Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Withdrawal Is Associated with Higher Mortality in Hospitalized Patients with COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Main Outcomes
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Study Population
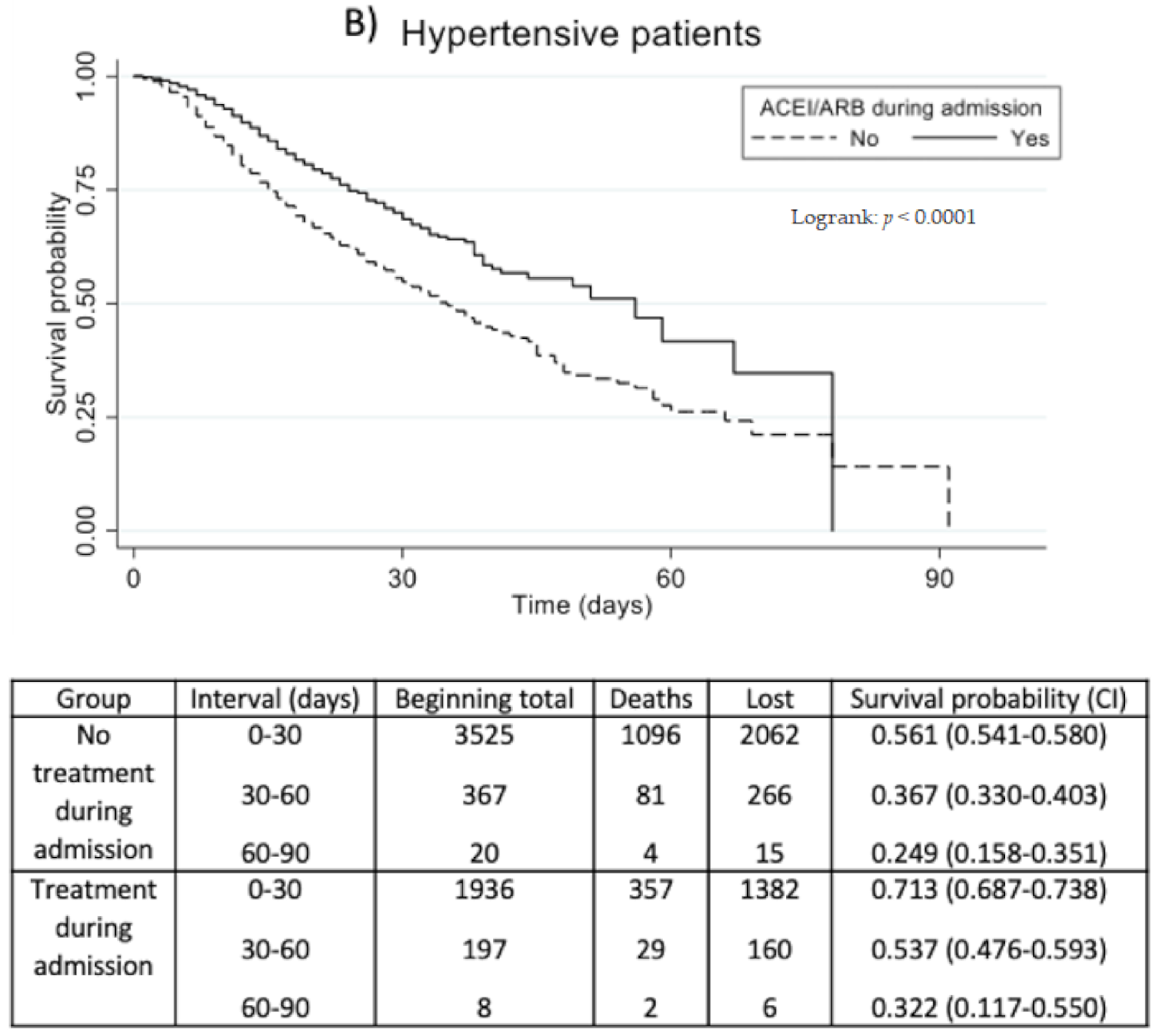
3.2. Outcomes of Prognosis
3.3. Major Adverse Cardiovascular Events
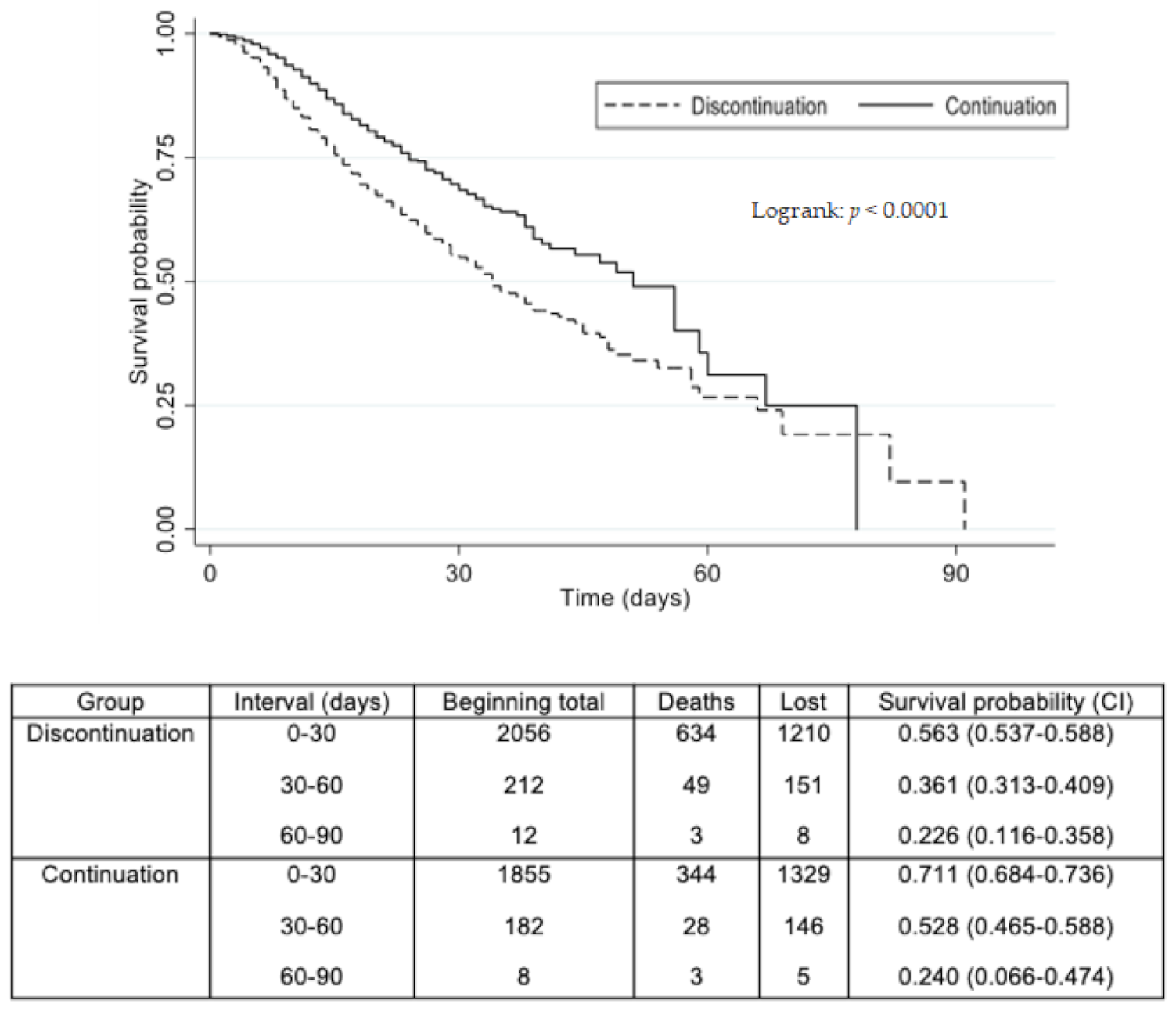
3.4. ACEI/ARB Continuation Versus Withdrawal during Hospitalization
3.5. Comparison between ACEI and ARB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, H.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.B.; Iturrate, E.; Johnson, S.B.; Hausvater, A.; Newman, J.D.; Berger, J.S.; Bangalore, S.; et al. Renin–Angiotensin–Aldosterone System Inhibitors and Risk of Covid-19. N. Engl. J. Med. 2020, 382, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Fosbøl, E.L.; Butt, J.H.; Østergaard, L.; Andersson, C.; Selmer, C.; Kragholm, K.; Schou, M.; Phelps, M.; Gislason, G.H.; Gerds, T.A.; et al. Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use With COVID-19 Diagnosis and Mortality. JAMA 2020, 324, 168. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Benson, L.; Sundström, J.; Lund, L.H. Association between renin–angiotensin–aldosterone system inhibitor use and COVID-19 hospitalization and death: A 1.4 million patient nationwide registry analysis. Eur. J. Heart Fail. 2020, 23, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Rea, F.; Ludergnani, M.; Apolone, G.; Corrao, G. Renin–Angiotensin–Aldosterone System Blockers and the Risk of Covid-19. N. Engl. J. Med. 2020, 382, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- ESC Council on Hypertension. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers 2020. 2020. Available online: https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang (accessed on 13 September 2020).
