The Antihypertensive Effects and Safety of LCZ696 in Patients with Hypertension: A Systemic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Selection, Search Strategy, and Outcome Measures
2.2. Data Synthesis and Statistical Analyses
3. Results
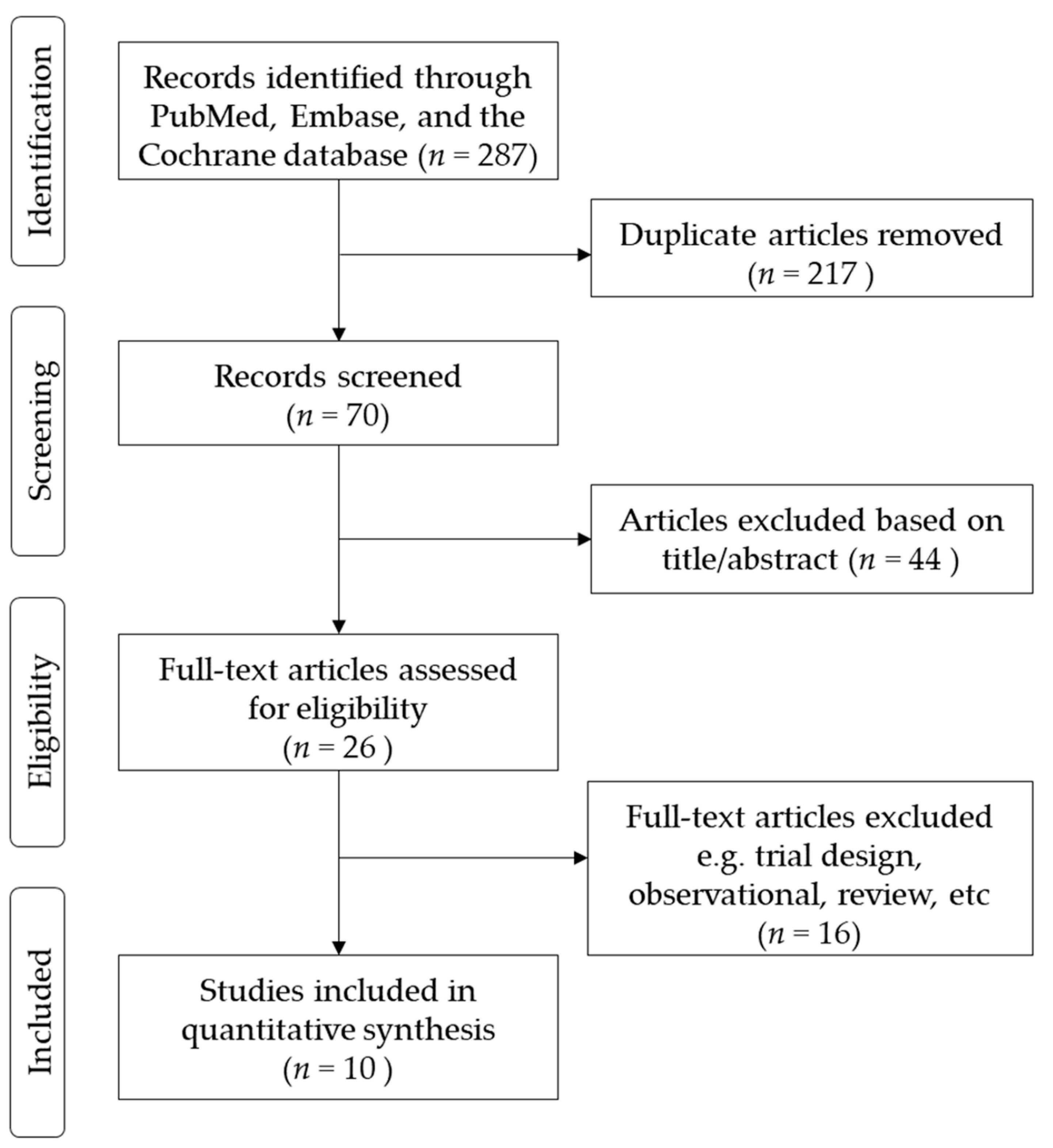
3.1. Enrollment of Studies
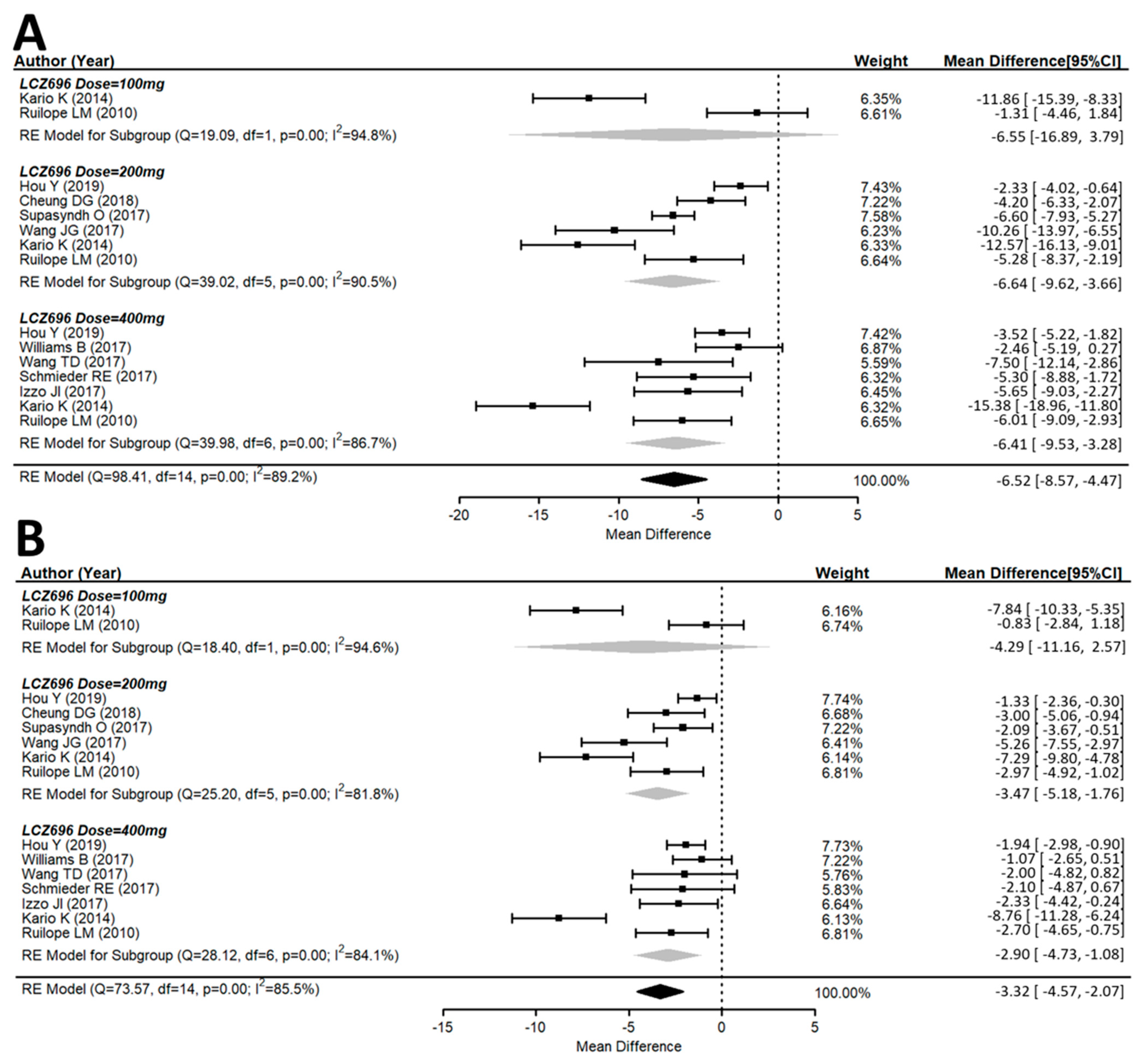
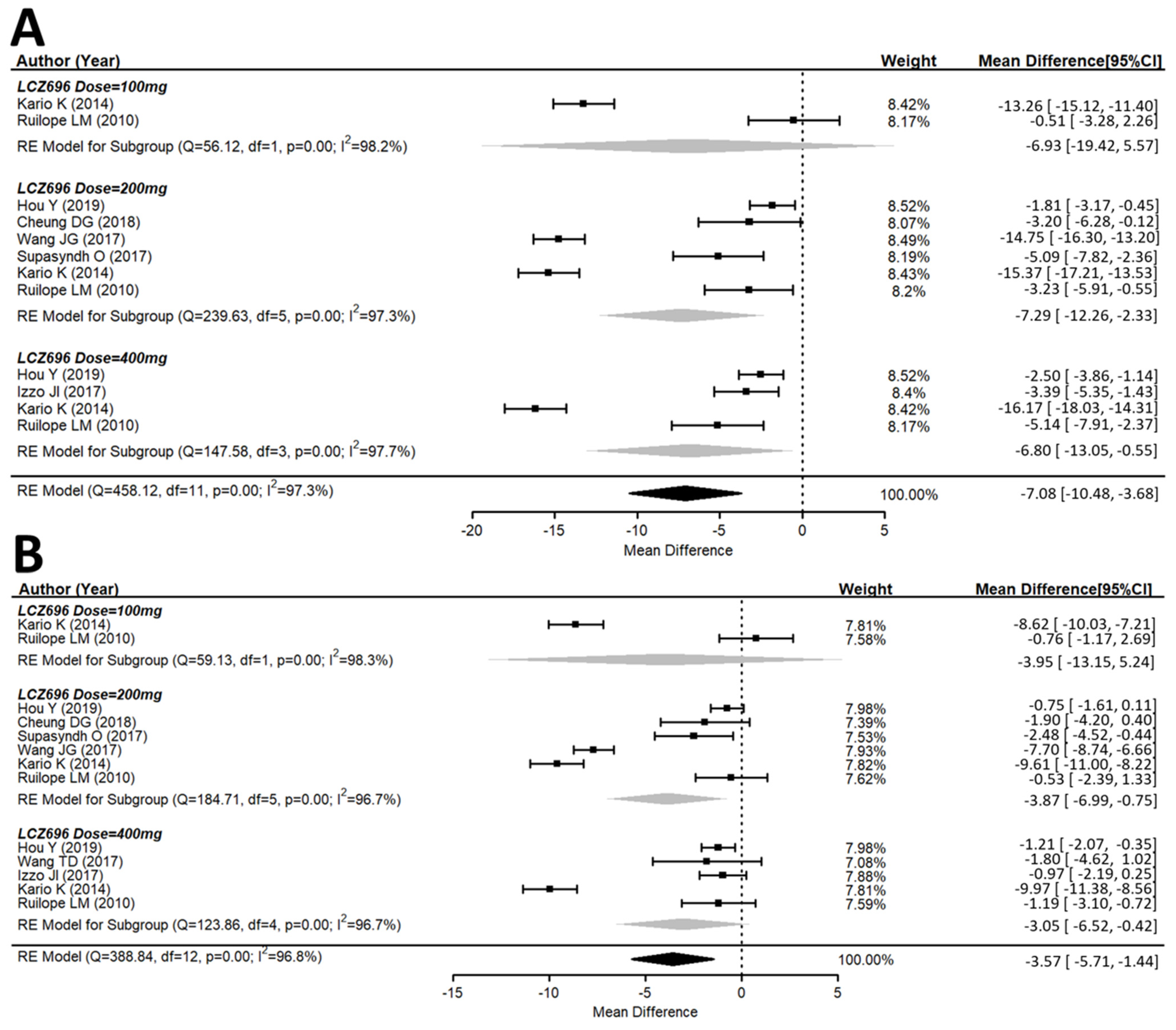
3.2. Efficacy of the Antihypertensive Effect of Sacubitril/Valsartan (LCZ696)
3.3. Effects of Different Doses of Sacubitril/Valsartan (LCZ696) Versus the Placebo Group
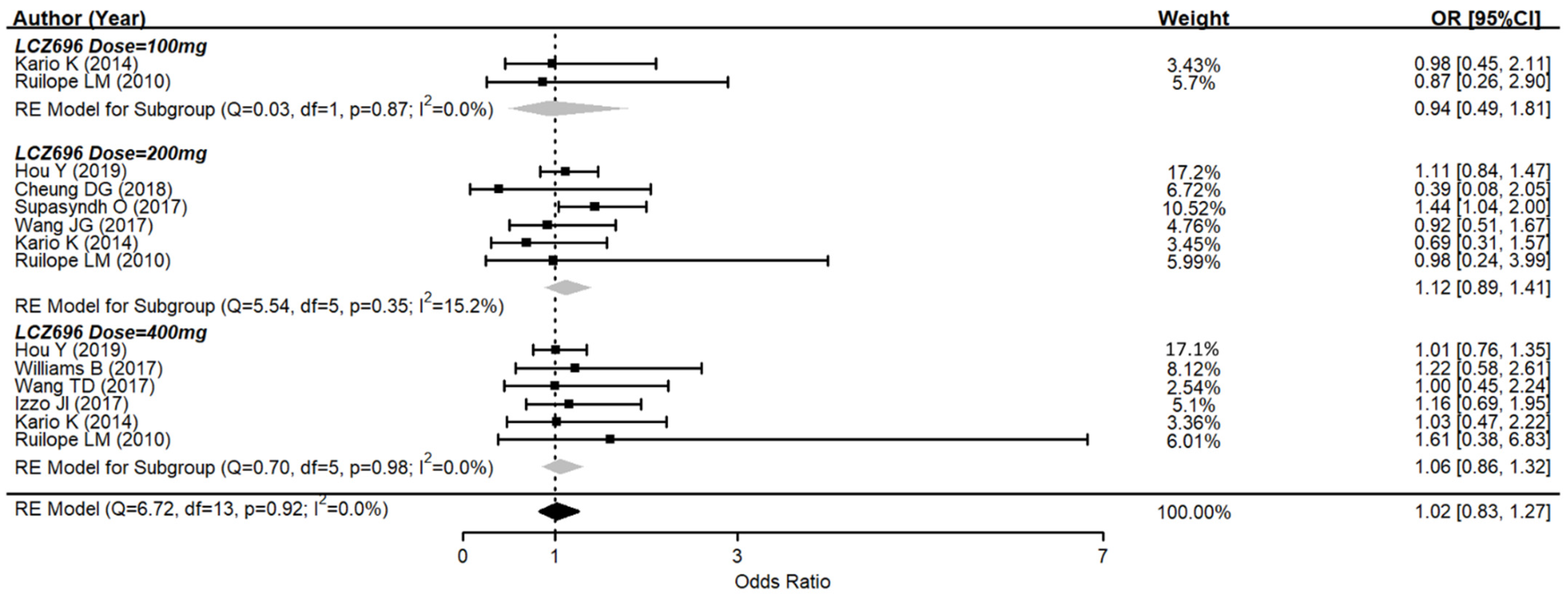
3.4. Adverse Effects of Sacubitril/Valsartan (LCZ696)
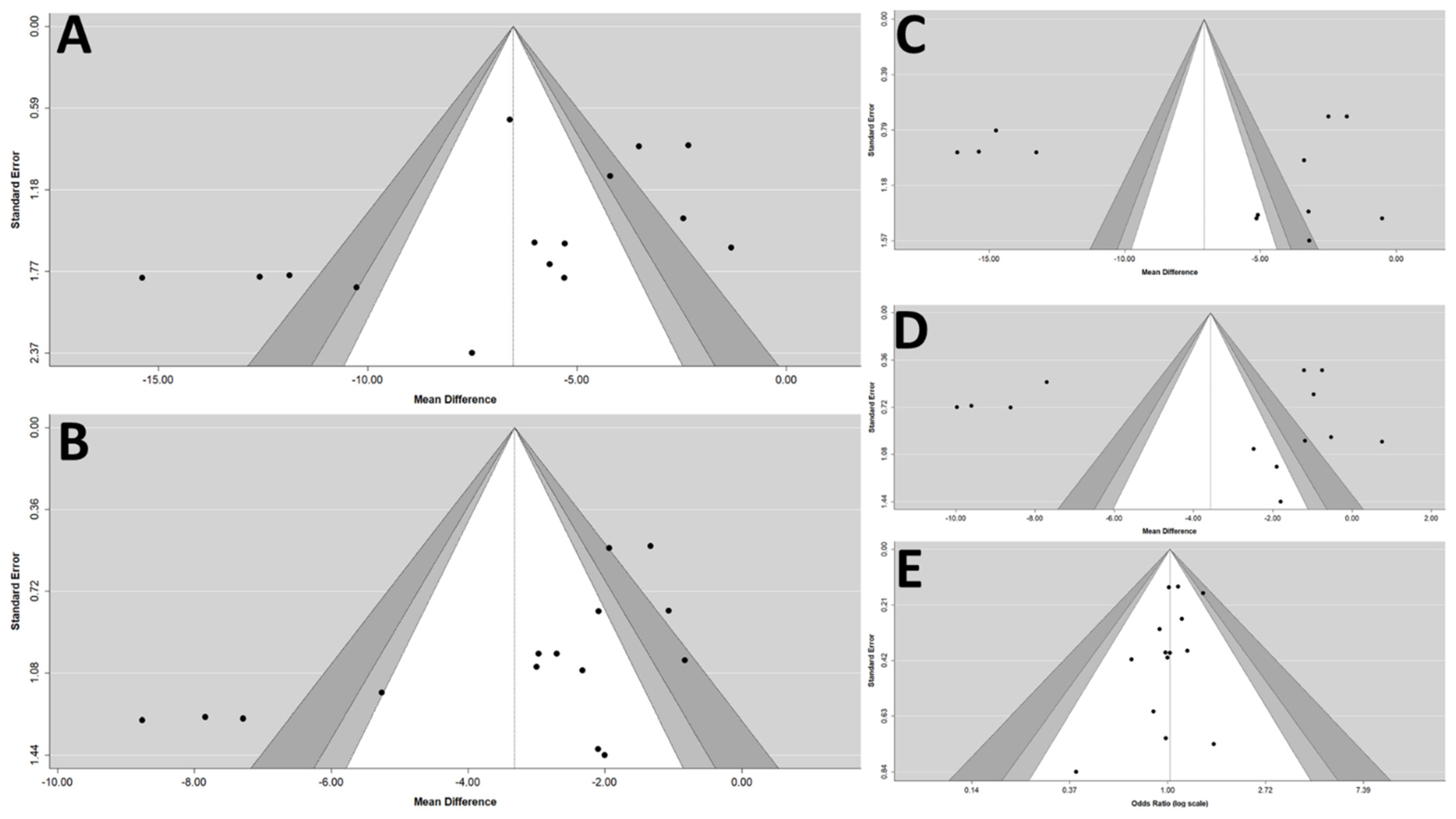
3.5. Adjunctive Evaluations Concerning the Risk of Publication Bias and the Stability of Results
4. Discussion
4.1. Antihypertensive Effectiveness of LCZ696
4.2. Mechanisms of LCZ696
4.3. Adverse Effects of Sacubitril/Valsartan (LCZ696) in Hypertensive Patients
4.4. Clinical Implications
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawes, C.M.M.; Hoorn, S.V.; Rodgers, A. Global burden of blood-pressure-related disease, 2001. Lancet 2008, 371, 1513–1518. [Google Scholar] [CrossRef]
