Therapeutic Efficacy of Autologous Platelet Concentrate Injection on Macular Holes with High Myopia, Large Macular Holes, or Recurrent Macular Holes: A Multicenter Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
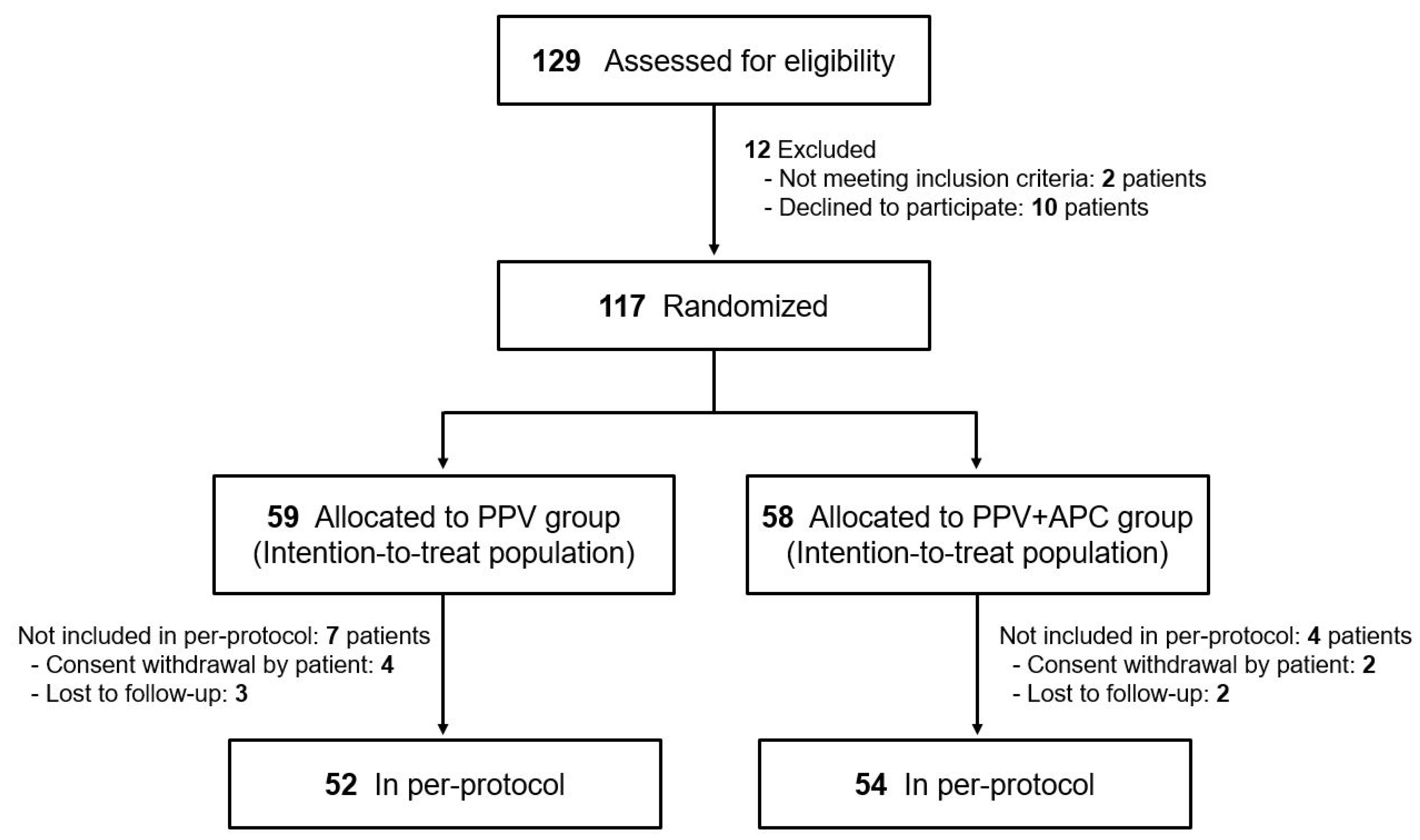
2.2. Randomization and Masking
2.3. Intervention: Surgical Techniques
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Outcomes
3.2.1. Primary Outcome
3.2.2. Secondary Outcomes
3.3. Safety Outcomes
3.4. Component Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, R.N.; Gass, J.D. Idiopathic macular holes. Observations, stages of formation, and implications for surgical intervention. Ophthalmology 1988, 95, 917–924. [Google Scholar] [CrossRef]
- Casuso, L.A.; Scott, I.U.; Flynn, H.W., Jr.; Gass, J.D.; Smiddy, W.E.; Lewis, M.L.; Schiffman, J. Long-term follow-up of unoperated macular holes. Ophthalmology 2001, 108, 1150–1155. [Google Scholar] [CrossRef]
- Tam, A.L.C.; Yan, P.; Gan, N.Y.; Lam, W.C. The Current Surgical Management of Large, Recurrent, or Persistent Macular Holes. Retina 2018, 38, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Ezra, E. Idiopathic full thickness macular hole: Natural history and pathogenesis. Br. J. Ophthalmol. 2001, 85, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnell, P.J.; Fine, S.L.; Hillis, A.I. Clinical features of idiopathic macular cysts and holes. Am. J. Ophthalmol. 1982, 93, 777–786. [Google Scholar] [CrossRef]
- Brooks, H.L., Jr. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology 2000, 107, 1939–1948, discussion 1948 1939. [Google Scholar] [CrossRef]
- Christmas, N.J.; Smiddy, W.E.; Flynn, H.W., Jr. Reopening of macular holes after initially successful repair. Ophthalmology 1998, 105, 1835–1838. [Google Scholar] [CrossRef]
- Sheidow, T.G.; Blinder, K.J.; Holekamp, N.; Joseph, D.; Shah, G.; Grand, M.G.; Thomas, M.A.; Bakal, J.; Sharma, S. Outcome results in macular hole surgery: An evaluation of internal limiting membrane peeling with and without indocyanine green. Ophthalmology 2003, 110, 1697–1701. [Google Scholar] [CrossRef]
- Gupta, B.; Laidlaw, D.A.; Williamson, T.H.; Shah, S.P.; Wong, R.; Wren, S. Predicting visual success in macular hole surgery. Br. J. Ophthalmol. 2009, 93, 1488–1491. [Google Scholar] [CrossRef] [Green Version]
- Ch’ng, S.W.; Patton, N.; Ahmed, M.; Ivanova, T.; Baumann, C.; Charles, S.; Jalil, A. The Manchester Large Macular Hole Study: Is it Time to Reclassify Large Macular Holes? Am. J. Ophthalmol. 2018, 195, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Michalewska, Z.; Michalewski, J.; Adelman, R.A.; Nawrocki, J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology 2010, 117, 2018–2025. [Google Scholar] [CrossRef]
- Steel, D.H.; Donachie, P.H.J.; Aylward, G.W.; Laidlaw, D.A.; Williamson, T.H.; Yorston, D. Factors affecting anatomical and visual outcome after macular hole surgery: Findings from a large prospective UK cohort. Eye (Lond.) 2021, 35, 316–325. [Google Scholar] [CrossRef]