- American Heart Association; Heart Failure Society of America; American College of Cardiology. HFSA/ACC/AHA Satement Addresses Concerns Regarding Using RAAS Antagonists in COVID 19. 2020. Available online: https://professional.heart.org/en/science-news/hfsa-acc-aha-statement-addresses-concerns-regarding-using-raas-antagonists-in-covid-19 (accessed on 13 September 2020).
- Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2021. Available online: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (accessed on 10 March 2021).
- Casas-Rojo, J.M.; Antón-Santos, J.M.; Millán-Núñez-Cortés, J.; Lumbreras-Bermejo, C.; Ramos-Rincón, J.M.; Roy-Vallejo, E.; Artero-Mora, A.; Arnalich-Fernández, F.; García-Bruñén, J.M.; Vargas-Núñez, J.A.; et al. Características clínicas de los pacientes hospitalizados con COVID-19 en España: Resultados del Registro SEMI-COVID-19. Rev. Clínica Española 2020, 220, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Rodilla, E.; Saura, A.; Jiménez, I.; Mendizábal, A.; Pineda-Cantero, A.; Lorenzo-Hernández, E. Association of Hypertension with All-Cause Mortality among Hospitalized Patients with COVID-19. J. Clin. Med. 2020, 9, 3136. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Trifirò, G.; Massari, M.; Da Cas, R.; Ippolito, F.M.; Sultana, J.; Crisafulli, S.; Rossi, P.G.; Marino, M.; Zorzi, M.; Bovo, E.; et al. Renin–Angiotensin–Aldosterone System Inhibitors and Risk of Death in Patients Hospitalised with COVID-19: A Retrospective Italian Cohort Study of 43,000 Patients. Drug Saf. 2020, 43, 1297–1308. [Google Scholar] [CrossRef]
- Semenzato, L.; Botton, J.; Drouin, J.; Baricault, B.; Vabre, C.; Cuenot, F.; Penso, L.; Herlemont, P.; Sbidian, E.; Weill, A.; et al. Antihypertensive Drugs and COVID-19 Risk. Hypertension 2021, 77, 833–842. [Google Scholar] [CrossRef]