- Muntner, P.; Carey, R.M.; Gidding, S.; Jones, D.W.; Taler, S.J.; Wright, J.T., Jr.; Whelton, P.K. Potential U.S. population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018, 137, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Kario, K.; Wang, J.G. Systolic hypertension: An increasing clinical challenge in Asia. Hypertens. Res. 2015, 38, 227–236. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, D.K.; Wang, T.J. Natriuretic Peptides and Cardiometabolic Health. Circ. J. 2015, 79, 1647–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, G. Omapatrilat Reduces Pulse Pressure and Proximal Aortic Stiffness in Patients with Systolic Hypertension: Results of the Conduit Hemodynamics of Omapatrilat International Research Study. Circulation 2002, 105, 2955–2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruilope, L.M.; Dukat, A.; Bohm, M.; Lacourciere, Y.; Gong, J.; Lefkowitz, M.P. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double-blind, placebo-controlled, active comparator study. Lancet 2010, 375, 1255–1266. [Google Scholar] [CrossRef]
- Kario, K.; Sun, N.; Chiang, F.T.; Supasyndh, O.; Baek, S.H.; Inubushi-Molessa, A.; Zhang, Y.; Gotou, H.; Lefkowitz, M.; Zhang, J. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: A randomized, double-blind, placebo-controlled study. Hypertension 2014, 63, 698–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.G.; Yukisada, K.; Sibulo, A., Jr.; Hafeez, K.; Jia, Y.; Zhang, J. Efficacy and safety of sacubitril/valsartan (LCZ696) add-on to amlodipine in Asian patients with systolic hypertension uncontrolled with amlodipine monotherapy. J. Hypertens. 2017, 35, 877–885. [Google Scholar] [CrossRef]
