Polycystic Ovary Syndrome Susceptibility Loci Inform Disease Etiological Heterogeneity
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of PCOS-Associated Genetic Variants, Traits and Disease Outcomes
2.2. Clustering Analysis
2.3. Trait and Disease Associations with Each Cluster
2.4. Mendelian Randomization Analysis
3. Results
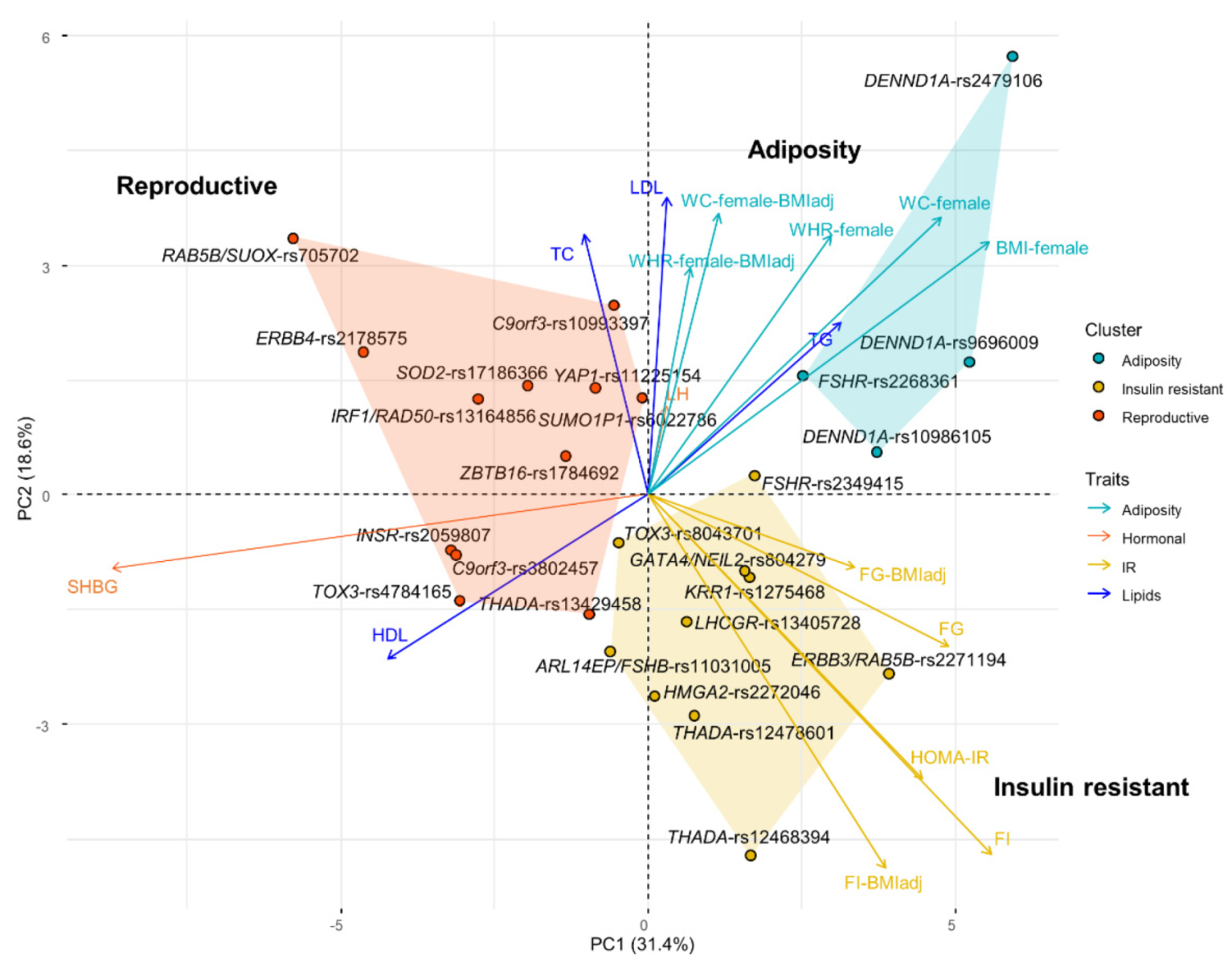
3.1. Clustering Suggests Mechanistic Heterogeneity for PCOS Etiology
3.2. Mendelian Randomization Suggests a Causal Role of SHBG and BMI on PCOS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Zawadzki, J.K.; Dunaif, A. Diagnostic Criteria for Polycystic Ovary Syndrome: Towards a Rational Approach; Dunaif, A.G.J., Haseltine, F.P., Merriam, G.R., Eds.; Blackwell Scientific Publications: Boston, MA, USA, 1992. [Google Scholar]
- Rotterdam ESHRE; ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE; ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Dapas, M.; Lin, F.T.J.; Nadkarni, G.N.; Sisk, R.; Legro, R.S.; Urbanek, M.; Hayes, M.G.; Dunaif, A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 2020, 17, e1003132. [Google Scholar] [CrossRef]
- Vink, J.M.; Sadrzadeh, S.; Lambalk, C.B.; Boomsma, D.I. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J. Clin. Endocrinol. Metab. 2006, 91, 2100–2104. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Zhao, H.; He, L.; Shi, Y.; Qin, Y.; Shi, Y.; Li, Z.; You, L.; Zhao, J.; Liu, J.; et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat. Genet. 2011, 43, 55–59. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, H.; Shi, Y.; Cao, Y.; Yang, D.; Li, Z.; Zhang, B.; Liang, X.; Li, T.; Chen, J.; et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat. Genet. 2012, 44, 1020–1025. [Google Scholar] [CrossRef]
- Day, F.R.; Hinds, D.A.; Tung, J.Y.; Stolk, L.; Styrkarsdottir, U.; Saxena, R.; Bjonnes, A.; Broer, L.; Dunger, D.B.; Halldorsson, B.V.; et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat. Commun. 2015, 6, 8464. [Google Scholar] [CrossRef]
- Hayes, M.G.; Urbanek, M.; Ehrmann, D.A.; Armstrong, L.L.; Lee, J.Y.; Sisk, R.; Karaderi, T.; Barber, T.M.; McCarthy, M.I.; Franks, S.; et al. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat. Commun. 2015, 6, 7502. [Google Scholar] [CrossRef] [PubMed]
