Hypercoagulability Evaluation in Antiphospholipid Syndrome without Anticoagulation Treatment with Thrombin Generation Assay: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
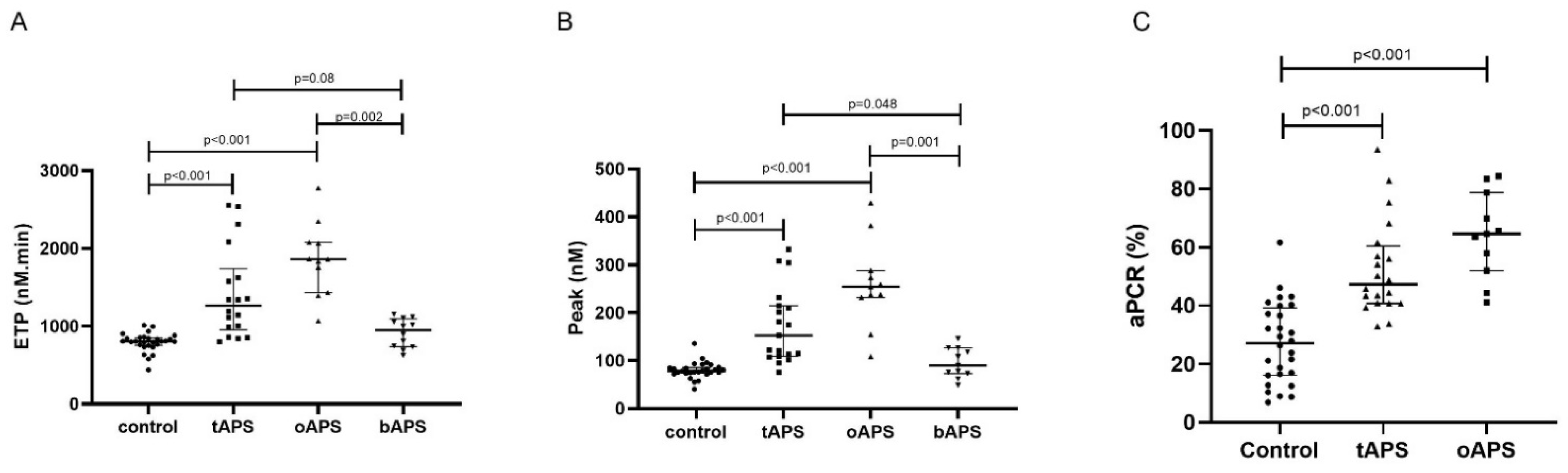
Hypercoagulability in Clinical Antiphospholipid Syndrome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uthman, I.; Noureldine, M.H.A.; Ruiz-Irastorza, G.; Khamashta, M. Management of Antiphospholipid Syndrome. Ann. Rheum. Dis. 2018. [Google Scholar] [CrossRef]
- Cervera, R.; Serrano, R.; Pons-Estel, G.J.; Ceberio-Hualde, L.; Shoenfeld, Y.; de Ramón, E.; Buonaiuto, V.; Jacobsen, S.; Zeher, M.M.; Tarr, T.; et al. Morbidity and Mortality in the Antiphospholipid Syndrome during a 10-Year Period: A Multicentre Prospective Study of 1000 Patients. Ann. Rheum. Dis. 2015, 74, 1011–1018. [Google Scholar] [CrossRef]
- Belizna, C.; Stojanovich, L.; Cohen-Tervaert, J.W.; Fassot, C.; Henrion, D.; Loufrani, L.; Nagy, G.; Muchardt, C.; Hasan, M.; Ungeheuer, M.N.; et al. Primary Antiphospholipid Syndrome and Antiphospholipid Syndrome Associated to Systemic Lupus: Are They Different Entities? Autoimmun. Rev. 2018, 17, 739–745. [Google Scholar] [CrossRef]
- Pons-Estel, G.J.; Andreoli, L.; Scanzi, F.; Cervera, R.; Tincani, A. The Antiphospholipid Syndrome in Patients with Systemic Lupus Erythematosus. J. Autoimmun. 2017, 76, 10–20. [Google Scholar] [CrossRef] [PubMed]
- De Jesús, G.R.; Sciascia, S.; Andrade, D.; Nascimento, I.S.; Rosa, R.; Barbhaiya, M.; Tektonidou, M.; Banzato, A.; Pengo, V.; Ji, L.; et al. Factors Associated with First Thrombosis in Patients Presenting with Obstetric Antiphospholipid Syndrome in APS Alliance For Clinical Trials & International Networking (APS ACTION) Clinical Database And Repository: A Retrospective Study. BJOG Int. J. Obstet. Gynaecol. 2018. [Google Scholar] [CrossRef]
- Pengo, V.; Testa, S.; Martinelli, I.; Ghirarduzzi, A.; Legnani, C.; Gresele, P.; Passamonti, S.M.; Bison, E.; Denas, G.; Jose, S.P.; et al. Incidence of a First Thromboembolic Event in Carriers of Isolated Lupus Anticoagulant. Thromb. Res. 2015, 135, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Billoir, P.; Duflot, T.; Fresel, M.; Chrétien, M.H.; Barbay, V.; Le Cam Duchez, V. Thrombin Generation Profile in Non-Thrombotic Factor V Leiden Carriers. J. Thromb. Thrombolysis 2019. [Google Scholar] [CrossRef]
- Billoir, P.; Alexandre, K.; Duflot, T.; Roger, M.; Miranda, S.; Goria, O.; Joly, L.M.; Demeyere, M.; Feugray, G.; Brunel, V.; et al. Investigation of Coagulation Biomarkers to Assess Clinical Deterioration in SARS-CoV-2 Infection. Front. Med. 2021, 8. [Google Scholar] [CrossRef]
