The Clinical Significance of DJ1 and L1CAM Serum Level Monitoring in Patients with Endometrial Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Management
2.3. Clinical Data
2.4. Serum CA125, HE4, DJ1 and L1CAM Level Measurement
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. DJ1, L1CAM, CA125 and HE4 Serial Serum Levels in Correlation with Disease Status
3.3. DJ1, L1CAM, CA125 and HE4 Serum Levels in Correlation with Patient-Related Characteristics
3.4. DJ1, L1CAM, CA125 and HE4 Serum Levels in Correlation with Tumor Clinicopathological Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bidart, J.-M.; Thuillier, F.; Augereau, C.; Chalas, J.; Daver, A.; Jacob, N.; Labrousse, F.; Voitot, H. Kinetics of Serum Tumor Marker Concentrations and Usefulness in Clinical Monitoring. Clin. Chem. 1999, 45, 1695–1707. [Google Scholar] [CrossRef]
- Ayhan, A.; Reed, N.; Gultekin, M.; Dursun, P. (Eds.) Textbook of Gynaecological Oncology; Günes Publishing: Ankara, Turkey, 2017; ISBN 978-975-277-682-1. [Google Scholar]
- Bast, J.R.; Xu, F.-J.; Yu, Y.-H.; Barnhill, S.; Zhang, Z.; Mills, G. CA 125: The past and the Future. Int. J. Biol. Mark. 1998, 13, 179–187. [Google Scholar] [CrossRef]
- Sood, A.K.; Buller, R.E.; Burger, R.A.; Dawson, J.D.; Sorosky, J.I.; Berman, M. Value of Preoperative CA 125 Level in the Management of Uterine Cancer and Prediction of Clinical Outcome. Obstet. Gynecol. 1997, 90, 441–447. [Google Scholar] [CrossRef]
- Reijnen, C.; Visser, N.C.; Kasius, J.C.; Boll, D.; Geomini, P.M.; Ngo, H.; Van Hamont, D.; Pijlman, B.M.; Vos, M.C.; Bulten, J.; et al. Improved preoperative risk stratification with CA-125 in low-grade endometrial cancer: A multicenter prospective cohort study. J. Gynecol. Oncol. 2019, 30, e70. [Google Scholar] [CrossRef]
- Fischerova, D. Ultrasound scanning of the pelvis and abdomen for staging of gynecological tumors: A review. Ultrasound Obstet. Gynecol. 2011, 38, 246–266. [Google Scholar] [CrossRef]
- Kalogera, E.; Scholler, N.; Powless, C.; Weaver, A.; Drapkin, R.; Li, J.; Jiang, S.-W.; Podratz, K.; Urban, N.; Dowdy, S.C. Correlation of serum HE4 with tumor size and myometrial invasion in endometrial cancer. Gynecol. Oncol. 2012, 124, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.G.; Brown, A.K.; Miller, M.C.; Badgwell, D.; Lu, Z.; Allard, W.J.; Granai, C.; Bast, R.; Lu, K. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol. Oncol. 2008, 110, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Lo, S.; Cheng, D.; Ng, T.; Wong, L.; Ngan, H. Prognostic Significance of Tumour Markers in Endometrial Cancer. Tumor Biol. 1997, 18, 241–249. [Google Scholar] [CrossRef]