- Day, F.; Karaderi, T.; Jones, M.R.; Meun, C.; He, C.; Drong, A.; Kraft, P.; Lin, N.; Huang, H.; Broer, L.; et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018, 14, e1007813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ho, K.; Keaton, J.M.; Hartzel, D.N.; Day, F.; Justice, A.E.; Josyula, N.S.; Pendergrass, S.A.; Actkins, K.; Davis, L.K.; et al. A genome-wide association study of polycystic ovary syndrome identified from electronic health records. Am. J. Obstet. Gynecol. 2020, 223, 559.e1–559.e21. [Google Scholar] [CrossRef]
- Udler, M.S.; Kim, J.; von Grotthuss, M.; Bonas-Guarch, S.; Cole, J.B.; Chiou, J.; on Behalf of METASTROKE and the ISGC; Boehnke, M.; Laakso, M.; Atzmon, G.; et al. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: A soft clustering analysis. PLoS Med. 2018, 15, e1002654. [Google Scholar] [CrossRef]
- Yaghootkar, H.; Scott, R.A.; White, C.C.; Zhang, W.; Speliotes, E.; Munroe, P.B.; Ehret, G.B.; Bis, J.C.; Fox, C.S.; Walker, M.; et al. Genetic evidence for a normal-weight “metabolically obese” phenotype linking insulin resistance, hypertension, coronary artery disease, and type 2 diabetes. Diabetes 2014, 63, 4369–4377. [Google Scholar] [CrossRef] [PubMed]
- Dimas, A.S.; Lagou, V.; Barker, A.; Knowles, J.W.; Magi, R.; Hivert, M.F.; Benazzo, A.; Rybin, D.; Jackson, A.U.; Stringham, H.M.; et al. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes 2014, 63, 2158–2171. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Shungin, D.; Winkler, T.W.; Croteau-Chonka, D.C.; Ferreira, T.; Locke, A.E.; Magi, R.; Strawbridge, R.J.; Pers, T.H.; Fischer, K.; Justice, A.E.; et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015, 518, 187–196. [Google Scholar] [CrossRef]
- Sun, B.B.; Maranville, J.C.; Peters, J.E.; Stacey, D.; Staley, J.R.; Blackshaw, J.; Burgess, S.; Jiang, T.; Paige, E.; Surendran, P.; et al. Genomic atlas of the human plasma proteome. Nature 2018, 558, 73–79. [Google Scholar] [CrossRef]
- Manning, A.K.; Hivert, M.F.; Scott, R.A.; Grimsby, J.L.; Bouatia-Naji, N.; Chen, H.; Rybin, D.; Liu, C.T.; Bielak, L.F.; Prokopenko, I.; et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat. Genet. 2012, 44, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.; Langenberg, C.; Prokopenko, I.; Saxena, R.; Soranzo, N.; Jackson, A.U.; Wheeler, E.; Glazer, N.L.; Bouatia-Naji, N.; Gloyn, A.L.; et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 2010, 42, 105–116. [Google Scholar] [CrossRef]
- Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; Buchkovich, M.L.; Mora, S.; et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013, 45, 1274–1283. [Google Scholar] [CrossRef]