- Joseph, L.; Izzo, J.; Zappe, D.H.; Jia, Y.; Hafeez, K.; Zhang, J. Efficacy and Safety of Crystalline Valsartan/Sacubitril (LCZ696) Compared With Placebo and Combinations of Free Valsartan and Sacubitril in Patients With Systolic Hypertension: The RATIO Study. J. Cardiovasc. Pharmacol. 2017, 69, 374–381. [Google Scholar]
- Schmieder, R.E.; Wagner, F.; Mayr, M.; Delles, C.; Ott, C.; Keicher, C.; Hrabak-Paar, M.; Heye, T.; Aichner, S.; Khder, Y.; et al. The effect of sacubitril/valsartan compared to olmesartan on cardiovascular remodelling in subjects with essential hypertension: The results of a randomized, double-blind, active-controlled study. Eur. Heart J. 2017, 38, 3308–3317. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.D.; Tan, R.S.; Lee, H.Y.; Ihm, S.H.; Rhee, M.Y.; Tomlinson, B.; Pal, P.; Yang, F.; Hirschhorn, E.; Prescott, M.F.; et al. Effects of Sacubitril/Valsartan (LCZ696) on Natriuresis, Diuresis, Blood Pressures, and NT-proBNP in Salt-Sensitive Hypertension. Hypertension 2017, 69, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Cockcroft, J.R.; Kario, K.; Zappe, D.H.; Brunel, P.C.; Wang, Q.; Guo, W. Effects of Sacubitril/Valsartan Versus Olmesartan on Central Hemodynamics in the Elderly With Systolic Hypertension: The PARAMETER Study. Hypertension 