Low-Intensity Continuous Ultrasound Therapies—A Systematic Review of Current State-of-the-Art and Future Perspectives
Abstract
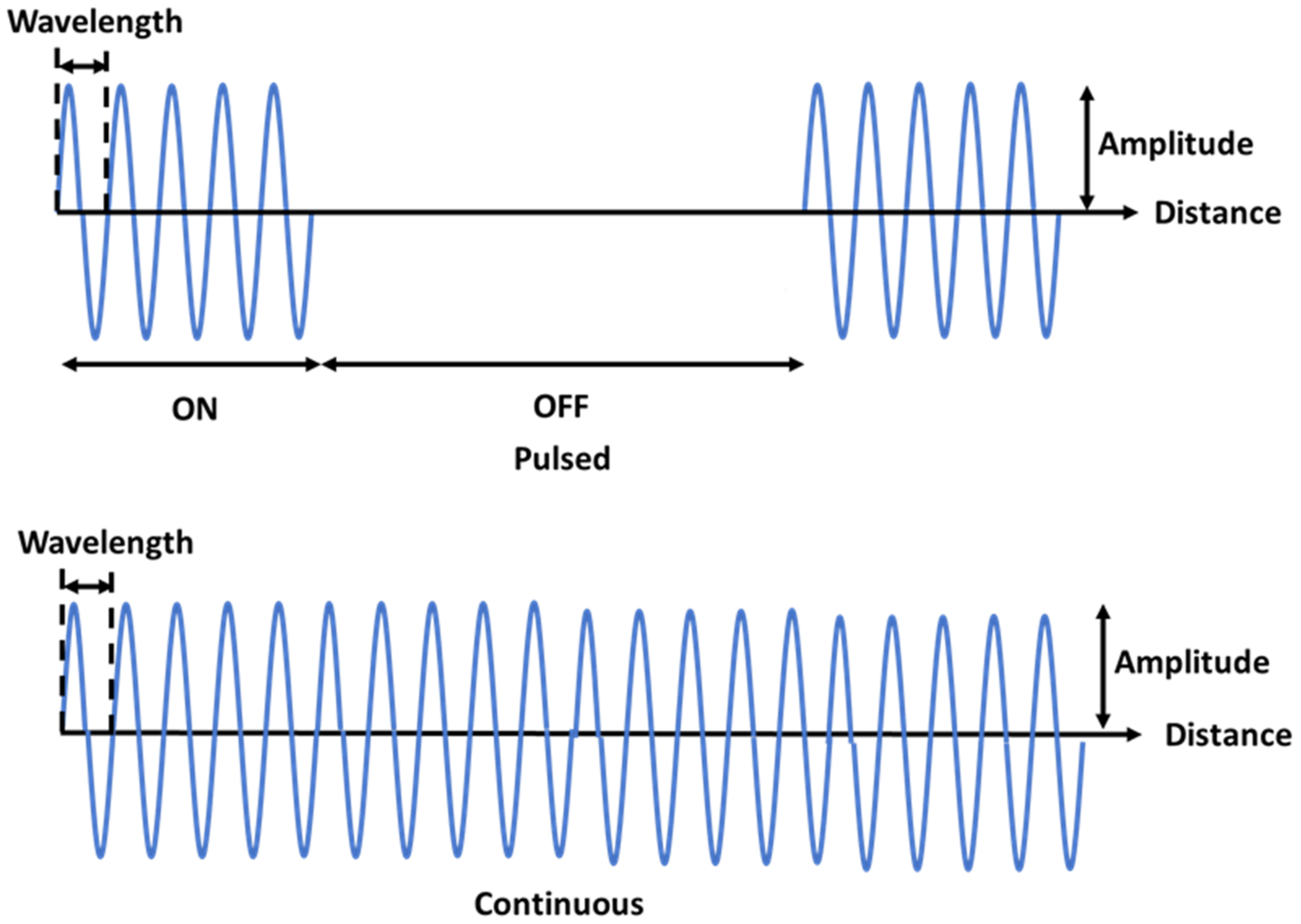
1. Introduction
2. Methods of How the Search Was Conducted for the Review Article: Materials and Methods
3. Applications of Low-Intensity Continuous Ultrasound
3.1. LICUS Effects on Tissue Regeneration
3.2. LICUS Role in Pain Management
3.3. Regulation of Neuromodulation
3.4. LICUS Effectiveness in Thrombosis
3.5. Sonophoresis and Drug Delivery
3.6. Cancer Treatment
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Agostino, M.C.; Craig, K.; Tibalt, E.; Respizzi, S. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int. J. Surg. 2015, 24, 147–153. [Google Scholar] [CrossRef]
- Enwemeka, C.S.; Rodriguez, O.; Mendosa, S. The biomechanical effects of low-intensity ultrasound on healing tendons. Ultrasound Med. Biol. 1990, 16, 801–807. [Google Scholar] [CrossRef]
- Kasturi, G.; Adler, R.A. Mechanical means to improve bone strength: Ultrasound and vibration. Curr. Rheumatol. Rep. 2011, 13, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.N.; Huang, Y.C.; Ni, G.X. Mechanotransduction of stem cells for tendon repair. World J. Stem Cells 2020, 12, 952–965. [Google Scholar] [CrossRef]
- Yamashiro, Y.; Yanagisawa, H. The molecular mechanism of mechanotransduction in vascular homeostasis and disease. Clin. Sci. 2020, 134, 2399–2418. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.S.; Chen, Y.Z.; Huang, T.H.; Tang, C.H.; Fu, W.M.; Lu, B.Y.; Lin, W.L. The effects of low-intensity ultrasound on growing bone after sciatic neurectomy. Ultrasound Med. Biol. 2005, 31, 431–437. [Google Scholar] [CrossRef]
- Whitney, N.P.; Lamb, A.C.; Louw, T.M.; Subramanian, A. Integrin-mediated mechanotransduction pathway of low-intensity continuous ultrasound in human chondrocytes. Ultrasound Med. Biol. 2012, 38, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.J.; Reader, J.S.; Tzima, E. Mechanical Forces and Their Effect on the Ribosome and Protein Translation Machinery. Cells 2020, 9, 650. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, I.; McCollum, D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. J. Biol. Chem. 2019, 294, 17693–17706. [Google Scholar] [CrossRef]
- Charras, G.; Yap, A.S. Tensile Forces and Mechanotransduction at Cell-Cell Junctions. Curr. Biol. 2018, 28, R445–R457. [Google Scholar] [CrossRef]
- Huseman, C.J.; Sigler, D.H.; Welsh, T.H.; Suva, L.J.; Vogelsang, M.M.; Dominguez, B.J.; Huggins, S.; Paulk, C. Skeletal response to whole body vibration and dietary calcium and phosphorus in growing pigs. J. Anim. Sci. 2019, 97, 3369–3378. [Google Scholar] [CrossRef] [PubMed]
- ElDeeb, A.M.; Abdel-Aziem, A.A. Effect of Whole-Body Vibration Exercise on Power Profile and Bone Mineral Density in Postmenopausal Women with Osteoporosis: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2020, 43, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Peretti, A.L.; Kakihata, C.M.M.; Tavares, A.L.F.; de Oliveira, C.M.T.; Guimaraes, A.T.B.; Costa, R.M.; Ribeiro, L.F.C.; Bertolini, G.R.F. Short-term effects of whole-body vibration on the soleus of ooforectomized rats: Histomorphometric analysis and oxidative stress in an animal model. Acta Histochem. 2020, 122, 151598. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Peng, N.; Zhou, M.; Liu, P.P.; Qi, X.L.; Wang, N.; Wang, G.; Wu, Z.P. Tai Chi and whole-body vibrating therapy in sarcopenic men in advanced old age: A clinical randomized controlled trial. Eur. J. Ageing 2019, 16, 273–282. [Google Scholar] [CrossRef]
- Sapoval, J.; Singh, V.; Carter, R.E. Ultrasound Biophysical Profile; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Miller, D.L.; Smith, N.B.; Bailey, M.R.; Czarnota, G.J.; Hynynen, K.; Makin, I.R.; Bioeffects Committee of the American Institute of Ultrasound in Medicine. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012, 31, 623–634. [Google Scholar] [CrossRef]
- Carovac, A.; Smajlovic, F.; Junuzovic, D. Application of ultrasound in medicine. Acta Inform. Med. 2011, 19, 168–171. [Google Scholar] [CrossRef]
- Kingwill, A.; Barker, G.; Wong, A. Point-of-care ultrasound: Its growing application in hospital medicine. Br. J. Hosp. Med. 2017, 78, 492–496. [Google Scholar] [CrossRef]
- Lele, P.P. Application of ultrasound in medicine. N. Engl. J. Med. 1972, 286, 1317–1318. [Google Scholar] [CrossRef]
- Cheung, V.Y.T. High-intensity focused ultrasound therapy. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 46, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Popert, R. High-intensity focussed ultrasound. Clin. Oncol. R. Coll. Radiol. 2011, 23, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Quadri, S.A.; Waqas, M.; Khan, I.; Khan, M.A.; Suriya, S.S.; Farooqui, M.; Fiani, B. High-intensity focused ultrasound: Past, present, and future in neurosurgery. Neurosurg. Focus 2018, 44, E16. [Google Scholar] [CrossRef]