- Xue, A.; Wu, Y.; Zhu, Z.; Zhang, F.; Kemper, K.E.; Zheng, Z.; Yengo, L.; Lloyd-Jones, L.R.; Sidorenko, J.; Wu, Y.; et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat. Commun. 2018, 9, 2941. [Google Scholar] [CrossRef] [PubMed]
- Van der Harst, P.; Verweij, N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ. Res. 2018, 122, 433–443. [Google Scholar] [CrossRef]
- Michailidou, K.; Lindstrom, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemacon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-base platform supports systematic causal inference across the human phenome. Elife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Poler, S.M.; Li, J.; Abedi, V.; Pendergrass, S.A.; Williams, M.S.; Lee, M.T.M. Dissecting genetic factors affecting phenylephrine infusion rates during anesthesia: A genome-wide association study employing EHR data. BMC Med. 2019, 17, 168. [Google Scholar] [CrossRef] [PubMed]
- Malika Charrad, N.G.; Boiteau, V.; Niknafs, A. NbClust: An R package for determining the relevant number of clusters in a data set. J. Stat. Softw. 2014, 61, 20875. [Google Scholar] [CrossRef]
- Lotta, L.A.; Gulati, P.; Day, F.R.; Payne, F.; Ongen, H.; van de Bunt, M.; Gaulton, K.J.; Eicher, J.D.; Sharp, S.J.; Luan, J.; et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 2017, 49, 17–26. [Google Scholar] [CrossRef]
- Wang, Q.; Holmes, M.V.; Davey Smith, G.; Ala-Korpela, M. Genetic support for a causal role of insulin resistance on circulating branched-chain amino acids and inflammation. Diabetes Care 2017, 40, 1779–1786. [Google Scholar] [CrossRef]
- Willer, C.J.; Li, Y.; Abecasis, G.R. METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010, 26, 2190–2191. [Google Scholar] [CrossRef]
- Li, L.; Cheng, W.Y.; Glicksberg, B.S.; Gottesman, O.; Tamler, R.; Chen, R.; Bottinger, E.P.; Dudley, J.T. Identification of type 2 diabetes subgroups through topological analysis of patient similarity. Sci. Transl. Med. 2015, 7, 311ra174. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Karajamaki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Y.; Wang, X.; Xu, L.; Chen, J.; Gao, C.; Wu, C.; Pan, D.; Zhang, Q.; Zhou, J.; et al. Body mass index and polycystic ovary syndrome: A 2-sample bidirectional mendelian randomization study. J. Clin. Endocrinol. Metab. 2020, 105, dgaa125. [Google Scholar] [CrossRef] [PubMed]
- Brower, M.A.; Hai, Y.; Jones, M.R.; Guo, X.; Chen, Y.I.; Rotter, J.I.; Krauss, R.M.; Legro, R.S.; Azziz, R.; Goodarzi, M.O. Bidirectional Mendelian randomization to explore the causal relationships between body mass index and polycystic ovary syndrome. Hum. Reprod. 2019, 34, 127–136. [Google Scholar] [CrossRef]