2017, 69, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Li, W.; Webb, R.; Zhao, L.; Wang, Q.; Guo, W. Efficacy and safety of sacubitril/valsartan compared with olmesartan in Asian patients with essential hypertension: A randomized, double-blind, 8-week study. J. Clin. Hypertens. 2019, 21, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, D.G.; Aizenberg, D.; Gorbunov, V.; Hafeez, K.; Chen, C.W.; Zhang, J. Efficacy and safety of sacubitril/valsartan in patients with essential hypertension uncontrolled by olmesartan: A randomized, double-blind, 8-week study. J. Clin. Hypertens. 2018, 20, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supasyndh, O.; Wang, J.; Hafeez, K.; Zhang, Y.; Zhang, J.; Rakugi, H. Efficacy and Safety of Sacubitril/Valsartan (LCZ696) Compared With Olmesartan in Elderly Asian Patients (>/=65 Years) With Systolic Hypertension. Am. J. Hypertens. 2017, 30, 1163–1169. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Wang, J.; Chen, Q.; Yang, X. LCZ696, a promising novel agent in treating hypertension (a meta-analysis of randomized controlled trials). Oncotarget 2017, 8, 107991–108005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, H.; Zhao, X.; Ma, R.; Li, N.; Yu, J. The Effects of LCZ696 in Patients with Hypertension Compared with Angiotensin Receptor Blockers: A Meta-Analysis of Randomized Controlled Trials. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 447–457. [Google Scholar] [CrossRef]