- Ryan, P.; Finelli, A.; Lawrentschuk, N.; Fleshner, N.; Sweet, J.; Cheung, C.; van der Kwast, T.; Evans, A. Prostatic needle biopsies following primary high intensity focused ultrasound (HIFU) therapy for prostatic adenocarcinoma: Histopathological features in tumour and non-tumour tissue. J. Clin. Pathol. 2012, 65, 729–734. [Google Scholar] [CrossRef]
- Jiang, X.; Savchenko, O.; Li, Y.; Qi, S.; Yang, T.; Zhang, W.; Chen, J. A Review of Low-Intensity Pulsed Ultrasound for Therapeutic Applications. IEEE Trans. Biomed. Eng. 2019, 66, 2704–2718. [Google Scholar] [CrossRef]
- Mason, T.J. Therapeutic ultrasound an overview. Ultrason. Sonochem. 2011, 18, 847–852. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, S.; Lv, Z.; Kan, S.; Wu, Q.; Song, W.; Ning, G.; Feng, S. Effects of therapeutic ultrasound for knee osteoarthritis: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 1863–1875. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.K.; Sehgal, C.M. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med. Biol. 2015, 41, 905–928. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Zhang, X.X.; Yu, G.Y.; Zhang, Z.C.; Wang, F.; Yang, Y.L.; Li, M.; Wei, X.Z. Effects of Low-Intensity Pulsed Ultrasound on Knee Osteoarthritis: A Meta-Analysis of Randomized Clinical Trials. Biomed. Res. Int. 2018, 2018, 7469197. [Google Scholar] [CrossRef]
- Bowary, P.; Greenberg, B.D. Noninvasive Focused Ultrasound for Neuromodulation: A Review. Psychiatr. Clin. N. Am. 2018, 41, 505–514. [Google Scholar] [CrossRef] [PubMed]
- D’Vaz, A.P.; Ostor, A.J.; Speed, C.A.; Jenner, J.R.; Bradley, M.; Prevost, A.T.; Hazleman, B.L. Pulsed low-intensity ultrasound therapy for chronic lateral epicondylitis: A randomized controlled trial. Rheumatology 2006, 45, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, S.; Matsui, N.; Kokubu, T.; Tsunoda, M.; Fujioka, H.; Mizuno, K.; Azuma, Y. Low-intensity pulsed ultrasound initiates bone healing in rat nonunion fracture model. J. Ultrasound Med. 2001, 20, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Behrens, S.; Spengos, K.; Daffertshofer, M.; Wirth, S.; Hennerici, M. Potential use of therapeutic ultrasound in ischemic stroke treatment. Echocardiography 2001, 18, 259–263. [Google Scholar] [CrossRef]
- Daffertshofer, M.; Fatar, M. Therapeutic ultrasound in ischemic stroke treatment: Experimental evidence. Eur. J. Ultrasound 2002, 16, 121–130. [Google Scholar] [CrossRef]
- Machet, L.; Boucaud, A. Phonophoresis: Efficiency, mechanisms and skin tolerance. Int. J. Pharm. 2002, 243, 1–15. [Google Scholar] [CrossRef]
- Ahmadi, F.; McLoughlin, I.V.; Chauhan, S.; ter-Haar, G. Bio-effects and safety of low-intensity, low-frequency ultrasonic exposure. Prog. Biophys. Mol. Biol. 2012, 108, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Feril, L.B., Jr.; Tachibana, K.; Ikeda-Dantsuji, Y.; Endo, H.; Harada, Y.; Kondo, T.; Ogawa, R. Therapeutic potential of low-intensity ultrasound (part 2): Biomolecular effects, sonotransfection, and sonopermeabilization. J. Med. Ultrason. 2008, 35, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Feril, L.B., Jr.; Tachibana, K.; Ogawa, K.; Yamaguchi, K.; Solano, I.G.; Irie, Y. Therapeutic potential of low-intensity ultrasound (part 1): Thermal and sonomechanical effects. J. Med. Ultrason. 2008, 35, 153–160. [Google Scholar] [CrossRef]
- De Lucas, B.; Perez, L.M.; Bernal, A.; Galvez, B.G. Ultrasound Therapy: Experiences and Perspectives for Regenerative Medicine. Genes 2020, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.M.Z.; Komatsu, D.E. Therapeutic Potential Low-Intensity Pulsed Ultrasound for Osteoarthritis: Pre-clinical and Clinical Perspectives. Ultrasound Med. Biol. 2020, 46, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Lin, G.; Lei, H.; Lue, T.F.; Guo, Y. Clinical applications of low-intensity pulsed ultrasound and its potential role in urology. Transl. Androl. Urol. 2016, 5, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chow, S.K.; Leung, K.S.; Cheung, W.H. Ultrasound as a stimulus for musculoskeletal disorders. J. Orthop. Transl. 2017, 9, 52–59. [Google Scholar] [CrossRef]
- Ennis, W.J.; Valdes, W.; Gainer, M.; Meneses, P. Evaluation of clinical effectiveness of MIST ultrasound therapy for the healing of chronic wounds. Adv. Skin Wound Care 2006, 19, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, G.N.; Pires-De-Campos, M.S.; Leonardi, G.R.; Dib-Giusti, H.H.; Polacow, M.L. Effect of ultrasound and dexpanthenol on collagen organization in tegumentary lesions. Rev. Bras. Fisioter. 2011, 15, 227–232. [Google Scholar] [PubMed]
- Zhou, S.; Schmelz, A.; Seufferlein, T.; Li, Y.; Zhao, J.; Bachem, M.G. Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts. J. Biol. Chem. 2004, 279, 54463–54469. [Google Scholar] [CrossRef] [PubMed]