- Bignotti, E.; Ragnoli, M.; Zanotti, L.; Calza, S.; Falchetti, M.L.; Lonardi, S.; Bergamelli, S.; Bandiera, E.; Tassi, R.A.; Romani, C.; et al. Diagnostic and prognostic impact of serum HE4 detection in endometrial carcinoma patients. Br. J. Cancer 2011, 104, 1418–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanotti, L.; Bignotti, E.; Calza, S.; Bandiera, E.; Ruggeri, G.; Galli, C.; Tognon, G.; Ragnoli, M.; Romani, C.; Tassi, R.A.; et al. Human epididymis protein 4 as a serum marker for diagnosis of endometrial carcinoma and prediction of clinical outcome. Clin. Chem. Lab. Med. 2012, 50, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.J.; Hackethal, A.; Mann, K.P.; Mutz-Dehbalaie, I.; Fiegl, H.; Marth, C.; Obermair, A. Serum HE4 detects recurrent endometrial cancer in patients undergoing routine clinical surveillance. BMC Cancer 2015, 15, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbink, K.; Zusterzeel, P.L.; Geurts-Moespot, A.J.; van Herwaarden, A.E.; Pijnenborg, J.M.; Sweep, F.; Massuger, L.F. HE4 is superior to CA125 in the detection of recurrent disease in high-risk endometrial cancer patients. Tumor Biol. 2018, 40, 101042831875710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morelli, M.; Scumaci, D.; Di Cello, A.; Venturella, R.; Donato, G.; Faniello, M.C.; Quaresima, B.; Cuda, G.; Zullo, F.; Costanzo, F.S. DJ-1 in Endometrial Cancer: A Possible Biomarker to Improve Differential Diagnosis Between Subtypes. Int. J. Gynecol. Cancer 2014, 24, 649–658. [Google Scholar] [CrossRef]
- Di Cello, A.; Di Sanzo, M.; Perrone, F.M.; Santamaria, G.; Rania, E.; Angotti, E.; Venturella, R.; Mancuso, S.; Zullo, F.; Cuda, G.; et al. DJ-1 is a reliable serum biomarker for discriminating high-risk endometrial cancer. Tumor Biol. 2017, 39, 101042831770574. [Google Scholar] [CrossRef] [Green Version]
- Benati, M.; Montagnana, M.; Danese, E.; Paviati, E.; Giudici, S.; Ruzzenente, O.; Franchi, M.; Lippi, G. The clinical significance of DJ-1 and HE4 in patients with endometrial cancer. J. Clin. Lab. Anal. 2018, 32, e22223. [Google Scholar] [CrossRef] [Green Version]
- Fogel, M.; Gutwein, P.; Mechtersheimer, S.; Riedle, S.; Stoeck, A.; Smirnov, A.; Edler, L.; Ben-Arie, A.; Huszar, M.; Altevogt, P. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet 2003, 362, 869–875. [Google Scholar] [CrossRef]