- De Vecchis, R.; Soreca, S.; Ariano, C. Anti-Hypertensive Effect of Sacubitril/Valsartan: A Meta-Analysis of Randomized Controlled Trials. Cardiol. Res. 2019, 10, 24–33. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar]
- Bohm, M.; Young, R.; Jhund, P.S.; Solomon, S.D.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Swedberg, K.; et al. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: Results from PARADIGM-HF. Eur. Heart J. 2017, 38, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J. Neprilysin inhibition to treat heart failure: A tale of science, serendipity, and second chances. Eur. J. Heart Fail. 2015, 17, 242–247. [Google Scholar] [CrossRef]
- Nickenig, G. Should angiotensin II receptor blockers and statins be combined? Circulation 2004, 110, 1013–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steckelings, U.M.; Paulis, L.; Namsolleck, P.; Unger, T. AT2 receptor agonists: Hypertension and beyond. Curr. Opin. Nephrol. Hypertens. 2012, 21, 142–146. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Race | Patients’ Characteristic | Age, Years | Number, (Female/Male) | LCZ696 Dose | Comparison Therapy | Observation Periods | Severe Adverse Events | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Ruilope LM et al., 2010 [7] | not restricted | Mild-to-moderate essential hypertension | 18–75 | 1328 patients (568/760) | 100 mg, 200 mg, 400 mg | Valsartan 80 mg, 160 mg, 320 mg | 8 weeks | Nil | 3/3 |