- Hemker, H.C.; Al Dieri, R.; De Smedt, E.; Béguin, S. Thrombin Generation, a Function Test of the Haemostatic-Thrombotic System. Thromb. Haemost. 2006, 96, 553–561. [Google Scholar]
- Regnault, V.; Béguin, S.; Wahl, D.; de Maistre, E.; Coenraad Hemker, H.; Lecompte, T. Thrombinography Shows Acquired Resistance to Activated Protein C in Patients with Lupus Anticoagulants. Thromb. Haemost. 2003, 89, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuily, S.; Ait Aissa, K.; Membre, A.; Regnault, V.; Lecompte, T.; Wahl, D. Thrombin Generation in Antiphospholipid Syndrome. Lupus 2012, 21, 758–760. [Google Scholar] [CrossRef]
- Billoir, P.; Miranda, S.; Damian, L.; Richard, V.; Benhamou, Y.; Le Cam Duchez, V. Development of a Thrombin Generation Test in Cultured Endothelial Cells: Evaluation of the Prothrombotic Effects of Antiphospholipid Antibodies. Thromb. Res. 2018, 169, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Billoir, P.; Damian, L.; Thiebaut, P.A.; Schapman, D.; Le Besnerais, M.; Jouen, F.; Galas, L.; Levesque, H.; Le Cam-Duchez, V.; et al. Hydroxychloroquine Reverses the Prothrombotic State in a Mouse Model of Antiphospholipid Syndrome: Role of Reduced Inflammation and Endothelial Dysfunction. PLoS ONE 2019, 14, e0212614. [Google Scholar] [CrossRef] [PubMed]
- Billoir, P.; Blandinières, A.; Gendron, N.; Chocron, R.; Gunther, S.; Philippe, A.; Guerin, C.L.; Israël-Biet, D.; Smadja, D.M. Endothelial Colony-Forming Cells from Idiopathic Pulmonary Fibrosis Patients Have a High Procoagulant Potential. Stem Cell Rev. Rep. 2020. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International Consensus Statement on an Update of the Classification Criteria for Definite Antiphospholipid Syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V.; Tripodi, A.; Reber, G.; Rand, J.H.; Ortel, T.L.; Galli, M.; De Groot, P.G. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis Update of the Guidelines for Lupus Anticoagulant Detection. J. Thromb. Haemost. 2009, 7, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Erkan, D. Diagnosis and Management of the Antiphospholipid Syndrome. N. Engl. J. Med. 2018, 379, 1290. [Google Scholar] [CrossRef]
- Pereira, J.; Alfaro, G.; Goycoolea, M.; Quiroga, T.; Ocqueteau, M.; Massardo, L.; Pérez, C.; Sáez, C.; Panes, O.; Matus, V.; et al. Circulating Platelet-Derived Microparticles in Systemic Lupus Erythematosus. Association with Increased Thrombin Generation and Procoagulant State. Thromb. Haemost. 2006, 95, 94–99. [Google Scholar]