- Van Gool, I.C.; Stelloo, E.; Nout, R.A.; Nijman, H.W.; Edmondson, R.; Church, D.N.; Mackay, H.J.; Leary, A.; Powell, M.E.; Mileshkin, L.; et al. Prognostic significance of L1CAM expression and its association with mutant p53 expression in high-risk endometrial cancer. Mod. Pathol. 2016, 29, 174–181. [Google Scholar] [CrossRef]
- Wojciechowski, M.; Głowacka, E.; Wilczyński, M.; Pękala-Wojciechowska, A.; Malinowski, A. The sL1CAM in sera of patients with endometrial and ovarian cancers. Arch. Gynecol. Obstet. 2016, 295, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Tangen, I.L.; Kopperud, R.K.; Visser, N.C.; Staff, A.C.; Tingulstad, S.; Marcickiewicz, J.; Amant, F.; Bjørge, L.; Pijnenborg, J.M.; Salvesen, H.B.; et al. Expression of L1CAM in curettage or high L1CAM level in preoperative blood samples predicts lymph node metastases and poor outcome in endometrial cancer patients. Br. J. Cancer 2017, 117, 840–847. [Google Scholar] [CrossRef]
- Frühauf, F.; Dvořák, M.; Haaková, L.; Hašlík, L.; Herboltová, P.; Chaloupková, B.; Kožnarová, J.; Kubešová, B.; Lukáčová, I.; Marek, R.; et al. Ultrasound staging of endometrial cancer—Recommended methodology of examination. Ceska Gynekol. 2014, 79, 466–476. [Google Scholar]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; Wiley: Somerset, UK, 2011; ISBN 978-1-4443-5896-4. [Google Scholar]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO–ESGO–ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Radiother. Oncol. 2015, 117, 559–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurman, R.J.; International Agency for Research on Cancer; World Health Organization (Eds.) WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; World Health Organization classification of tumours; International Agency for Research on Cancer: Lyon, France, 2014; ISBN 978-92-832-2435-8. [Google Scholar]
- FIGO Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int. J. Gynecol. Obstet. 2014, 125, 97–98. [Google Scholar] [CrossRef]
- Diamandis, E.P. Tumor Markers: Physiology, Pathobiology, Technology and Clinical Application; AACC Press: Washington, WA, USA, 2002. [Google Scholar]
- Burke, W.M.; Orr, J.; Leitao, M.; Salom, E.; Gehrig, P.; Olawaiye, A.B.; Brewer, M.; Boruta, D.; Villella, J.; Herzog, T.; et al. Endometrial cancer: A review and current management strategies: Part I. Gynecol. Oncol. 2014, 134, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.M.; Orr, J.; Leitao, M.; Salom, E.; Gehrig, P.; Olawaiye, A.B.; Brewer, M.; Boruta, D.; Herzog, T.J.; Abu Shahin, F. Endometrial cancer: A review and current management strategies: Part II. Gynecol. Oncol. 2014, 134, 393–402. [Google Scholar] [CrossRef]