- Velazquez, E.M.; Mendoza, S.; Hamer, T.; Sosa, F.; Glueck, C.J. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 1994, 43, 647–654. [Google Scholar] [CrossRef]
- Facchinetti, F.; Unfer, V.; Dewailly, D.; Kamenov, Z.A.; Diamanti-Kandarakis, E.; Lagana, A.S.; Nestler, J.E.; Soulage, C.O.; Group of ‘Inositol in, PCOS and Reproduction’. Inositols in polycystic ovary syndrome: An overview on the advances. Trends Endocrinol. Metab. 2020, 31, 435–447. [Google Scholar] [CrossRef]
- Lagana, A.S.; Rossetti, P.; Sapia, F.; Chiofalo, B.; Buscema, M.; Valenti, G.; Rapisarda, A.M.C.; Vitale, S.G. Evidence-based and patient-oriented inositol treatment in polycystic ovary syndrome: Changing the perspective of the disease. Int. J. Endocrinol. Metab. 2017, 15, e43695. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, D.A.; Barnes, R.B.; Rosenfield, R.L.; Cavaghan, M.K.; Imperial, J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 1999, 22, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Legro, R.S.; Kunselman, A.R.; Dodson, W.C.; Dunaif, A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: A prospective, controlled study in 254 affected women. J. Clin. Endocrinol. Metab. 1999, 84, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.J.; Subtil, S.; Rodrigues, A.; Oliveira, J.; Figueiredo-Dias, M. Controversial association between polycystic ovary syndrome and breast cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 243, 125–132. [Google Scholar] [CrossRef]
- Harris, H.R.; Terry, K.L. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: A systematic review. Fertil. Res. Pract. 2016, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Mihm, M.; Gangooly, S.; Muttukrishna, S. The normal menstrual cycle in women. Anim. Reprod. Sci. 2011, 124, 229–236. [Google Scholar] [CrossRef] [PubMed]
| Adiposity | Insulin Resistant | Reproductive | ||||
|---|---|---|---|---|---|---|
| β | p-Value | β | p-Value | β | p-Value | |
| SHBG | −0.198 | 7.377 × 10−6 | −0.069 | 0.0052 | 0.111 | 1.70 × 10−6 |
| LH | 0.025 | 0.1462 | 0.002 | 0.8053 | −0.001 | 0.8898 |
| FI | 0.004 | 0.0855 | 0.005 | 1.93E-05 | −0.003 | 0.0101 |
| FI-BMI adj. | 0.002 | 0.3702 | 0.004 | 2.12E-05 | −0.001 | 0.1558 |
| FG | 0.004 | 0.0519 | 0.004 | 0.0004 | −0.002 | 0.0653 |
| FG-BMI adj. | 0.003 | 0.1947 | 0.003 | 0.0138 | −0.001 | 0.3067 |
| HOMA-IR | 0.003 | 0.2389 | 0.005 | 0.0013 | −0.003 | 0.0633 |
| HDL | −0.008 | 0.0035 | 0 | 0.9734 | 0.003 | 0.0411 |
