Corneal Biomechanical Parameters and Central Corneal Thickness in Glaucoma Patients, Glaucoma Suspects, and a Healthy Population
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Examination Techniques
2.3. Statistical Analysis
3. Results
3.1. Subjects
3.1.1. CH
3.1.2. CRF
3.1.3. IOPg
3.1.4. IOPcc
3.1.5. CCT
4. Discussion
4.1. Control Group
4.2. Glaucoma, FHG, and Glaucoma Suspect Groups
4.2.1. CH
4.2.2. CRF
4.2.3. CCT
4.2.4. CH and CCT
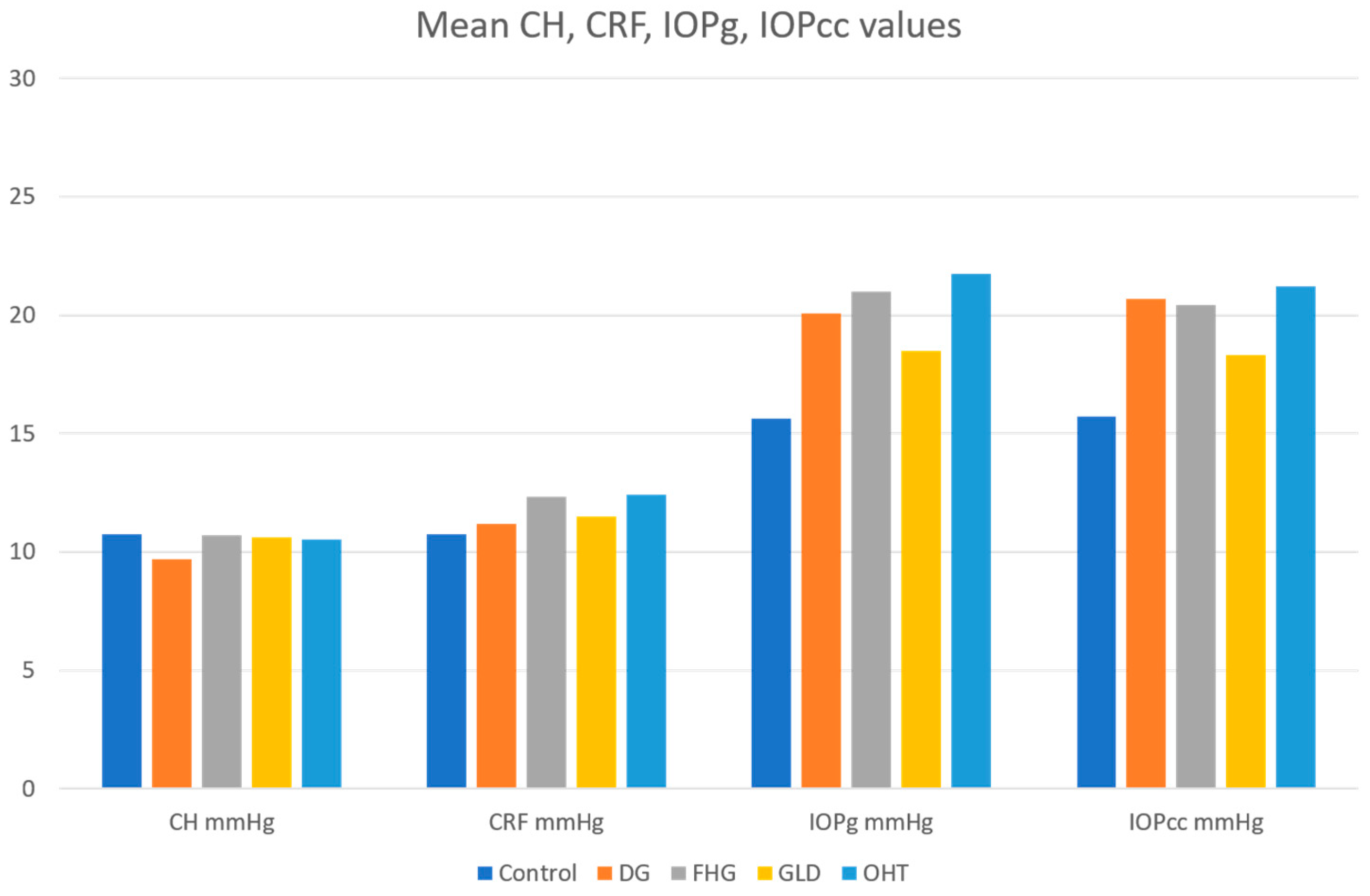
- A mean CH and CRF value of 10.75 mmHg was established in the healthy control population, which can be a reference for the Spanish population. The mean IOPg (15.63 mmHg) and IOPcc (15.72) values estimated by the ORA, in patients without ocular pathology, were similar to the mean GAT values. IOPg and IOPcc values were similar when the biomechanical properties of the cornea were within normal limits.