- Salani, R.; Backes, F.J.; Fung, M.F.K.; Holschneider, C.H.; Parker, L.P.; Bristow, R.E.; Goff, B.A. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am. J. Obstet. Gynecol. 2011, 204, 466–478. [Google Scholar] [CrossRef]
- Patsner, B.; Orr, J.W.; Mann, W.J. Use of serum CA 125 measurement in posttreatment surveillance of early-stage endometrial carcinoma. Am. J. Obstet. Gynecol. 1990, 162, 427–429. [Google Scholar] [CrossRef]
- Rose, P.G.; Sommers, R.M.; Reale, F.R.; Hunter, R.E.; Fournier, L.; Nelson, B.E. Serial serum CA 125 measurements for evaluation of recurrence in patients with endometrial carcinoma. Obstet. Gynecol. 1994, 84, 12–16. [Google Scholar]
- Fung-Kee-Fung, M.; Dodge, J.; Elit, L.; Lukka, H.; Chambers, A.; Oliver, T. Follow-up after primary therapy for endometrial cancer: A systematic review. Gynecol. Oncol. 2006, 101, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Creutzberg, C.L.; van Putten, W.L.; Koper, P.C.; Lybeert, M.L.; Jobsen, J.J.; Wárlám-Rodenhuis, C.C.; De Winter, K.A.; Lutgens, L.C.; Bergh, A.C.V.D.; van der Steen-Banasik, E.; et al. Survival after relapse in patients with endometrial cancer: Results from a randomized trial. Gynecol. Oncol. 2003, 89, 201–209. [Google Scholar] [CrossRef]
- Smith, C.J.; Heeren, M.; Nicklin, J.L.; Perrin, L.; Land, R.; Crandon, A.J.; Obermair, A. Efficacy of routine follow-up in patients with recurrent uterine cancer. Gynecol. Oncol. 2007, 107, 124–129. [Google Scholar] [CrossRef]
- Angioli, R.; Capriglione, S.; Scaletta, G.; Aloisi, A.; Miranda, A.; Nardone, C.D.C.; Terranova, C.; Plotti, F. The role of HE4 in endometrial cancer recurrence: How to choose the optimal follow-up program. Tumor Biol. 2015, 37, 4973–4978. [Google Scholar] [CrossRef] [PubMed]
- Coppolino, G.; Bolignano, D.; Rivoli, L.; Mazza, G.; Presta, P.; Fuiano, G. Tumour Markers and Kidney Function: A Systematic Review. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chovanec, J.; Selingerova, I.; Greplova, K.; Antonsen, S.L.; Nalezinska, M.; Høgdall, C.; Høgdall, E.; Søgaard-Andersen, E.; Jochumsen, K.; Fabian, P.; et al. Adjustment of serum HE4 to reduced glomerular filtration and its use in biomarker-based prediction of deep myometrial invasion in endometrial cancer. Oncotarget 2017, 8, 108213–108222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saarelainen, S.K.; Peltonen, N.; Lehtimäki, T.; Perheentupa, A.; Vuento, M.H.; Mäenpää, J.U. Predictive value of serum human epididymis protein 4 and cancer antigen 125 concentrations in endometrial carcinoma. Am. J. Obstet. Gynecol. 2013, 209, 142.e1–142.e6. [Google Scholar] [CrossRef]