| LDL | 0.003 | 0.335 | −0.004 | 0.0152 | −0.002 | 0.2336 |
| TG | 0.001 | 0.6019 | −0.003 | 0.0499 | −0.004 | 0.0207 |
| TC | −0.001 | 0.7885 | −0.006 | 0.0009 | −0.001 | 0.5062 |
| BMI | 0.015 | 2.59 × 10−7 | 0 | 0.9586 | −0.001 | 0.4855 |
| WC | 0.017 | 1.67 × 10−7 | 0 | 0.892 | 0.001 | 0.7129 |
| WC-BMI adj. | 0.01 | 0.001 | 0 | 0.8755 | 0.004 | 0.0264 |
| WHR | 0.011 | 0.0008 | −0.002 | 0.3264 | 0 | 0.8949 |
| WHR-BMI adj. | 0.006 | 0.0807 | −0.002 | 0.251 | 0.001 | 0.5162 |
| CAD | −0.007 | 0.1482 | 0 | 0.8988 | 0 | 0.8444 |
| T2DM | 0.009 | 0.0726 | −0.004 | 0.2279 | −0.005 | 0.0964 |
| Breast cancer | 0.014 | 0.0008 | 0.007 | 0.0106 | 0.006 | 0.0192 |
| ER positive | 0.012 | 0.0201 | 0.008 | 0.0146 | 0.005 | 0.0864 |
| ER negative | 0.016 | 0.0423 | 0.002 | 0.6789 | 0.003 | 0.4304 |
| Trait | Method | nSNV | β | SE | OR (95%CI) | p-Value |
|---|---|---|---|---|---|---|
| SHBG | MR Egger | 171 | −0.0140 | 0.0067 | 0.986 [0.973, 0.999] | 3.926 × 10−2 |
| SHBG | Weighted median | 171 | −0.0162 | 0.0052 | 0.984 [0.974, 0.994] | 1.785 × 10−3 |
| SHBG | IVW | 171 | −0.0120 | 0.0035 | 0.988 [0.981, 0.995] | 6.694 × 10−4 |
| BMI—female | MR Egger | 35 | 1.2206 | 0.3224 | 3.389 [1.802, 6.376] | 6.157 × 10−4 |
| BMI—female | Weighted median | 35 | 0.9210 | 0.1887 | 2.512 [1.735, 3.636] | 1.056 × 10−6 |
| BMI—female | IVW | 35 | 0.8842 | 0.1209 | 2.421 [1.910, 3.068] | 2.611 × 10−13 |
| Insulin resistance | MR Egger | 51 | 0.5460 | 0.4531 | 1.726 [0.710, 4.196] | 2.340 × 10−1 |
| Insulin resistance | Weighted median | 51 | 0.1011 | 0.2687 | 1.106 [0.653, 1.873] | 7.067 × 10−1 |
| Insulin resistance | IVW | 51 | 0.5267 | 0.2220 | 1.693 [1.096, 2.616] | 1.768 × 10−2 |
| WC—female | MR Egger | 18 | 0.9313 | 0.7627 | 2.538 [0.569, 11.316] | 2.398 × 10−1 |
| WC—female | Weighted median | 18 | 0.6591 | 0.2646 | 1.933 [1.151, 3.247] | 1.276 × 10−2 |
| WC—female | IVW | 18 | 0.5738 | 0.2112 | 1.775 [1.173, 2.685] | 6.596 × 10−3 |
| BMI adj. WC—female | MR Egger | 24 | 0.6659 | 0.7861 | 1.946 [0.417, 9.085] | 4.061 × 10−1 |
| BMI adj. WC—female | Weighted median | 24 | 0.2865 | 0.2213 | 1.332 [0.863, 2.055] | 1.955 × 10−1 |
| BMI adj. WC—female | IVW | 24 | 0.3255 | 0.1774 | 1.385 [0.978, 1.961] | 6.650 × 10−2 |
| WHR—female | MR Egger | 20 | −0.7122 | 1.1173 | 0.491 [0.055, 4.383] | 5.319 × 10−1 |
| WHR—female | Weighted median | 20 | 0.2172 | 0.2383 | 1.243 [0.779, 1.982] | 3.619 × 10−1 |
| WHR—female | IVW | 20 | 0.3912 | 0.2220 | 1.479 [0.957, 2.285] | 7.806 × 10−2 |