- Collins, C. Wound Care and Healing. Available online: https://www.defensemedianetwork.com/stories/wound-care-and-healing/ (accessed on 2 May 2021).
- Jarvinen, T.A.; Jarvinen, T.L.; Kaariainen, M.; Aarimaa, V.; Vaittinen, S.; Kalimo, H.; Jarvinen, M. Muscle injuries: Optimising recovery. Best Pract. Res. Clin. Rheumatol. 2007, 21, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Altland, O.D.; Dalecki, D.; Suchkova, V.N.; Francis, C.W. Low-intensity ultrasound increases endothelial cell nitric oxide synthase activity and nitric oxide synthesis. J. Thromb. Haemost. 2004, 2, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Viljoen, H.J.; Subramanian, A. Continuous low-intensity ultrasound attenuates IL-6 and TNFalpha-induced catabolic effects and repairs chondral fissures in bovine osteochondral explants. BMC Musculoskelet. Disord. 2019, 20, 193. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Mizuno, S.; Nakayama, N.; Iwaki, T.; Murakami, E.; Wang, Z.; Endoh, R.; Furuhata, H. Nitric oxide generation directly responds to ultrasound exposure. Ultrasound Med. Biol. 2008, 34, 487–493. [Google Scholar] [CrossRef]
- Huang, J.J.; Shi, Y.Q.; Li, R.L.; Hu, A.; Lu, Z.Y.; Weng, L.; Wang, S.Q.; Han, Y.P.; Zhang, L.; Li, B.; et al. Angiogenesis effect of therapeutic ultrasound on HUVECs through activation of the PI3K-Akt-eNOS signal pathway. Am. J. Transl. Res. 2015, 7, 1106–1115. [Google Scholar]
- Karnes, J.L.; Burton, H.W. Continuous therapeutic ultrasound accelerates repair of contraction-induced skeletal muscle damage in rats. Arch. Phys. Med. Rehabil. 2002, 83, 1–4. [Google Scholar] [CrossRef]
- Vasquez, B.; Navarrete, J.; Farfan, E.; Cantin, M. Effect of pulsed and continuous therapeutic ultrasound on healthy skeletal muscle in rats. Int. J. Clin. Exp. Pathol. 2014, 7, 779–783. [Google Scholar]
- Best, T.M.; Moore, B.; Jarit, P.; Moorman, C.T.; Lewis, G.K. Sustained acoustic medicine: Wearable, long duration ultrasonic therapy for the treatment of tendinopathy. Phys. Sportsmed. 2015, 43, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Best, T.M.; Wilk, K.E.; Moorman, C.T.; Draper, D.O. Low Intensity Ultrasound for Promoting Soft Tissue Healing: A Systematic Review of the Literature and Medical Technology. Intern. Med. Rev. 2016, 2, 271. [Google Scholar]
- Wiltink, A.; Nijweide, P.J.; Oosterbaan, W.A.; Hekkenberg, R.T.; Helders, P.J. Effect of therapeutic ultrasound on endochondral ossification. Ultrasound Med. Biol. 1995, 21, 121–127. [Google Scholar] [CrossRef]
- Noriega, S.; Mamedov, T.; Turner, J.A.; Subramanian, A. Intermittent applications of continuous ultrasound on the viability, proliferation, morphology, and matrix production of chondrocytes in 3D matrices. Tissue Eng. 2007, 13, 611–618. [Google Scholar] [CrossRef]
- Sahu, N.; Miller, A.; Viljoen, H.J.; Subramanian, A. Continuous Low-Intensity Ultrasound Promotes Native-to-Native Cartilage Integration. Tissue Eng. Part A 2019, 25, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, K.J.; Finucane, S.D.; Owen, J.R.; Wayne, J.S. The effects of low-intensity ultrasound on medial collateral ligament healing in the rabbit model. Am. J. Sports Med. 2005, 33, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Draper, D.O.; Wells, A.; Wilk, K. Efficacy of Sustained Acoustic Medicine as an Add-on to Traditional Therapy in Treating Sport-related Injuries: Case Reports. Glob. J. Orthop. Res. 2020, 2, 545. [Google Scholar]
- Ennis, W.J.; Foremann, P.; Mozen, N.; Massey, J.; Conner-Kerr, T.; Meneses, P. Ultrasound therapy for recalcitrant diabetic foot ulcers: Results of a randomized, double-blind, controlled, multicenter study. Ostomy Wound Manag. 2005, 51, 24–39. [Google Scholar]
- Rubin, C.; Bolander, M.; Ryaby, J.P.; Hadjiargyrou, M. The use of low-intensity ultrasound to accelerate the healing of fractures. J. Bone Jt. Surg. Am. 2001, 83, 259–270. [Google Scholar] [CrossRef]
- Higgins, A.; Glover, M.; Yang, Y.; Bayliss, S.; Meads, C.; Lord, J. EXOGEN ultrasound bone healing system for long bone fractures with non-union or delayed healing: A NICE medical technology guidance. Appl. Health Econ. Health Policy 2014, 12, 477–484. [Google Scholar] [CrossRef]
- El-Bialy, T.H.; Elgazzar, R.F.; Megahed, E.E.; Royston, T.J. Effects of ultrasound modes on mandibular osteodistraction. J. Dent. Res. 2008, 87, 953–957. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef] [PubMed]
- Landry, B.W.; Fischer, P.R.; Driscoll, S.W.; Koch, K.M.; Harbeck-Weber, C.; Mack, K.J.; Wilder, R.T.; Bauer, B.A.; Brandenburg, J.E. Managing Chronic Pain in Children and Adolescents: A Clinical Review. PM&R 2015, 7, S295–S315. [Google Scholar]
- Gaskin, D.J.; Richard, P. The economic costs of pain in the United States. J. Pain 2012, 13, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Hylands-White, N.; Duarte, R.V.; Raphael, J.H. An overview of treatment approaches for chronic pain management. Rheumatol. Int. 2017, 37, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Bell, A. The neurobiology of acute pain. Vet. J. 2018, 237, 55–62. [Google Scholar] [CrossRef]