- D’Souza, M.J.; Chaudhary, V.; Devenyi, R.; Kertes, P.J.; Lam, W.C. Re-operation of idiopathic full-thickness macular holes after initial surgery with internal limiting membrane peel. Br. J. Ophthalmol. 2011, 95, 1564–1567. [Google Scholar] [CrossRef]
- Che, X.; He, F.; Lu, L.; Zhu, D.; Xu, X.; Song, X.; Fan, X.; Wang, Z. Evaluation of secondary surgery to enlarge the peeling of the internal limiting membrane following the failed surgery of idiopathic macular holes. Exp. Ther. Med. 2014, 7, 742–746. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.T.; Kung, Y.H.; Chang, C.Y.; Chang, S.P. Surgical Outcomes in Eyes with Extremely High Myopia for Macular Hole without Retinal Detachment. Retina 2018, 38, 2051–2055. [Google Scholar] [CrossRef] [PubMed]
- Sulkes, D.J.; Smiddy, W.E.; Flynn, H.W.; Feuer, W. Outcomes of macular hole surgery in severely myopic eyes: A case-control study. Am. J. Ophthalmol. 2000, 130, 335–339. [Google Scholar] [CrossRef]
- Patel, S.C.; Loo, R.H.; Thompson, J.T.; Sjaarda, R.N. Macular hole surgery in high myopia. Ophthalmology 2001, 108, 377–380. [Google Scholar] [CrossRef]
- Suda, K.; Hangai, M.; Yoshimura, N. Axial length and outcomes of macular hole surgery assessed by spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2011, 151, 118–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalewska, Z.; Michalewski, J.; Dulczewska-Cichecka, K.; Nawrocki, J. Inverted internal limiting membrane flap technique for surgical repair of myopic macular holes. Retina 2014, 34, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Yang, C.M. Lens Capsular Flap Transplantation in the Management of Refractory Macular Hole from Multiple Etiologies. Retina 2016, 36, 163–170. [Google Scholar] [CrossRef]
- Kumar, A.; Tinwala, S.I.; Gogia, V.; Sehra, S.V. Tapping of Macular Hole Edges: The Outcomes of a Novel Technique for Large Macular Holes. Asia Pac. J. Ophthalmol. (Phila) 2013, 2, 305–309. [Google Scholar] [CrossRef]
- Ezra, E.; Aylward, W.G.; Gregor, Z.J. Membranectomy and autologous serum for the retreatment of full-thickness macular holes. Arch. Ophthalmol. 1997, 115, 1276–1280. [Google Scholar] [CrossRef]
- Ezra, E.; Gregor, Z.J. Surgery for idiopathic full-thickness macular hole: Two-year results of a randomized clinical trial comparing natural history, vitrectomy, and vitrectomy plus autologous serum: Morfields Macular Hole Study Group RAeport no. 1. Arch. Ophthalmol. 2004, 122, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, D.; Melean, P.; Calvo, R.; Vaisman, A.; Zilleruelo, N.; Figueroa, F.; Villalón, I. Magnetic resonance imaging evaluation of the integration and maturation of semitendinosus-gracilis graft in anterior cruciate ligament reconstruction using autologous platelet concentrate. Arthroscopy 2010, 26, 1318–1325. [Google Scholar] [CrossRef]