| Kario K et al., 2014 [8] | only Asians | mild-to-moderate essential hypertension | ≥18 | 389 patients (114/275) | 100 mg, 200 mg, 400 mg | Placebo | 8 weeks | Nil | 2/3 |
| Wang JG et al., 2017 [9] | only Asians | mean clinic SBP 145–180mmHg | ≥18 | 266 patients (110/156) | 200 mg | Amlodipine 5 mg | 8 weeks | Nil | 3/2 |
| Izzo JI et al., 2017 [10] | not restricted | mild-to-moderate systolic hypertension | ≥18 | 907 patients (412/495) | 400 mg | Valsartan 320 mg | 8 weeks | 1 angioedema and 1 CV death in LCZ696 group | 3/3 |
| Schmieder RE et al., 2017 [11] | not restricted | essential hypertension Stage 1 and 2 | ≥18 | 114 patients (37/77) | 400 mg | Olmesartan 40 mg | 52 weeks | NA | 2/2 |
| Wang TD et al., 2017 [12] | only Asians | patient with salt-sensitive hypertension | ≥18 | 72 patients (26/46) | 400 mg | Valsartan 320 mg | 4 weeks | Nil | 2/2 |
| Williams B et al., 2017 [13] | not restricted | msSBP 140–179 mm Hg and PP > 60 mm Hg | ≥60 | 454 patients (217/237) | 400mg | Olmesartan 40 mg | 12 weeks | NA | 3/3 |
| Supasyndh et al., 2017 [16] | only Asians | elderly Asian (≥65 years) | ≥65 | 588 patients (294/294) | 100 mg, 200 mg, 400 mg | Olmesartan 10 mg, 20 mg, 40 mg | 14 weeks | Nil | 3/3 |