- Aiyer, R.; Noori, S.A.; Chang, K.V.; Jung, B.; Rasheed, A.; Bansal, N.; Ottestad, E.; Gulati, A. Therapeutic Ultrasound for Chronic Pain Management in Joints: A Systematic Review. Pain Med. 2020, 21, 1437–1448. [Google Scholar] [CrossRef]
- Ay, S.; Dogan, S.K.; Evcik, D.; Baser, O.C. Comparison the efficacy of phonophoresis and ultrasound therapy in myofascial pain syndrome. Rheumatol. Int. 2011, 31, 1203–1208. [Google Scholar] [CrossRef]
- Cetin, N.; Aytar, A.; Atalay, A.; Akman, M.N. Comparing hot pack, short-wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: A single-blind, randomized, controlled trial. Am. J. Phys. Med. Rehabil. 2008, 87, 443–451. [Google Scholar] [CrossRef]
- Chung, J.I.; Min, B.H.; Baik, E.J. Effect of Continuous-Wave Low-Intensity Ultrasound in Inflammatory Resolution of Arthritis-Associated Synovitis. Phys. Ther. 2016, 96, 808–817. [Google Scholar] [CrossRef]
- Draper, D.O.; Mahaffey, C.; Kaiser, D.; Eggett, D.; Jarmin, J. Thermal ultrasound decreases tissue stiffness of trigger points in upper trapezius muscles. Physiother. Theory Pract. 2010, 26, 167–172. [Google Scholar] [CrossRef]
- Ebadi, S.; Ansari, N.N.; Henschke, N.; Naghdi, S.; van Tulder, M.W. The effect of continuous ultrasound on chronic low back pain: Protocol of a randomized controlled trial. BMC Musculoskelet. Disord. 2011, 12, 59. [Google Scholar] [CrossRef]
- Ebadi, S.; Ansari, N.N.; Naghdi, S.; Jalaei, S.; Sadat, M.; Bagheri, H.; Vantulder, M.W.; Henschke, N.; Fallah, E. The effect of continuous ultrasound on chronic non-specific low back pain: A single blind placebo-controlled randomized trial. BMC Musculoskelet. Disord. 2012, 13, 192. [Google Scholar] [CrossRef]
- Goren, A.; Yildiz, N.; Topuz, O.; Findikoglu, G.; Ardic, F. Efficacy of exercise and ultrasound in patients with lumbar spinal stenosis: A prospective randomized controlled trial. Clin. Rehabil. 2010, 24, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Said Ahmed, H.-A.S.; Ali, O.I.; ElLaithy, M.H.G. Continuous versus pulsed ultrasound on myofascial pain syndrome: Randomized single blind controlled trial. Int. J. Ther. Rehabil. Res. 2017, 17, 2. [Google Scholar]
- Ilter, L.; Dilek, B.; Batmaz, I.; Ulu, M.A.; Sariyildiz, M.A.; Nas, K.; Cevik, R. Efficacy of Pulsed and Continuous Therapeutic Ultrasound in Myofascial Pain Syndrome: A Randomized Controlled Study. Am. J. Phys. Med. Rehabil. 2015, 94, 547–554. [Google Scholar] [CrossRef]
- Petterson, S.; Plancher, K.; Klyve, D.; Draper, D.; Ortiz, R. Low-Intensity Continuous Ultrasound for the Symptomatic Treatment of Upper Shoulder and Neck Pain: A Randomized, Double-Blind Placebo-Controlled Clinical Trial. J. Pain Res. 2020, 13, 1277. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P. Management of myofascial pain of upper trapezius: A three group comparison study. Glob. J. Health Sci. 2012, 4, 46–52. [Google Scholar] [CrossRef]
- Lewis, G.; Guarino, S.; Ortiz, R. Wearable long-duration ultrasound treatment of chronic trapezius myalgia. J. Pain 2012, 13, S94. [Google Scholar] [CrossRef]
- Lewis, G.K., Jr.; Langer, M.D.; Henderson, C.R., Jr.; Ortiz, R. Design and evaluation of a wearable self-applied therapeutic ultrasound device for chronic myofascial pain. Ultrasound Med. Biol. 2013, 39, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Manca, A.; Limonta, E.; Pilurzi, G.; Ginatempo, F.; De Natale, E.R.; Mercante, B.; Tolu, E.; Deriu, F. Ultrasound and laser as stand-alone therapies for myofascial trigger points: A randomized, double-blind, placebo-controlled study. Physiother. Res. Int. 2014, 19, 166–175. [Google Scholar] [CrossRef]
- Noori, S.A.; Rasheed, A.; Aiyer, R.; Jung, B.; Bansal, N.; Chang, K.V.; Ottestad, E.; Gulati, A. Therapeutic Ultrasound for Pain Management in Chronic Low Back Pain and Chronic Neck Pain: A Systematic Review. Pain Med. 2020, 21, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, M.A.; Ones, K.; Goksenoglu, G. Effectiveness of Ultrasound Therapy on Myofascial Pain Syndrome of the Upper Trapezius: Randomized, Single-Blind, Placebo-Controlled Study. Arch. Rheumatol. 2018, 33, 418–423. [Google Scholar] [CrossRef]
- Muftic, M.; Miladinovic, K. Therapeutic ultrasound and pain in degenerative diseases of musculoskeletal system. Acta Inform. Med. 2013, 21, 170–172. [Google Scholar] [CrossRef]
- Srbely, J.Z.; Dickey, J.P.; Lowerison, M.; Edwards, A.M.; Nolet, P.S.; Wong, L.L. Stimulation of myofascial trigger points with ultrasound induces segmental antinociceptive effects: A randomized controlled study. Pain 2008, 139, 260–266. [Google Scholar] [CrossRef]