- Horstmann, W.G.; Slappendel, R.; van Hellemondt, G.G.; Wymenga, A.W.; Jack, N.; Everts, P.A. Autologous platelet gel in total knee arthroplasty: A prospective randomized study. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 115–121. [Google Scholar] [CrossRef]
- Lindeboom, J.A.; Mathura, K.R.; Aartman, I.H.; Kroon, F.H.; Milstein, D.M.; Ince, C. Influence of the application of platelet-enriched plasma in oral mucosal wound healing. Clin. Oral Implant. Res. 2007, 18, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Hersant, B.; SidAhmed-Mezi, M.; Picard, F.; Hermeziu, O.; Rodriguez, A.M.; Ezzedine, K.; Meningaud, J.P. Efficacy of Autologous Platelet Concentrates as Adjuvant Therapy to Surgical Excision in the Treatment of Keloid Scars Refractory to Conventional Treatments: A Pilot Prospective Study. Ann. Plast. Surg. 2018, 81, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Velotta, J.; Brinton, T.J.; Wang, X.; Chang, S.; Palmer, O.; Sheikh, A.; Chung, J.; Yang, P.C.; Robbins, R.; et al. RevaTen platelet-rich plasma improves cardiac function after myocardial injury. Cardiovasc. Revascularization Med. 2011, 12, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Gaudric, A.; Massin, P.; Paques, M.; Santiago, P.Y.; Guez, J.E.; Le Gargasson, J.F.; Mundler, O.; Drouet, L. Autologous platelet concentrate for the treatment of full-thickness macular holes. Graefes Arch. Clin. Exp. Ophthalmol. 1995, 233, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Paques, M.; Chastang, C.; Mathis, A.; Sahel, J.; Massin, P.; Dosquet, C.; Korobelnik, J.F.; Le Gargasson, J.F.; Gaudric, A. Effect of autologous platelet concentrate in surgery for idiopathic macular hole: Results of a multicenter, double-masked, randomized trial. Platelets in Macular Hole Surgery Group. Ophthalmology 1999, 106, 932–938. [Google Scholar] [CrossRef]
- Gaudric, A.; Paques, M.; Massin, P.; Santiago, P.Y.; Dosquet, C. Use of autologous platelet concentrate in macular hole surgery: Report of 77 cases. Dev. Ophthalmol. 1997, 29, 30–35. [Google Scholar] [PubMed]
- Mulhern, M.G.; Cullinane, A.; Cleary, P.E. Visual and anatomical success with short-term macular tamponade and autologous platelet concentrate. Graefes Arch. Clin. Exp. Ophthalmol. 2000, 238, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Mangione, C.M.; Lee, P.P.; Gutierrez, P.R.; Spritzer, K.; Berry, S.; Hays, R.D. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch. Ophthalmol. 2001, 119, 1050–1058. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, B.J.; Kim, M.N.; Mun, S.K. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: A simultaneous split-face trial. Dermatol. Surg. 2011, 37, 931–938. [Google Scholar] [CrossRef]
- Freeman, W.R.; Azen, S.P.; Kim, J.W.; el-Haig, W.; Mishell, D.R., 3rd; Bailey, I. Vitrectomy for the treatment of full-thickness stage 3 or 4 macular holes. Results of a multicentered randomized clinical trial. The Vitrectomy for Treatment of Macular Hole Study Group. Arch. Ophthalmol. 1997, 115, 11–21. [Google Scholar] [CrossRef]