| Cheung et al., 2018 [15] | not restricted | mild-to-moderate hypertension | ≥18 | 375 patients (183/192) | 200 mg | Olmesartan 20 mg | 8 weeks | Nil | 2/3 |
| Hou Y et al., 2019 [14] | only Asians | mild-to-moderate hypertension | ≥18 | 1438 patients (682/756) | 200 mg, 400 mg | Olmesartan 20 mg | 8 weeks | 1 Angioedema in each group | 3/3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chua, S.-K.; Lai, W.-T.; Chen, L.-C.; Hung, H.-F. The Antihypertensive Effects and Safety of LCZ696 in Patients with Hypertension: A Systemic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 2824. https://doi.org/10.3390/jcm10132824
Chua S-K, Lai W-T, Chen L-C, Hung H-F. The Antihypertensive Effects and Safety of LCZ696 in Patients with Hypertension: A Systemic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2021; 10(13):2824. https://doi.org/10.3390/jcm10132824
Chicago/Turabian StyleChua, Su-Kiat, Wei-Ting Lai, Lung-Ching Chen, and Huei-Fong Hung. 2021. "The Antihypertensive Effects and Safety of LCZ696 in Patients with Hypertension: A Systemic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 10, no. 13: 2824. https://doi.org/10.3390/jcm10132824
APA StyleChua, S.-K., Lai, W.-T., Chen, L.-C., & Hung, H.-F. (2021). The Antihypertensive Effects and Safety of LCZ696 in Patients with Hypertension: A Systemic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 10(13), 2824. https://doi.org/10.3390/jcm10132824