- Warden, S.J.; Metcalf, B.R.; Kiss, Z.S.; Cook, J.L.; Purdam, C.R.; Bennell, K.L.; Crossley, K.M. Low-intensity pulsed ultrasound for chronic patellar tendinopathy: A randomized, double-blind, placebo-controlled trial. Rheumatology 2008, 47, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.A.; Bradley, J.; Walsh, D.M.; Baxter, G.D.; Allen, J.M. Delayed onset muscle soreness: Lack of effect of therapeutic ultrasound in humans. Arch. Phys. Med. Rehabil. 1999, 80, 318–323. [Google Scholar] [CrossRef]
- Huang, M.H.; Lin, Y.S.; Lee, C.L.; Yang, R.C. Use of ultrasound to increase effectiveness of isokinetic exercise for knee osteoarthritis. Arch. Phys. Med. Rehabil. 2005, 86, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Yang, R.C.; Lee, C.L.; Chen, T.W.; Wang, M.C. Preliminary results of integrated therapy for patients with knee osteoarthritis. Arthritis Rheum. 2005, 53, 812–820. [Google Scholar] [CrossRef]
- Langer, M.D.; Levine, V.; Taggart, R.; Lewis, G.K.; Hernandez, L.; Ortiz, R. Pilot Clinical Studies of Long Duration, Low Intensity Therapeutic Ultrasound for Osteoarthritis. In Proceedings of the 40th Annual Northeast Bioengineering Conference (NEBEC), Boston, MA, USA, 25–27 April 2014. [Google Scholar]
- Zeng, C.; Li, H.; Yang, T.; Deng, Z.H.; Yang, Y.; Zhang, Y.; Ding, X.; Lei, G.H. Effectiveness of continuous and pulsed ultrasound for the management of knee osteoarthritis: A systematic review and network meta-analysis. Osteoarthr. Cartil. 2014, 22, 1090–1099. [Google Scholar] [CrossRef]
- Park, S.R.; Jang, K.W.; Park, S.H.; Cho, H.S.; Jin, C.Z.; Choi, M.J.; Chung, S.I.; Min, B.H. The effect of sonication on simulated osteoarthritis. Part I: Effects of 1 MHz ultrasound on uptake of hyaluronan into the rabbit synovium. Ultrasound Med. Biol. 2005, 31, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Park, S.R.; Park, S.H.; Jang, K.W.; Cho, H.S.; Cui, J.H.; An, H.J.; Choi, M.J.; Chung, S.I.; Min, B.H. The effect of sonication on simulated osteoarthritis. Part II: Alleviation of osteoarthritis pathogenesis by 1 MHz ultrasound with simultaneous hyaluronate injection. Ultrasound Med. Biol. 2005, 31, 1559–1566. [Google Scholar] [CrossRef]
- Uddin, S.M.; Richbourgh, B.; Ding, Y.; Hettinghouse, A.; Komatsu, D.E.; Qin, Y.X.; Liu, C.J. Chondro-protective effects of low intensity pulsed ultrasound. Osteoarthr. Cartil. 2016, 24, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Tascioglu, F.; Kuzgun, S.; Armagan, O.; Ogutler, G. Short-term effectiveness of ultrasound therapy in knee osteoarthritis. J. Int. Med. Res. 2010, 38, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Draper, D.O.; Klyve, D.; Ortiz, R.; Best, T.M. Effect of low-intensity long-duration ultrasound on the symptomatic relief of knee osteoarthritis: A randomized, placebo-controlled double-blind study. J. Orthop. Surg. Res. 2018, 13, 257. [Google Scholar] [CrossRef]
- Fomenko, A.; Neudorfer, C.; Dallapiazza, R.F.; Kalia, S.K.; Lozano, A.M. Low-intensity ultrasound neuromodulation: An overview of mechanisms and emerging human applications. Brain Stimul. 2018, 11, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Babakhanian, M.; Yang, L.; Nowroozi, B.; Saddik, G.; Boodaghians, L.; Blount, P.; Grundfest, W. Effects of Low Intensity Focused Ultrasound on Liposomes Containing Channel proteins. Sci. Rep. 2018, 8, 17250. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Lee, J.M.; Kim, H.B.; Lee, J.; Han, S.; Bae, J.Y.; Hong, G.S.; Koh, W.; Kwon, J.; Hwang, E.S.; et al. Ultrasonic Neuromodulation via Astrocytic TRPA1. Curr. Biol. 2019, 29, 3386–3401. [Google Scholar] [CrossRef]
- Kim, H.B.; Swanberg, K.M.; Han, H.S.; Kim, J.C.; Kim, J.W.; Lee, S.; Lee, C.J.; Maeng, S.; Kim, T.S.; Park, J.H. Prolonged stimulation with low-intensity ultrasound induces delayed increases in spontaneous hippocampal culture spiking activity. J. Neurosci. Res. 2017, 95, 885–896. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Yan, J.; Wang, X.; Yuan, Y.; Li, X. Seizure control by low-intensity ultrasound in mice with temporal lobe epilepsy. Epilepsy Res. 2019, 154, 1–7. [Google Scholar] [CrossRef]
- Blackmore, J.; Shrivastava, S.; Sallet, J.; Butler, C.R.; Cleveland, R.O. Ultrasound Neuromodulation: A Review of Results, Mechanisms and Safety. Ultrasound Med. Biol. 2019, 45, 1509–1536. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, Y.; Shi, A.; Zhang, L.; Guo, S.; Wan, M. Neuroprotective Effect of Low-Intensity Pulsed Ultrasound Against MPP(+)-Induced Neurotoxicity in PC12 Cells: Involvement of K2P Channels and Stretch-Activated Ion Channels. Ultrasound Med. Biol. 2017, 43, 1986–1999. [Google Scholar] [CrossRef]
- Liu, S.H.; Lai, Y.L.; Chen, B.L.; Yang, F.Y. Ultrasound Enhances the Expression of Brain-Derived Neurotrophic Factor in Astrocyte Through Activation of TrkB-Akt and Calcium-CaMK Signaling Pathways. Cereb. Cortex 2017, 27, 3152–3160. [Google Scholar] [CrossRef]