- Ip, M.S.; Baker, B.J.; Duker, J.S.; Reichel, E.; Baumal, C.R.; Gangnon, R.; Puliafito, C.A. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch. Ophthalmol. 2002, 120, 29–35. [Google Scholar] [CrossRef]
- Ksander, G.A.; Sawamura, S.J.; Ogawa, Y.; Sundsmo, J.; McPherson, J.M. The effect of platelet releasate on wound healing in animal models. J. Am. Acad. Dermatol. 1990, 22, 781–791. [Google Scholar] [CrossRef]
- Martin, P. Wound healing--aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Kakudo, N.; Matsui, M.; Ogura, T.; Hara, T.; Suzuki, K.; Yamamoto, M.; Tabata, Y.; Kusumoto, K. Exploratory clinical trial of combination wound therapy with a gelatin sheet and platelet-rich plasma in patients with chronic skin ulcers: Study protocol. BMJ Open 2015, 5, e007733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayanagi, Y.; Kato, S.; Okada, M. Benefits of autologous platelet tissue graft in wound healing after corneal refractive surgery: A case report. J. Med. Case Rep. 2021, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Ski. Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef] [PubMed]

| PPV Group (n = 59) | PPV+APC Group (n = 58) | p Value | |
|---|---|---|---|
| Age | 63.4 ± 8.0 | 65.0 ± 7.4 | 0.371 * |
| Sex, male (%) | 46 (78.0%) | 37 (63.8%) | 0.091 † |
| Right side, n (%) | 27 (45.8%) | 32 (55.2%) | 0.309 † |
| Main symptom | 0.091 † | ||
| Decreased vision | 41 (69.5%) | 46 (79.3%) | |
| Metamorphopsia | 9 (15.3%) | 2 (3.4%) | |
| Both | 9 (15.3%) | 10 (17.2%) | |
| Systolic blood pressure, mmHg | 122.3 ± 14.0 | 126.2 ± 13.7 | 0.228 * |
| Diastolic blood pressure, mmHg | 74.3 ± 10.3 | 75.9 ± 8.7 | 0.531 * |
| Ocular examinations | |||
| Axial length, mm | 24.7 ± 2.6 | 24.8 ± 2.6 | 0.749 * |
| Spherical equivalent, diopter | −1.1 ± 2.7 | −1.7 ± 4.2 | 0.788 * |
| Intraocular pressure, mmHg | 14.8 ± 2.8 | 14.4 ± 3.4 | 0.259 * |
| BCVA (logMAR) | 0.8 ± 0.5 | 0.8 ± 0.6 | 0.415 * |
| Lens status, n (%) | 0.397 † | ||
| Phakic | 20 (33.9%) | 22 (37.9%) | |
| Pseudophakic | 39 (66.1%) | 36 (62.1%) |
| PPV Group (n = 59) | PPV+APC Group (n = 58) | p Value | |
|---|---|---|---|
| Status of macular hole | 0.134 | ||
| Closed | 47 (79.7%) | 52 (89.7%) | |
| Unclosed | 12 (20.3%) | 6 (10.3%) |
| Baseline | 1 Month | 2 Months | 4 Months | 6 Months | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Group (G) | Time (T) | G × T | |||||||
| Minimum Diameter | PPV | 537.8 ± 26.4 | 78.0 ± 30.5 | 61.7 ± 29.1 | 51.8 ± 27.2 | 57.2 ± 30.2 | 0.964 | <0.001 | 0.072 |
| PPV+APC | 589.6 ± 29.2 | 57.3 ± 24.3 | 34.0 ± 20.1 | 39.1 ± 23.2 | 43.6 ± 22.1 | ||||
| Bonferroni post hoc test of the p value | 0.192 | 0.595 | 0.432 | 0.724 | 0.714 | ||||
| Base Diameter | PPV | 967.0 ± 42.7 | 139.9 ± 50.4 | 110.8 ± 47.4 | 102.2 ± 48.3 | 101.9 ± 45.3 | 0.949 | <0.001 | 0.050 |