- Schreiber, K.; Breen, K.; Parmar, K.; Rand, J.H.; Wu, X.-X.; Hunt, B.J. The Effect of Hydroxychloroquine on Haemostasis, Complement, Inflammation and Angiogenesis in Patients with Antiphospholipid Antibodies. Rheumatol. Oxf. Engl. 2018, 57, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Kassis, J.; Neville, C.; Rauch, J.; Busque, L.; Chang, E.R.; Joseph, L.; Le Comte, M.; Subang, R.; Fortin, P.R. Antiphospholipid Antibodies and Thrombosis: Association with Acquired Activated Protein C Resistance in Venous Thrombosis and with Hyperhomocysteinemia in Arterial Thrombosis. Thromb. Haemost. 2004, 92, 1312–1319. [Google Scholar] [CrossRef] [Green Version]
- Zuily, S.; Regnault, V.; Guillemin, F.; Kaminsky, P.; Rat, A.-C.; Lecompte, T.; Wahl, D. Superficial Vein Thrombosis, Thrombin Generation and Activated Protein C Resistance as Predictors of Thromboembolic Events in Lupus and Antiphospholipid Patients. A Prospective Cohort Study. Thromb. Res. 2013, 132, e1–e7. [Google Scholar] [CrossRef] [PubMed]
| Control | tAPS | oAPS | bAPS | |
|---|---|---|---|---|
| N | 28 | 19 | 11 | 11 |
| Age (years) | 49.5 ± 18.3 | 36.3 ± 8.8 | 57.1 ± 19.7 | |
| Sex (women/men) | 16/3 | 11 | 10/1 | |
| Thrombotic event | ||||
| Venous thrombosis | 10 | |||
| Arterial thrombosis | 7 | |||
| Both | 2 | |||
| Obstetrical complication | ||||
| EPL | 4 | |||
| Late miscarriages | 4 | |||
| HELLP syndrome | 2 | |||
| IGR | 1 | |||
| APL | ||||
| LA | 4 | 2 | 8 | |
| aCL IgM/IgG | 7/5 | 1/6 | 5/3 | |
| Anti-β2GPI IgM/IgG | 0/4 | 1/1 | 3/0 | |
| Double positivity | 1 | 1 | ||
| Triple positivity | 2 | 2 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billoir, P.; Miranda, S.; Levesque, H.; Benhamou, Y.; Le Cam Duchez, V. Hypercoagulability Evaluation in Antiphospholipid Syndrome without Anticoagulation Treatment with Thrombin Generation Assay: A Preliminary Study. J. Clin. Med. 2021, 10, 2728. https://doi.org/10.3390/jcm10122728
Billoir P, Miranda S, Levesque H, Benhamou Y, Le Cam Duchez V. Hypercoagulability Evaluation in Antiphospholipid Syndrome without Anticoagulation Treatment with Thrombin Generation Assay: A Preliminary Study. Journal of Clinical Medicine. 2021; 10(12):2728. https://doi.org/10.3390/jcm10122728
Chicago/Turabian StyleBilloir, Paul, Sébastien Miranda, Herve Levesque, Ygal Benhamou, and Véronique Le Cam Duchez. 2021. "Hypercoagulability Evaluation in Antiphospholipid Syndrome without Anticoagulation Treatment with Thrombin Generation Assay: A Preliminary Study" Journal of Clinical Medicine 10, no. 12: 2728. https://doi.org/10.3390/jcm10122728
APA StyleBilloir, P., Miranda, S., Levesque, H., Benhamou, Y., & Le Cam Duchez, V. (2021). Hypercoagulability Evaluation in Antiphospholipid Syndrome without Anticoagulation Treatment with Thrombin Generation Assay: A Preliminary Study. Journal of Clinical Medicine, 10(12), 2728. https://doi.org/10.3390/jcm10122728