- Barochiner, J.; Martínez, R. Use of inhibitors of the renin-angiotensin system in hypertensive patients and COVID-19 severity: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2020, 45, 1244–1252. [Google Scholar] [CrossRef]
- Xu, J.; Teng, Y.; Shang, L.; Gu, X.; Fan, G.; Chen, Y.; Tian, R.; Zhang, S.; Cao, B. The Effect of Prior ACEI/ARB Treatment on COVID-19 Susceptibility and Outcome: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, B.; Li, Y.; Zhang, L.; Wang, Y.; Yang, S.; Xiao, X.; Qin, Q. The use of renin–angiotensin–aldosterone system (RAAS) inhibitors is associated with a lower risk of mortality in hypertensive COVID-19 patients: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 1370–1377. [Google Scholar] [CrossRef]
- Lopes, R.D.; Macedo, A.V.; Silva, P.G.; Moll-Bernardes, R.J.; Dos Santos, T.M.; Mazza, L.; Feldman, A.; Arruda, G.D.; Denílson, C.; Camiletti, A.S.; et al. Effect of Discontinuing vs. Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 254. [Google Scholar] [CrossRef]
- Meng, J.; Xiao, G.; Zhang, J.; He, X.; Ou, M.; Bi, J.; Yang, R.; Di, W.; Wang, Z.; Li, Z.; et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microbes Infect. 2020, 9, 757–760. [Google Scholar] [CrossRef]
- Lam, K.W.; Chow, K.W.; Vo, J.; Hou, W.; Li, H.; Richman, P.S.; Mallipattu, S.K.; Skopicki, H.A.; Singer, A.J.; Duong, T.Q.; et al. Continued In-Hospital Angiotensin-Converting Enzyme Inhibitor and Angiotensin II Receptor Blocker Use in Hypertensive COVID-19 Patients Is Associated With Positive Clinical Outcome. J. Infect. Dis. 2020, 222, 1256–1264. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Y.M.; Xie, J.; Li, H.; Lei, F.; Yang, H.; Qin, J.J.; Cai, J.; Zhang, X.J.; Wu, B.; et al. Comparative Impacts of ACE (Angiotensin-Converting Enzyme) Inhibitors Versus Angiotensin II Receptor Blockers on the Risk of COVID-19 Mortality. Hypertension 2020, 76, e15–e17. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.J.; Xie, J.; Liu, Y.M.; Zhao, Y.C.; Huang, X.; Lin, L. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef]
- Gurwitz, D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020, 81, 537–540. [Google Scholar] [CrossRef]
- Oussalah, A.; Gleye, S.; Clerc Urmes, I.; Laugel, E.; Callet, J.; Barbé, F.; Orlowski, S.; Malaplate, C.; Aimone-Gastin, I.; Caillierez, B.M.; et al. Long-term ACE Inhibitor/ARB Use Is Associated With Severe Renal Dysfunction and Acute Kidney Injury in Patients With Severe COVID-19: Results From a Referral Center Cohort in the Northeast of France. Clin. Infect. Dis. 2020, 7, 2447–2456. [Google Scholar] [CrossRef]
- Sleight, P. Angiotensin II and trials of cardiovascular outcomes1. Am. J. Cardiol. 2002, 89, 11–16. [Google Scholar] [CrossRef]
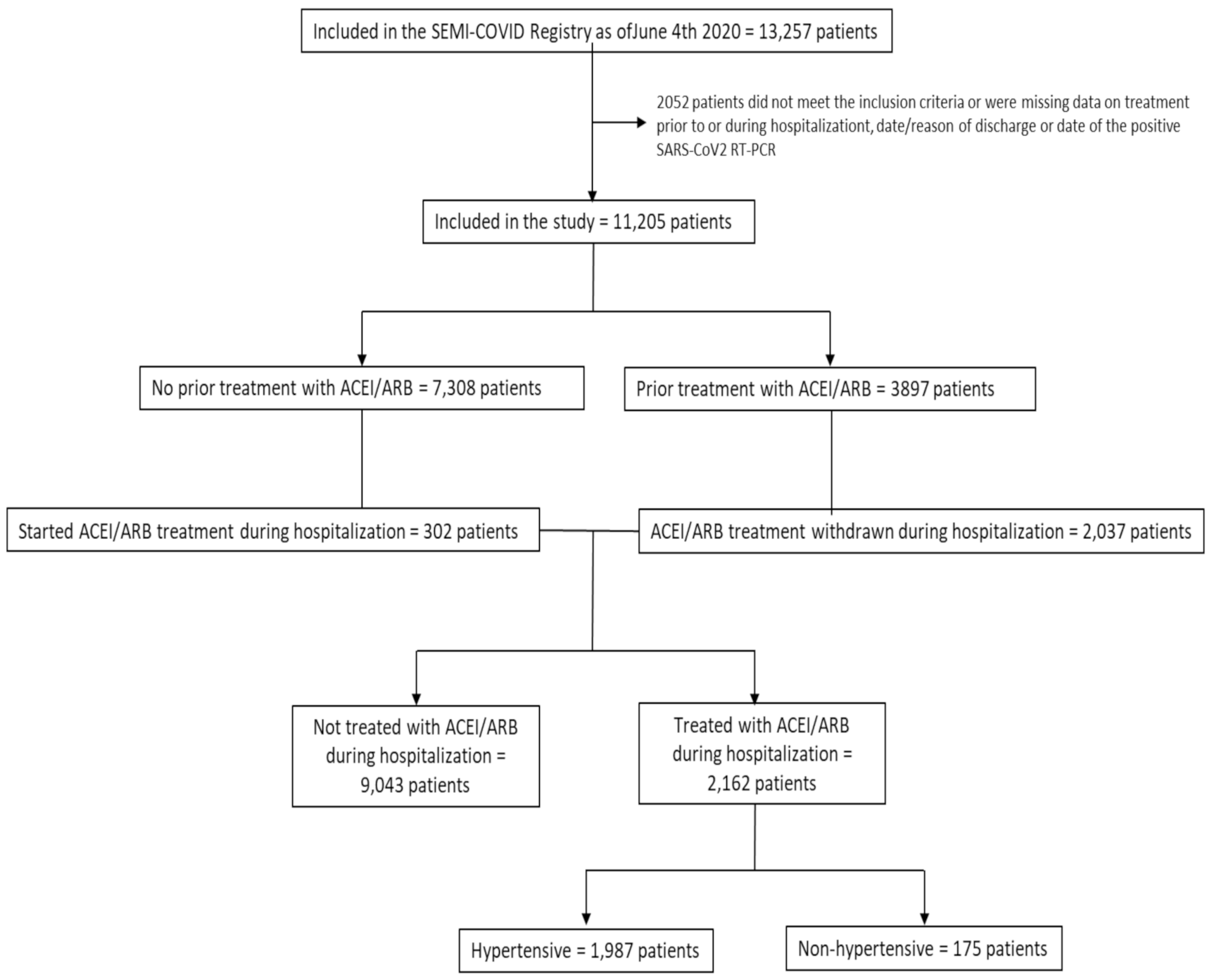

| Total Population (n = 11,205) | Non-ACEI/ARB Group (n = 9043) | ACEI/ARB Group (n = 2162) | p-Value | |
|---|---|---|---|---|
| Age (years): mean (SD) | 67.0 (16.3) | 65.7 (16.8) | 72.5 (12.5) | <0.0001 |
| Female sex (%) | 4827/11,190 (43.1%) | 3944/9030 (43.7%) | 883/2160 (40.9%) | 0.018 |
| Race/Ethnicity (%) | ||||
| Caucasian | 9831/11,014 (89.3%) | 7836/8886 (88.2%) | 1995/2128 (93.8%) | <0.001 * |
| African | 45/11,014 (0.4%) | 36/8886 (0.4%) | 9/2128 (0.4%) | |
| Latin American | 990/11,014 (9.0%) | 892/8886 (10.0%) | 98/2128 (4.6%) | |
| Asian | 50/11,014 (0.5%) | 43/8886 (0.5) | 7/2128 (0.3%) | |
| Other | 98/11,014 (0.9%) | 79/8886 (0.9%) | 19/2128 (0.9%) | |
| Smoking (%) | ||||
| Non-smoker | 7438/10,699 (69.5%) | 6125/8617 (71.1%) | 1313/2082 (63.1%) | <0.001 * |