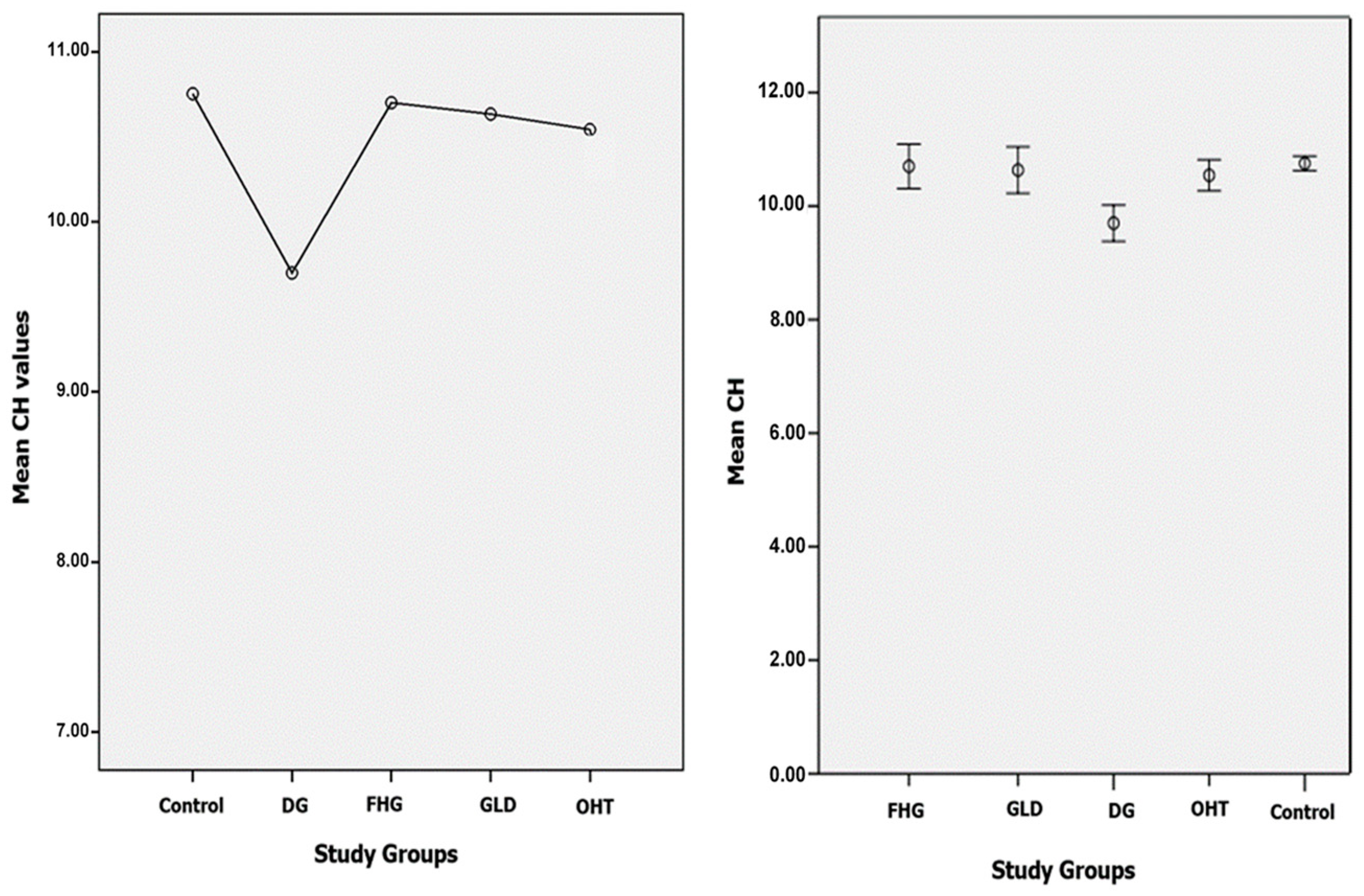
- The IOPg and IOPcc means were significantly higher than those of the control group in all glaucoma and glaucoma suspect groups. There was a significant decrease in CH in the DG group compared to the control group, and with respect to the three glaucoma suspect groups. However, the CH values in the three glaucoma suspect groups (FHG, GLD, and OHT) did not show statistically significant differences between them, or with respect to the control group. No statistically significant differences in CRF values between the DG group and the control group were found. However, elevated CRF values in all the glaucoma suspect groups were found, such differences being statistically significant with respect to the control group. There was no CCT alteration in the DG group. POAG showed its own biomechanical profile (normal or high CCT, normal or high CRF, and low CH with IOPcc > IOPg).