- King, R.L.; Brown, J.R.; Newsome, W.T.; Pauly, K.B. Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med. Biol. 2013, 39, 312–331. [Google Scholar] [CrossRef]
- Kim, H.; Chiu, A.; Lee, S.D.; Fischer, K.; Yoo, S.S. Focused ultrasound-mediated non-invasive brain stimulation: Examination of sonication parameters. Brain Stimul. 2014, 7, 748–756. [Google Scholar] [CrossRef]
- Downs, M.E.; Lee, S.A.; Yang, G.; Kim, S.; Wang, Q.; Konofagou, E.E. Non-invasive peripheral nerve stimulation via focused ultrasound in vivo. Phys. Med. Biol. 2018, 63, 035011. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.J.; Wang, X.D.; Zhao, Y.H.; Sun, H.L.; Hu, Y.M.; Yao, J.; Wang, Y. The Effect of Low-Intensity Ultrasound on Brain-Derived Neurotropic Factor Expression in a Rat Sciatic Nerve Crushed Injury Model. Ultrasound Med. Biol. 2017, 43, 461–468. [Google Scholar] [CrossRef]
- Gavrilov, L.R. Use of focused ultrasound for stimulation of nerve structures. Ultrasonics 1984, 22, 132–138. [Google Scholar] [CrossRef]
- Kim, T.; Park, C.; Chhatbar, P.Y.; Feld, J.; Mac Grory, B.; Nam, C.S.; Wang, P.; Chen, M.; Jiang, X.; Feng, W. Effect of Low Intensity Transcranial Ultrasound Stimulation on Neuromodulation in Animals and Humans: An Updated Systematic Review. Front. Neurosci. 2021, 15, 620863. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, J.L.; Hameroff, S.; Smith, E.E.; Sato, T.; Daft, C.M.W.; Tyler, W.J.; Allen, J.J.B. Transcranial Focused Ultrasound to the Right Prefrontal Cortex Improves Mood and Alters Functional Connectivity in Humans. Front. Hum. Neurosci. 2020, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, H.; Lee, S.; Yoo, S.S.; Chung, Y.A. Creation of various skin sensations using pulsed focused ultrasound: Evidence for functional neuromodulation. Int. J. Imaging Syst. Technol. 2014, 24, 167–174. [Google Scholar] [CrossRef]
- Daffertshofer, M.; Hennerici, M.G. Sonothrombolysis: Experimental evidence. Front. Neurol. Neurosci. 2006, 21, 140–149. [Google Scholar] [PubMed]
- Francis, C.W. Ultrasound-enhanced thrombolysis. Echocardiography 2001, 18, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Nedelmann, M.; Eicke, B.M.; Lierke, E.G.; Heimann, A.; Kempski, O.; Hopf, H.C. Low-frequency ultrasound induces nonenzymatic thrombolysis in vitro. J. Ultrasound Med. 2002, 21, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Saguchi, T.; Onoue, H.; Urashima, M.; Ishibashi, T.; Abe, T.; Furuhata, H. Effective and safe conditions of low-frequency transcranial ultrasonic thrombolysis for acute ischemic stroke: Neurologic and histologic evaluation in a rat middle cerebral artery stroke model. Stroke 2008, 39, 1007–1011. [Google Scholar] [CrossRef]
- Wu, J.; Xie, F.; Kumar, T.; Liu, J.; Lof, J.; Shi, W.; Everbach, E.C.; Porter, T.R. Improved sonothrombolysis from a modified diagnostic transducer delivering impulses containing a longer pulse duration. Ultrasound Med. Biol. 2014, 40, 1545–1553. [Google Scholar] [CrossRef]
- Blinc, A.; Francis, C.W.; Trudnowski, J.L.; Carstensen, E.L. Characterization of ultrasound-potentiated fibrinolysis in vitro. Blood 1993, 81, 2636–2643. [Google Scholar] [CrossRef]
- Trubestein, G.; Engel, C.; Etzel, F.; Sobbe, A.; Cremer, H.; Stumpff, U. Thrombolysis by ultrasound. Clin. Sci. Mol. Med. 1976, 3, 697s–698s. [Google Scholar] [CrossRef]
- Riggs, P.N.; Francis, C.W.; Bartos, S.R.; Penney, D.P. Ultrasound enhancement of rabbit femoral artery thrombolysis. Cardiovasc. Surg. 1997, 5, 201–207. [Google Scholar] [CrossRef]
- Suchkova, V.N.; Baggs, R.B.; Francis, C.W. Effect of 40-kHz ultrasound on acute thrombotic ischemia in a rabbit femoral artery thrombosis model: Enhancement of thrombolysis and improvement in capillary muscle perfusion. Circulation 2000, 101, 2296–2301. [Google Scholar] [CrossRef]
- Nedelmann, M.; Brandt, C.; Schneider, F.; Eicke, B.M.; Kempski, O.; Krummenauer, F.; Dieterich, M. Ultrasound-induced blood clot dissolution without a thrombolytic drug is more effective with lower frequencies. Cerebrovasc. Dis. 2005, 20, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, J. Transcranial Pulsed Ultrasound with Alteplase Intravenous Thrombolysis for Vascular Recanalisation in Acute Ischemic Stroke. J. Coll. Physicians Surg. Pak. 2020, 30, 765–767. [Google Scholar] [PubMed]
- Aguiar, M.O.D.; Tavares, B.G.; Tsutsui, J.M.; Fava, A.M.; Borges, B.C.; Oliveira, M.T., Jr.; Soeiro, A.; Nicolau, J.C.; Ribeiro, H.B.; Chiang, H.P.; et al. Sonothrombolysis Improves Myocardial Dynamics and Microvascular Obstruction Preventing Left Ventricular Remodeling in Patients with ST Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2020, 13, e009536. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Feiszthuber, H.; Bhatnagar, S.; Gyongy, M.; Coussios, C.C. Cavitation-enhanced delivery of insulin in agar and porcine models of human skin. Phys. Med. Biol. 2015, 60, 2421–2434. [Google Scholar] [CrossRef][Green Version]