| BMI adj. WHR—female | MR Egger | 32 | 0.0927 | 0.5285 | 1.097 [0.389, 3.091] | 8.620 × 10−1 |
| BMI adj. WHR—female | Weighted median | 32 | 0.1742 | 0.1743 | 1.190 [0.846, 1.675] | 3.175 × 10−1 |
| BMI adj. WHR—female | IVW | 32 | 0.2089 | 0.1387 | 1.232 [0.939, 1.617] | 1.322 × 10−1 |
| Outcome | Method | nSNP | β | SE | OR (95%CI) | p-Value |
| T2DM | MR Egger | 8 | 0.0244 | 0.1823 | 1.025 [0.717, 1.465] | 8.979 × 10−1 |
| T2DM | Weighted median | 8 | −0.0285 | 0.0355 | 0.972 [0.907, 1.042] | 4.208 × 10−1 |
| T2DM | IVW | 8 | −0.0325 | 0.0384 | 0.968 [0.898, 1.044] | 3.962 × 10−1 |
| CAD | MR Egger | 10 | −0.0226 | 0.1086 | 0.978 [0.790, 1.210] | 8.403 × 10−1 |
| CAD | Weighted median | 10 | −0.0474 | 0.0283 | 0.954 [0.902, 1.008] | 9.473 × 10−2 |
| CAD | IVW | 10 | −0.0349 | 0.0228 | 0.966 [0.923, 1.010] | 1.258 × 10−1 |
| BC | MR Egger | 9 | 0.0441 | 0.1278 | 1.045 [0.814, 1.343] | 7.403 × 10−1 |
| BC | Weighted median | 9 | 0.0727 | 0.0268 | 1.075 [1.020, 1.133] | 6.697 × 10−3 |
| BC | IVW | 9 | 0.0646 | 0.0277 | 1.067 [1.010, 1.126] | 1.950 × 10−2 |
| ER + BC | MR Egger | 9 | 0.0859 | 0.1501 | 1.090 [0.812, 1.462] | 5.850 × 10−1 |
| ER + BC | Weighted median | 9 | 0.0981 | 0.0341 | 1.103 [1.032, 1.179] | 4.015 × 10−3 |
| ER + BC | IVW | 9 | 0.0862 | 0.0324 | 1.090 [1.023, 1.161] | 7.900 × 10−3 |
| ER − BC | MR Egger | 9 | −0.0564 | 0.1630 | 0.945 [0.687, 1.301] | 7.394 × 10−1 |
| ER − BC | Weighted median | 9 | 0.0168 | 0.0399 | 1.017 [0.940, 1.100] | 6.744 × 10−1 |
| ER − BC | IVW | 9 | 0.0605 | 0.0368 | 1.062 [0.988, 1.142] | 1.001 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Movva, V.C.; Williams, M.S.; Lee, M.T.M. Polycystic Ovary Syndrome Susceptibility Loci Inform Disease Etiological Heterogeneity. J. Clin. Med. 2021, 10, 2688. https://doi.org/10.3390/jcm10122688
Zhang Y, Movva VC, Williams MS, Lee MTM. Polycystic Ovary Syndrome Susceptibility Loci Inform Disease Etiological Heterogeneity. Journal of Clinical Medicine. 2021; 10(12):2688. https://doi.org/10.3390/jcm10122688
Chicago/Turabian StyleZhang, Yanfei, Vani C. Movva, Marc S. Williams, and Ming Ta Michael Lee. 2021. "Polycystic Ovary Syndrome Susceptibility Loci Inform Disease Etiological Heterogeneity" Journal of Clinical Medicine 10, no. 12: 2688. https://doi.org/10.3390/jcm10122688
APA StyleZhang, Y., Movva, V. C., Williams, M. S., & Lee, M. T. M. (2021). Polycystic Ovary Syndrome Susceptibility Loci Inform Disease Etiological Heterogeneity. Journal of Clinical Medicine, 10(12), 2688. https://doi.org/10.3390/jcm10122688