| Former smoker | 2686/10,699 (25.0%) | 2030/8617 (23.6%) | 656/2082 (31.5%) | |
| Active smoker | 575%10,699 (5.4%) | 462/8617 (5.4%) | 113/2082 (15.4%) | |
| Alcohol use disorder (%) | 516/10,887 (4.7%) | 387/8780 (4.4%) | 129/2107 (6.1%) | 0.001 |
| Comorbidities (%) | ||||
| Hypertension | 5576/11,190 (49.8%) | 3589/9033 (39.7%) | 1987/2157 (92.1%) | <0.001 |
| Dyslipidemia | 4415/11,189 (39.5%) | 3231/9029 (35.8%) | 1184/2160 (54.8%) | <0.001 |
| Diabetes mellitus | 2095/11,178 (18.7%) | 1464/9022 (16.2%) | 631/2156 (29.3%) | <0.001 |
| Obesity | 2186/10,212 (21.4%) | 1618/8242 (19.6%) | 568/1970 (28.8%) | <0.001 |
| Heart failure | 811/11,186 (7.3%) | 594/9030 (6.6%) | 217/2156 (10.1%) | <0.001 |
| Ischemic heart disease | 880/11,193 (7.9%) | 591/9032 (6.5%) | 289/2161 (13.4%) | <0.001 |
| Cerebrovascular disease | 797/11,173 (7.1%) | 598/9018 (6.6%) | 199/2155 (9.2%) | <0.001 |
| Peripheral artery disease | 523/11,183 (4.7%) | 387/9026 (4.3%) | 136/2157 (6.3%) | <0.001 |
| Chronic kidney disease | 665/11,183 (5.9%) | 491/9026 (5.4%) | 174/2157 (8.1%) | <0.001 |
| Age-adjusted Charlson Comorbidity Index: points (SD) | 3.6 (2.7) | 3.4 (2.7) | 4.4 (2.47) | <0.0001 |
| Previous treatment (%) | ||||
| ACEI | 1890/11,205 (16,9%) | 993/9043 (11.0%) | 897/2162 (41.5%) | <0.001 |
| ARB | 2133/11,205 (19.0%) | 1103/9043 (12.2%) | 1030/2162 (47.6%) | <0.001 |
| Outcome | Non-ACEI/ARB Group | ACEI/ARB Group | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| RR (95%CI) | p-Value | RR (95%CI) | p-Value | |||
| Composite variable of prognosis: IMV, NIMV, ICU admission, or death * | 2443/8854 (27.6%) | 569/2107 (27.0%) | 0.98 (0.91–1.06) | 0.6064 | Normotensive: 1.12 (0.90–1.38) Hypertensive: 0.68 (0.62–0.75) | 0.3085 <0.0001 |