- Reijnen, C.; Gogou, E.; Visser, N.C.M.; Engerud, H.; Ramjith, J.; Van Der Putten, L.J.M.; Van De Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; et al. Preoperative risk stratification in endometrial cancer (ENDORISK) by a Bayesian network model: A development and validation study. PLoS Med. 2020, 17, e1003111. [Google Scholar] [CrossRef]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Murali, R.; Delair, D.F.; Bean, S.M.; Abu-Rustum, N.R.; Soslow, R.A. Evolving Roles of Histologic Evaluation and Molecular/Genomic Profiling in the Management of Endometrial Cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Urick, M.E.; Bell, D.W. Clinical actionability of molecular targets in endometrial cancer. Nat. Rev. Cancer 2019, 19, 510–521. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Travaglino, A.; Gabrielli, O.; Micheli, M.; Zuccalà, V.; Bitonti, G.; Camastra, C.; Gargiulo, V.; Insabato, L.; Zullo, F. Clinical features of ProMisE groups identify different phenotypes of patients with endometrial cancer. Arch. Gynecol. Obstet. 2021, 303, 1393–1400. [Google Scholar] [CrossRef]
- Santoro, A.; Angelico, G.; Travaglino, A.; Inzani, F.; Arciuolo, D.; Valente, M.; D’Alessandris, N.; Scaglione, G.; Fiorentino, V.; Raffone, A.; et al. New Pathological and Clinical Insights in Endometrial Cancer in View of the Updated ESGO/ESTRO/ESP Guidelines. Cancers 2021, 13, 2623. [Google Scholar] [CrossRef] [PubMed]
- Troisi, J.; Raffone, A.; Travaglino, A.; Belli, G.; Belli, C.; Anand, S.; Giugliano, L.; Cavallo, P.; Scala, G.; Symes, S.; et al. Development and Validation of a Serum Metabolomic Signature for Endometrial Cancer Screening in Postmenopausal Women. JAMA Netw. Open 2020, 3, e2018327. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Troisi, J.; Boccia, D.; Travaglino, A.; Capuano, G.; Insabato, L.; Mollo, A.; Guida, M.; Zullo, F. Metabolomics in endometrial cancer diagnosis: A systematic review. Acta Obstet. Gynecol. Scand. 2020, 99, 1135–1146. [Google Scholar] [CrossRef] [PubMed]

| Age (Years) Median (Min; Max) | 65 (30; 85) | |
|---|---|---|
| Menopausal status (n, %) | Pre-/perimenopausal | 11 (17%) |
| Postmenopausal | 54 (83%) | |
| Biometric data Median (min; max) | Weight (kg) | 82 (50; 121) |
| Height (cm) | 165 (146; 176) | |
| BMI (kg/m2) | 31.3 (17.3; 45.7) | |
| BSA (m2) | 1.89 (1.5; 2.33) | |
| Renal function, n = 64 Median (min; max) | Creat/S (umol/L) | 69 (52; 134) |
| CKD-EPI (mL/s) | 1.33 (0.52; 1.81) | |
| FIGO stage [25] (n; %) | I | 50 (77%) |
| II | 8 (12%) | |
