version]
- van der Heijde, D.; Deodhar, A.; Wei, J.C.; Drescher, E.; Fleishaker, D.; Hendrikx, T.; Li, D.; Menon, S.; Kanik, K.S. Tofacitinib in patients with ankylosing spondylitis: A phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann. Rheum. Dis. 2017, 76, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- van der Heijde, D.; Song, I.-H.; Pangan, A.L.; Deodhar, A.; van den Bosch, F.; Maksymowych, W.P.; Kim, T.-H.; Kishimoto, M.; Everding, A.; Sui, Y.; et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): A multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet 2019, 394, 2108–2117. [Google Scholar] [CrossRef]
- Wang, L.; Ping, X.; Chen, W.; Xing, W. Performance of Janus kinase inhibitors in psoriatic arthritis with axial involvement in indirect comparison with ankylosing spondylitis: A retrospective analysis from pooled data. Clin. Rheumatol. 2021, 40, 1725–1737. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannini, I.; Zabotti, A.; Cicciò, C.; Salgarello, M.; Cereser, L.; De Vita, S.; Tinazzi, I. Axial Psoriatic Disease: Clinical and Imaging Assessment of an Underdiagnosed Condition. J. Clin. Med. 2021, 10, 2845. https://doi.org/10.3390/jcm10132845
Giovannini I, Zabotti A, Cicciò C, Salgarello M, Cereser L, De Vita S, Tinazzi I. Axial Psoriatic Disease: Clinical and Imaging Assessment of an Underdiagnosed Condition. Journal of Clinical Medicine. 2021; 10(13):2845. https://doi.org/10.3390/jcm10132845
Chicago/Turabian StyleGiovannini, Ivan, Alen Zabotti, Carmelo Cicciò, Matteo Salgarello, Lorenzo Cereser, Salvatore De Vita, and Ilaria Tinazzi. 2021. "Axial Psoriatic Disease: Clinical and Imaging Assessment of an Underdiagnosed Condition" Journal of Clinical Medicine 10, no. 13: 2845. https://doi.org/10.3390/jcm10132845
APA StyleGiovannini, I., Zabotti, A., Cicciò, C., Salgarello, M., Cereser, L., De Vita, S., & Tinazzi, I. (2021). Axial Psoriatic Disease: Clinical and Imaging Assessment of an Underdiagnosed Condition. Journal of Clinical Medicine, 10(13), 2845. https://doi.org/10.3390/jcm10132845