| IMV * | 622/9024 (6.9%) | 115/2157 (5.3%) | 0.77 (0.64–0.94) | 0.0100 | Normotensive: 2.07 (1.42–3.02) Hypertensive: 0.50 (0.39–0.64) | 0.0002 <0.0001 |
| NIMV | 396/9026 (4.4%) | 130/2156 (6.0%) | 1.37 (1.13–1.67) | 0.0015 | 1.13 (0.90–1.43) | 0.2908 |
| ICU admission * | 767/9035 (8.5%) | 148/2161 (6.8%) | 0.81 (0.68–0.96) | 0.0140 | Normotensive: 1.76 (1.23–2.52) Hypertensive: 0.57 (0.46–0.71) | 0.0019 <0.0001 |
| Death | 1897/8853 (21.4%) | 436/2105 (20.7%) | 0.97 (0.88–1.06) | 0.4897 | 0.65 (0.59–0.72) | <0.0001 |
| Comparison | Adjusted HR | 95%CI | p-Value |
|---|---|---|---|
| ACEI/ARB vs. non-ACEI/ARB during hospitalization | From the onset of symptoms: At 7 days: 0.57 At 30 days: 0.68 | 0.49–0.66 0.55–0.85 | <0.001 0.001 |
| ACEI/ARB continued vs. withdrawn | From the onset of symptoms: At 7 days: 0.52 At 30 days: 0.79 | 0.44–0.60 0.60–1.05 | <0.001 0.110 |
| ARB vs. ACEI | 0.77 | 0.62–0.96 | 0.027 |
| Outcome | Non-ACEI/ARB Group | ACEI/ARB Group | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| RR (95%CI) | p-Value | RR (95%CI) | p-Value | |||
| Major adverse cardiovascular events (MACE): MI, HF, stroke, arrhythmia | 827/8986 (9.2%) | 257/2144 (12.0%) | 1.30 (1.14–1.49) | 0.0001 | 0.94 (0.81–1.09) | 0.4211 |
| MI | 60/9005 (0.7%) | 28/2151 (1.3%) | 1.95 (1.23–3.05) | 0.0043 | 1.64 (0.93–2.89) | 0.0877 |
| HF | 504/9010 (5.6%) | 160/2150 (7.4%) | 1.33 (1.12–1.58) | 0.0014 | 1.03 (0.85–1.26) | 0.7597 |
| Stroke | 51/9003 (0.6%) | 18/2153 (0.8%) | 1.48 (0.86–2.52) | 0.2005 | 0.90 (0.46–1.73) | 0.7435 |
| Arrhythmia | 347/9002 (3.9%) | 104/2152 (4.8%) | 1.25 (1.01–1.55) | 0.0446 | 0.86 (0.66–1.11) | 0.2335 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy-Vallejo, E.; Sánchez Purificación, A.; Torres Peña, J.D.; Sánchez Moreno, B.; Arnalich, F.; García Blanco, M.J.; López Miranda, J.; Romero-Cabrera, J.L.; Herrero Gil, C.R.; Bascunana, J.; et al. Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Withdrawal Is Associated with Higher Mortality in Hospitalized Patients with COVID-19. J. Clin. Med. 2021, 10, 2642. https://doi.org/10.3390/jcm10122642