| III | 5 (8%) | |
| IV | 2 (3%) | |
| Myometrial invasion (n; %) | <50% | 48 (74%) |
| ≥50% | 17 (26%) | |
| Histology (n; %) | E G1-2 | 51 (79%) |
| E G3, non-E | 14 (21%) | |
| Treatment (n; %) | HY and AE | 65 (100%) |
| PLN +/− PALN | 20 (31%) | |
| RT | 23 (35%) | |
| CHT | 7 (11%) | |
| Recurrence (n; %) | Local | 2 (3%) |
| Distant | 3 (5%) | |
| No | 60 (92%) | |
| Time (months) Median (min; max) Surgery—FU1 FU1–FU2, n = 56 Dg–last FU | ||
| 6.3 (2.6; 20.7) | ||
| 4.6 (1.7; 14.7) | ||
| 29.5 (13.7; 46.5) | ||
| Status at the end of FU (n; %) | Alive | 58 (89%) |
| Died of EC | 4 (6%) | |
| Died (another cause) | 3 (5%) | |
| Pts | Age | Histology | G | HY+AE | PLN/ PALN | RT | CHT | FIGO | Relapse | TTP (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| I | 75 | E | 2 | yes | no | yes | yes | IVB | Distant | 8 |
| II | 71 | E | 3 | yes | yes | yes | no | II | Local | 16 |
| III | 72 | E | 2 | yes | no | no | no | IA | Local, Distant | 11 |
| IV | 55 | E | 2 | yes | no | yes | yes | IVB | Distant | 13 |
| V | 66 | Non-E | 3 | yes | yes | no | yes | IA | Distant | 7 |
| Time of Collection | p Value | ||||
|---|---|---|---|---|---|
| Preoperative | FU1 | FU2 | |||
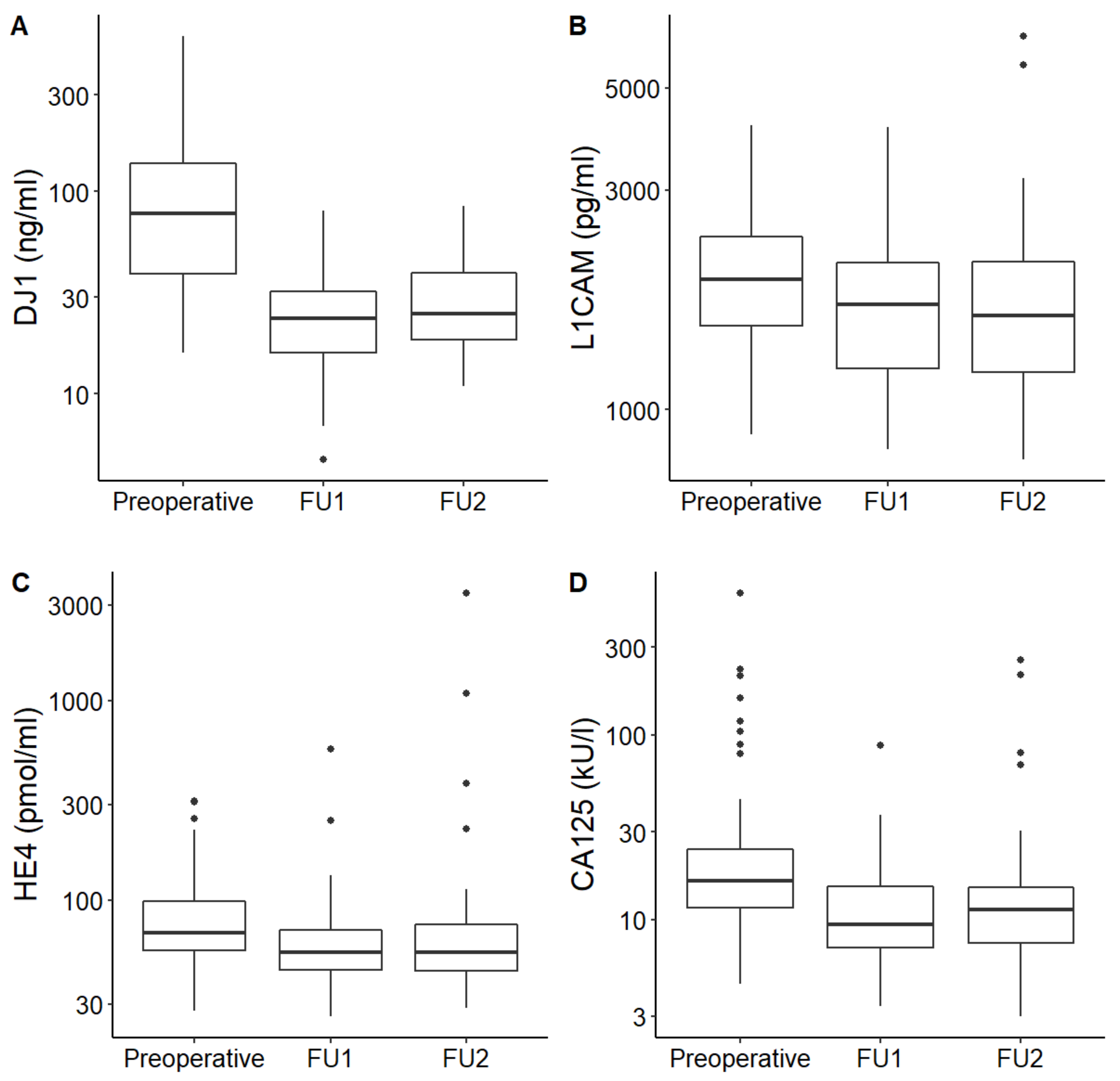
| DJ1 (ng/mL) | Valid n | 64 | 65 | 50 | <0.001 |
| Median (IQR) | 78 (38.4–139) | 23.5 (15.9–31.9) | 25 (18.4–40.1) | ||
| L1CAM (pg/mL) | Valid n | 64 | 65 | 50 | <0.001 |
| Median (IQR) | 1919 (1519–2387) | 1690 (1229–2087) | 1602.5 (1195–2105) | ||
| HE4 (pmol/L) | Valid n | 49 | 65 | 56 | <0.001 |
| Median (IQR) | 68.2 (55.8–98) | 54.5 (44.8–70.5) | 54.7 (44.2–76.7) | ||
| CA125 (kU/L) | Valid n | 60 | 65 | 56 | <0.001 |
| Median (IQR) | 16.3 (11.4–24.2) | 9.4 (7.1–15.2) | 11.3 (7.5–15.4) | ||
| Serum Levels at Follow-Up | p Value | |||
|---|---|---|---|---|
| Remission | Recurrence | |||
| DJ1 (ng/mL) | Valid n | 110 | 5 | 0.035 |
| Median (IQR) | 23.9 (16.8–32.8) | 60.1 (32.7–61) | ||
| L1CAM (pg/mL) | Valid n | 110 | 5 | 0.353 |
| Median (IQR) | 1650.5 (1203–2050) | 2630 (2474–2740) | ||
| HE4 (pmol/L) | Valid n | 116 | 5 | <0.001 |
| Median (IQR) | 53.5 (44.5–71.2) | 572 (110–1083) | ||
| CA125 (kU/L) | Valid n | 116 | 5 | <0.001 |
| Median (IQR) | 10 (7.5–14.8) | 34.4 (12.8–69.2) | ||
| Valid n | Median (IQR) | p Value | |||
|---|---|---|---|---|---|
| DJ1 (ng/mL) | Age | <60 years | 20 | 53.4 (31.4–103) | 0.152 |
| ≥60 years | 44 | 88 (43–151.8) | |||
| Weight | BMI < 27 kg/m2 | 18 | 52.9 (25.5–121.9) | 0.181 | |
| BMI ≥ 27 kg/m2 | 48 | 86.2 (44.4–141.7) | |||
| Renal function | CKD-EPI ≥ 1 mL/s | 56 | 83.4 (40.6–139) | 0.604 | |