| PPV+APC | 1078.3 ± 49.9 | 115.2 ± 47.3 | 69.9 ± 40.9 | 74.9 ± 44.0 | 88.5 ± 44.5 | ||||
| Bonferroni post hoc test of the p value | 0.094 | 0.721 | 0.514 | 0.676 | 0.833 | ||||
| Height | PPV | 438.3 ± 20.4 | 65.8 ± 24.1 | 50.9 ± 21.3 | 38.9 ± 17.2 | 36.1 ± 16.1 | 0.530 | <0.001 | 0.276 |
| PPV+APC | 464.4 ± 25.5 | 47.6 ± 19.2 | 26.9 ± 16.2 | 27.3 ± 16.1 | 38.5 ± 19.6 | ||||
| Bonferroni post hoc test of the p value | 0.428 | 0.554 | 0.369 | 0.624 | 0.927 | ||||
| Baseline | 1 Month | 2 Months | 4 Months | 6 Months | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Group (G) | Time (T) | G × T | |||||||
| BCVA | PPV | 0.84 ± 0.06 | 0.69 ± 0.06 | 0.69 ± 0.05 | 0.66 ± 0.05 | 0.68 ± 0.06 | 0.130 | <0.001 | 0.256 |
| PPV+APC | 0.85 ± 0.08 | 0.82 ± 0.07 | 0.77 ± 0.06 | 0.72 ± 0.05 | 0.68 ± 0.05 | ||||
| Bonferroni post hoc test of the p value | 0.908 | 0.175 | 0.371 | 0.402 | 0.979 | ||||
| Baseline | 2 Months | 6 Months | p Value | ||||
|---|---|---|---|---|---|---|---|
| Group (G) | Time (T) | G × T | |||||
| M-chart score | PPV | 0.82 ± 0.06 | 0.64 ± 0.05 | 0.52 ± 0.05 | 0.762 | <0.001 | 0.070 |
| PPV+APC | 0.72 ± 0.06 | 0.70 ± 0.07 | 0.62 ± 0.07 | ||||
| Bonferroni post hoc test of the p value | 0.296 | 0.463 | 0.268 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Won, J.-Y.; Choi, S.-Y.; Kim, M.; Ra, H.; Jee, D.; Kwon, J.-W.; Kang, K.-D.; Roh, Y.-J.; Park, Y.-G.; et al. Therapeutic Efficacy of Autologous Platelet Concentrate Injection on Macular Holes with High Myopia, Large Macular Holes, or Recurrent Macular Holes: A Multicenter Randomized Controlled Trial. J. Clin. Med. 2021, 10, 2727. https://doi.org/10.3390/jcm10122727
Kim M, Won J-Y, Choi S-Y, Kim M, Ra H, Jee D, Kwon J-W, Kang K-D, Roh Y-J, Park Y-G, et al. Therapeutic Efficacy of Autologous Platelet Concentrate Injection on Macular Holes with High Myopia, Large Macular Holes, or Recurrent Macular Holes: A Multicenter Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(12):2727. https://doi.org/10.3390/jcm10122727
Chicago/Turabian StyleKim, Mirinae, Jae-Yon Won, Seung-Yong Choi, Minhee Kim, Ho Ra, Donghyun Jee, Jin-Woo Kwon, Kui-Dong Kang, Young-Jung Roh, Young-Gun Park, and et al. 2021. "Therapeutic Efficacy of Autologous Platelet Concentrate Injection on Macular Holes with High Myopia, Large Macular Holes, or Recurrent Macular Holes: A Multicenter Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 12: 2727. https://doi.org/10.3390/jcm10122727
APA StyleKim, M., Won, J.-Y., Choi, S.-Y., Kim, M., Ra, H., Jee, D., Kwon, J.-W., Kang, K.-D., Roh, Y.-J., Park, Y.-G., Kang, S., Shin, J.-A., Yim, H.-W., & Park, Y.-H. (2021). Therapeutic Efficacy of Autologous Platelet Concentrate Injection on Macular Holes with High Myopia, Large Macular Holes, or Recurrent Macular Holes: A Multicenter Randomized Controlled Trial. Journal of Clinical Medicine, 10(12), 2727. https://doi.org/10.3390/jcm10122727