- Seah, B.C.; Teo, B.M. Recent advances in ultrasound-based transdermal drug delivery. Int. J. Nanomed. 2018, 13, 7749–7763. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, M.; Tachibana, K.; Pandit, A.B.; Yasui, K.; Tuziuti, T.; Towata, A.; Iida, Y. Transdermal drug delivery using ultrasound-theory, understanding and critical analysis. Cell Mol. Biol. 2005, 51, OL767–OL784. [Google Scholar]
- Smith, N.B. Perspectives on transdermal ultrasound mediated drug delivery. Int. J. Nanomed. 2007, 2, 585–594. [Google Scholar]
- Langer, M.S.L.; Fleshman, S.; Lewis, G. “SonoBandage” a transdermal ultrasound drug delivery system for peripheral neuropathy. Proc. Meet. Acoust. 2013, 19, 75074. [Google Scholar]
- Miyazaki, S.; Mizuoka, H.; Kohata, Y.; Takada, M. External control of drug release and penetration. VI. Enhancing effect of ultrasound on the transdermal absorption of indomethacin from an ointment in rats. Chem. Pharm. Bull. 1992, 40, 2826–2830. [Google Scholar] [CrossRef]
- Feril, L.B., Jr.; Kondo, T. Biological effects of low intensity ultrasound: The mechanism involved, and its implications on therapy and on biosafety of ultrasound. J. Radiat. Res. 2004, 45, 479–489. [Google Scholar] [CrossRef]
- Dromi, S.; Frenkel, V.; Luk, A.; Traughber, B.; Angstadt, M.; Bur, M.; Poff, J.; Xie, J.; Libutti, S.K.; Li, K.C.; et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin. Cancer Res. 2007, 13, 2722–2727. [Google Scholar] [CrossRef]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef]
- Dudelzak, J.; Hussain, M.; Phelps, R.G.; Gottlieb, G.J.; Goldberg, D.J. Evaluation of histologic and electron microscopic changes after novel treatment using combined microdermabrasion and ultrasound-induced phonophoresis of human skin. J. Cosmet. Laser Ther. 2008, 10, 187–192. [Google Scholar] [CrossRef]
- Cagnie, B.; Vinck, E.; Rimbaut, S.; Vanderstraeten, G. Phonophoresis versus topical application of ketoprofen: Comparison between tissue and plasma levels. Phys. Ther. 2003, 83, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Aldwaikat, M.; Alarjah, M. Investigating the sonophoresis effect on the permeation of diclofenac sodium using 3D skin equivalent. Ultrason. Sonochem. 2015, 22, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Masterson, J.; Kluge, B.; Burdette, A.; Sr, G.L. Sustained acoustic medicine; sonophoresis for nonsteroidal anti-inflammatory drug delivery in arthritis. Ther. Deliv. 2020, 11, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Madzia, A.; Agrawal, C.; Jarit, P.; Petterson, S.; Plancher, K.; Ortiz, R. Sustained Acoustic Medicine Combined with A Diclofenac Ultrasound Coupling Patch for the Rapid Symptomatic Relief of Knee Osteoarthritis: Multi-Site Clinical Efficacy Study. Open Orthop. J. 2020, 14, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Schoellhammer, C.M.; Polat, B.E.; Mendenhall, J.; Maa, R.; Jones, B.; Hart, D.P.; Langer, R.; Blankschtein, D. Rapid skin permeabilization by the simultaneous application of dual-frequency, high-intensity ultrasound. J. Control. Release 2012, 163, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Schoellhammer, C.M.; Srinivasan, S.; Barman, R.; Mo, S.H.; Polat, B.E.; Langer, R.; Blankschtein, D. Applicability and safety of dual-frequency ultrasonic treatment for the transdermal delivery of drugs. J. Control. Release 2015, 202, 93–100. [Google Scholar] [CrossRef]
- Yin, L.; Qin, F.H.; Zhou, Y.; Qi, X. Enhancing percutaneous permeability of sinomenine hydrochloride using dual-frequency sonophoresis. J. Drug Deliv. Sci. Technol. 2016, 36, 5. [Google Scholar] [CrossRef]
- Kline-Schoder, A.; Le, Z.; Zderic, V. Ultrasound-Enhanced Ciclopirox Delivery for Treatment of Onychomycosis. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2018, 2018, 5717–5720. [Google Scholar]
- Zderic, V.; Clark, J.I.; Vaezy, S. Drug delivery into the eye with the use of ultrasound. J. Ultrasound Med. 2004, 23, 1349–1359. [Google Scholar] [CrossRef]
- Pong, M.; Umchid, S.; Guarino, A.J.; Lewin, P.A.; Litniewski, J.; Nowicki, A.; Wrenn, S.P. In vitro ultrasound-mediated leakage from phospholipid vesicles. Ultrasonics 2006, 45, 133–145. [Google Scholar] [CrossRef] [PubMed]
- ter Haar, G. Therapeutic applications of ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Nanda, S. Sonophoresis: Recent advancements and future trends. J. Pharm. Pharmacol. 2009, 61, 689–705. [Google Scholar] [CrossRef]
- Wood, A.K.; Ansaloni, S.; Ziemer, L.S.; Lee, W.M.; Feldman, M.D.; Sehgal, C.M. The antivascular action of physiotherapy ultrasound on murine tumors. Ultrasound Med. Biol. 2005, 31, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Sawai, Y.; Murata, H.; Koto, K.; Matsui, T.; Horie, N.; Ashihara, E.; Maekawa, T.; Fushiki, S.; Kubo, T. Effects of low-intensity pulsed ultrasound on osteosarcoma and cancer cells. Oncol. Rep. 2012, 28, 481–486. [Google Scholar] [CrossRef]