| CKD-EPI < 1 mL/s | 7 | 74.1 (24.7–352) | |||
| Menopausal status | Pre/perimenopausal | 11 | 45.4 (24.4–142.4) | 0.393 | |
| Postmenopausal | 53 | 84.9 (41.4–135.5) | |||
| L1CAM (pg/mL) | Age | <60 years | 20 | 1546.5 (1273.5–1872.5) | 0.004 |
| ≥60 years | 44 | 2070 (1703.5–2447) | |||
| Weight | BMI < 27 kg/m2 | 16 | 1519.3 (1331.3–1804) | 0.002 | |
| BMI ≥ 27 kg/m2 | 48 | 2028 (1676.8–2507) | |||
| Renal function | CKD-EPI ≥ 1 mL/s | 56 | 1889.5 (1503.5–2350.3) | 0.105 | |
| CKD-EPI < 1 mL/s | 7 | 2247 (1878–3343.5) | |||
| Menopausal status | Pre/perimenopausal | 11 | 1528 (1173–1928) | 0.010 | |
| Postmenopausal | 53 | 2000 (1565–2424.5) | |||
| HE4 (pmol/L) | Age | <60 years | 15 | 65.7 (57.7–105) | 0.765 |
| ≥60 years | 34 | 68.4 (53.1–98) | |||
| Weight | BMI < 27 kg/m2 | 13 | 65.7 (59.1–104.3) | 0.570 | |
| BMI ≥ 27 kg/m2 | 36 | 68.7 (53.6–94) | |||
| Renal function | CKD-EPI ≥ 1 mL/s | 47 | 67.1 (53.1–98) | 0.196 | |
| CKD-EPI < 1 mL/s | 1 | 161 (161–161) | |||
| Menopausal status | Pre/perimenopausal | 8 | 63.4 (58.9–90.6) | 0.468 | |
| Postmenopausal | 41 | 68.6 (53.1–98) | |||
| CA125 (kU/L) | Age | <60 years | 17 | 17.6 (9.5–24.9) | 0.873 |
| ≥60 years | 43 | 15.8 (11.9–23.7) | |||
| Weight | BMI < 27 kg/m2 | 16 | 17.8 (13–24.8) | 0.481 | |
| BMI ≥ 27 kg/m2 | 44 | 15.4 (11.2–23.8) | |||
| Renal function | CKD-EPI ≥ 1 mL/s | 54 | 17.3 (12.4–24.6) | 0.128 | |
| CKD-EPI < 1 mL/s | 5 | 12.5 (9.9–15.8) | |||
| Menopausal status | Pre/perimenopausal | 9 | 17.6 (15.1–24.9) | 0.873 | |
| Postmenopausal | 51 | 15.8 (10.8–23.8) | |||
| Valid n | Median (IQR) | pValue | |||
| DJ1 (ng/mL) | Histological type | E G1-2 | 50 | 83.4 (41.4–151.5) | 0.191 |
| E G3, non-E | 14 | 69 (22.1–94.5) | |||
| Myometrial invasion | <50% | 47 | 63.9 (35.4–135.5) | 0.331 | |
| ≥50% | 17 | 91.1 (51.9–151.5) | |||
| LN involvement | No | 60 | 78 (38.4–145.2) | 0.688 | |
| Yes | 4 | 73.2 (36.4–111.3) | |||
| Distant metastasis | No | 62 | 83.4 (37.1–142.4) | 0.714 | |
| Yes | 2 | 56.7 (53.1–60.3) | |||
| Definitive risk | Low 1 | 34 | 57.8 (39.7–147.9) | 0.941 | |
| High 2 | 30 | 86.2 (37.1–128) | |||
| L1CAM (pg/mL) | Histological type | E G1-2 | 50 | 1889.5 (1523.5–2418) | 0.620 |
| E G3, non-E | 14 | 2132.5 (1460–2345.5) | |||
| Myometrial invasion | <50% | 47 | 1969 (1528–2355) | 0.721 | |
| ≥50% | 17 | 1680 (1515–2418) | |||
| LN involvement | No | 60 | 1919 (1519.3–2389.8) | 0.945 | |
| Yes | 4 | 2055.5 (1586.5–2381.8) | |||
| Distant metastasis | No | 62 | 1919 (1523.5–2355) | 0.772 | |