Roy-Vallejo E, Sánchez Purificación A, Torres Peña JD, Sánchez Moreno B, Arnalich F, García Blanco MJ, López Miranda J, Romero-Cabrera JL, Herrero Gil CR, Bascunana J, et al. Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Withdrawal Is Associated with Higher Mortality in Hospitalized Patients with COVID-19. Journal of Clinical Medicine. 2021; 10(12):2642. https://doi.org/10.3390/jcm10122642
Chicago/Turabian StyleRoy-Vallejo, Emilia, Aquilino Sánchez Purificación, José David Torres Peña, Beatriz Sánchez Moreno, Francisco Arnalich, María José García Blanco, José López Miranda, Juan Luis Romero-Cabrera, Carmen Rosario Herrero Gil, José Bascunana, and et al. 2021. "Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Withdrawal Is Associated with Higher Mortality in Hospitalized Patients with COVID-19" Journal of Clinical Medicine 10, no. 12: 2642. https://doi.org/10.3390/jcm10122642
APA StyleRoy-Vallejo, E., Sánchez Purificación, A., Torres Peña, J. D., Sánchez Moreno, B., Arnalich, F., García Blanco, M. J., López Miranda, J., Romero-Cabrera, J. L., Herrero Gil, C. R., Bascunana, J., Rubio-Rivas, M., Pintos Otero, S., Martínez Sempere, V., Ballano Rodríguez-Solís, J., Gil Sánchez, R., Luque del Pino, J., González Noya, A., Navas-Alcántara, M. S., Cortés Rodríguez, B., ... on behalf of the SEMI-COVID-19 Network. (2021). Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Withdrawal Is Associated with Higher Mortality in Hospitalized Patients with COVID-19. Journal of Clinical Medicine, 10(12), 2642. https://doi.org/10.3390/jcm10122642