- Zeng, Q.; Hong, S.; Wang, X.; Cheng, Y.; Sun, J.; Xia, W. Regulation of exosomes secretion by low-intensity pulsed ultrasound in lung cancer cells. Exp. Cell Res. 2019, 383, 111448. [Google Scholar] [CrossRef]
- Yu, T.; Huang, X.; Hu, K.; Bai, J.; Wang, Z. Treatment of transplanted adriamycin-resistant ovarian cancers in mice by combination of adriamycin and ultrasound exposure. Ultrason. Sonochem. 2004, 11, 287–291. [Google Scholar] [CrossRef]
- Jin, Z.H.; Miyoshi, N.; Ishiguro, K.; Umemura, S.; Kawabata, K.; Yumita, N.; Sakata, I.; Takaoka, K.; Udagawa, T.; Nakajima, S.; et al. Combination effect of photodynamic and sonodynamic therapy on experimental skin squamous cell carcinoma in C3H/HeN mice. J. Dermatol. 2000, 27, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Barati, A.H.; Mokhtari-Dizaji, M.; Mozdarani, H.; Bathaie, S.Z.; Hassan, Z.M. Treatment of murine tumors using dual-frequency ultrasound in an experimental in vivo model. Ultrasound Med. Biol. 2009, 35, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Barati, A.H.; Mokhtari-Dizaji, M.; Mozdarani, H.; Bathaie, Z.; Hassan, Z.M. Effect of exposure parameters on cavitation induced by low-level dual-frequency ultrasound. Ultrason. Sonochem. 2007, 14, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Yamamoto, M.; Tachibana, K.; Ueno, Y.; Bu, G.; Fukushima, T. Mechanism of photofrin-enhanced ultrasound-induced human glioma cell death. Anticancer Res. 2009, 29, 897–905. [Google Scholar] [PubMed]
- Yang, S.; Wang, P.; Wang, X.; Su, X.; Liu, Q. Activation of microbubbles by low-level therapeutic ultrasound enhances the antitumor effects of doxorubicin. Eur. Radiol. 2014, 24, 2739–2753. [Google Scholar] [CrossRef] [PubMed]
- Staples, B.J.; Pitt, W.G.; Roeder, B.L.; Husseini, G.A.; Rajeev, D.; Schaalje, G.B. Distribution of doxorubicin in rats undergoing ultrasonic drug delivery. J. Pharm. Sci. 2010, 99, 3122–3131. [Google Scholar] [CrossRef] [PubMed]
- Loria, R.; Giliberti, C.; Bedini, A.; Palomba, R.; Caracciolo, G.; Ceci, P.; Falvo, E.; Marconi, R.; Falcioni, R.; Bossi, G.; et al. Very low intensity ultrasounds as a new strategy to improve selective delivery of nanoparticles-complexes in cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 1. [Google Scholar] [CrossRef]
- Li, X.H.; Zhou, P.; Wang, L.H.; Tian, S.M.; Qian, Y.; Chen, L.R.; Zhang, P. The targeted gene (KDRP-CD/TK) therapy of breast cancer mediated by SonoVue and ultrasound irradiation in vitro. Ultrasonics 2012, 52, 186–191. [Google Scholar] [CrossRef]
- Wood, A.K.; Schultz, S.M.; Lee, W.M.; Bunte, R.M.; Sehgal, C.M. Antivascular ultrasound therapy extends survival of mice with implanted melanomas. Ultrasound Med. Biol. 2010, 36, 853–857. [Google Scholar] [CrossRef]
- Tardoski, S.; Ngo, J.; Gineyts, E.; Roux, J.P.; Clezardin, P.; Melodelima, D. Low-intensity continuous ultrasound triggers effective bisphosphonate anticancer activity in breast cancer. Sci. Rep. 2015, 5, 16354. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ng, W.T.; Chen, J. A Miniaturized Low-Intensity Ultrasound Device for Wearable Medical Therapeutic Applications. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Langer, M.D.; Lewis, G.K., Jr. Sustained Acoustic Medicine: A Novel Long Duration Approach to Biomodulation Utilizing Low Intensity Therapeutic Ultrasound. Proc. SPIE Int. Soc. Opt. Eng. 2015, 9467, 94670I. [Google Scholar]
- Rubira, A.; Rubira, M.C.; Rubira, L.A.; Comachio, J.; Magalhaes, M.O.; Marques, A.P. Comparison of the effects of low-level laser and pulsed and continuous ultrasound on pain and physical disability in chronic non-specific low back pain: A randomized controlled clinical trial. Adv. Rheumatol. 2019, 59, 57. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, S.M.Z.; Komatsu, D.E.; Motyka, T.; Petterson, S. Low-Intensity Continuous Ultrasound Therapies—A Systematic Review of Current State-of-the-Art and Future Perspectives. J. Clin. Med. 2021, 10, 2698. https://doi.org/10.3390/jcm10122698
Uddin SMZ, Komatsu DE, Motyka T, Petterson S. Low-Intensity Continuous Ultrasound Therapies—A Systematic Review of Current State-of-the-Art and Future Perspectives. Journal of Clinical Medicine. 2021; 10(12):2698. https://doi.org/10.3390/jcm10122698
Chicago/Turabian StyleUddin, Sardar M. Z., David E. Komatsu, Thomas Motyka, and Stephanie Petterson. 2021. "Low-Intensity Continuous Ultrasound Therapies—A Systematic Review of Current State-of-the-Art and Future Perspectives" Journal of Clinical Medicine 10, no. 12: 2698. https://doi.org/10.3390/jcm10122698
APA StyleUddin, S. M. Z., Komatsu, D. E., Motyka, T., & Petterson, S. (2021). Low-Intensity Continuous Ultrasound Therapies—A Systematic Review of Current State-of-the-Art and Future Perspectives. Journal of Clinical Medicine, 10(12), 2698. https://doi.org/10.3390/jcm10122698