| Yes | 2 | 2200.5 (1515–2886) | |||
| Definitive risk | Low 1 | 34 | 1948.5 (1580–2355) | 0.687 | |
| High 2 | 30 | 1837.75 (1469–2418) | |||
| HE4 (pmol/mL) | Histological type | E G1-2 | 38 | 65 (51.4–98) | 0.276 |
| E G3, non-E | 11 | 76.2 (60.2–153.7) | |||
| Myometrial invasion | <50% | 39 | 63.7 (51.4–79.2) | 0.002 | |
| ≥50% | 10 | 148.4 (68.6–255) | |||
| LN involvement | No | 45 | 65.7 (53.1–89.6) | 0.033 | |
| Yes | 4 | 148.4 (105.8–189.4) | |||
| Distant metastasis | No | 47 | 67.1 (53.1–90) | 0.021 | |
| Yes | 2 | 284.5 (255–314) | |||
| Definitive risk | Low 1 | 28 | 61.9 (48.3–79.9) | 0.020 | |
| High 2 | 21 | 76.2 (61.1–153.7) | |||
| CA125 (kU/L) | Histological type | E G1-2 | 47 | 15.8 (12.4–23.8) | 0.837 |
| E G3, non-E | 13 | 17.9 (10.8–24.7) | |||
| Myometrial invasion | <50% | 45 | 14.9 (10.5–20) | 0.009 | |
| ≥50% | 15 | 23.8 (16.9–119) | |||
| LN involvement | No | 56 | 15.8 (10.7–23.6) | 0.010 | |
| Yes | 4 | 192.4 (88.2–407.4) | |||
| Distant metastasis | No | 58 | 16 (10.8–23.8) | 0.104 | |
| Yes | 2 | 116.7 (23.4–210) | |||
| Definitive risk | Low 1 | 32 | 15.3 (10.2–19.8) | 0.135 | |
| High 2 | 28 | 19 (12.2–41.4) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarikova, M.; Vinklerova, P.; Gottwaldova, J.; Ovesna, P.; Hausnerova, J.; Minar, L.; Felsinger, M.; Valik, D.; Cermakova, Z.; Weinberger, V. The Clinical Significance of DJ1 and L1CAM Serum Level Monitoring in Patients with Endometrial Cancer. J. Clin. Med. 2021, 10, 2640. https://doi.org/10.3390/jcm10122640
Bednarikova M, Vinklerova P, Gottwaldova J, Ovesna P, Hausnerova J, Minar L, Felsinger M, Valik D, Cermakova Z, Weinberger V. The Clinical Significance of DJ1 and L1CAM Serum Level Monitoring in Patients with Endometrial Cancer. Journal of Clinical Medicine. 2021; 10(12):2640. https://doi.org/10.3390/jcm10122640
Chicago/Turabian StyleBednarikova, Marketa, Petra Vinklerova, Jana Gottwaldova, Petra Ovesna, Jitka Hausnerova, Lubos Minar, Michal Felsinger, Dalibor Valik, Zdenka Cermakova, and Vit Weinberger. 2021. "The Clinical Significance of DJ1 and L1CAM Serum Level Monitoring in Patients with Endometrial Cancer" Journal of Clinical Medicine 10, no. 12: 2640. https://doi.org/10.3390/jcm10122640
APA StyleBednarikova, M., Vinklerova, P., Gottwaldova, J., Ovesna, P., Hausnerova, J., Minar, L., Felsinger, M., Valik, D., Cermakova, Z., & Weinberger, V. (2021). The Clinical Significance of DJ1 and L1CAM Serum Level Monitoring in Patients with Endometrial Cancer. Journal of Clinical Medicine, 10(12), 2640. https://doi.org/10.3390/jcm10122640