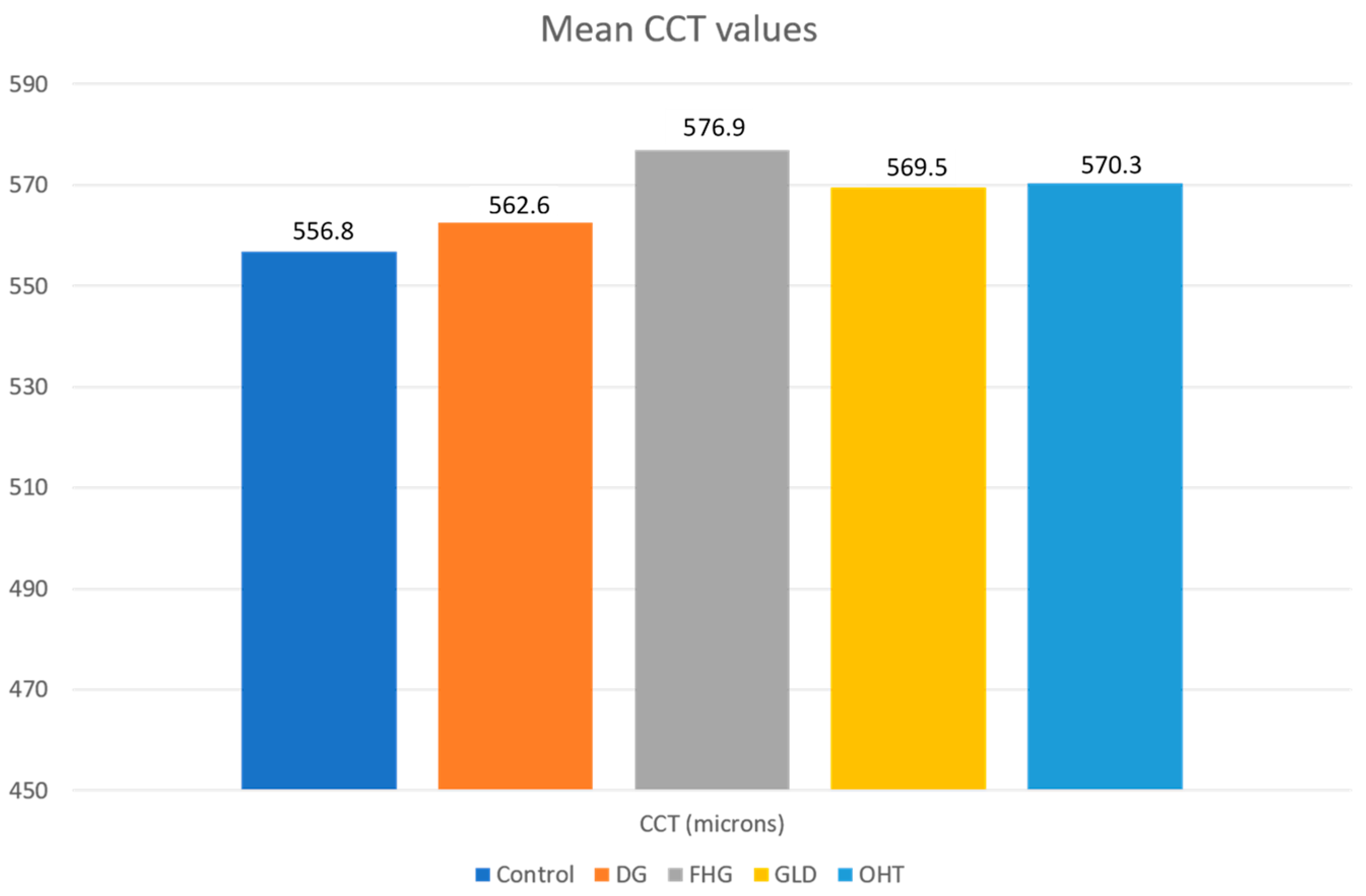
- The OHT and FHG suspect groups presented higher CRF values than the DG group and the differences were statistically significant. The mean CCT values were higher than the control group in all groups, although there were no statistically significant differences. Ocular hypertension suspect cases showed the highest CCT values. OHT showed its own biomechanical profile (high CCT, high CRF, and normal or high CH with IOPcc < IOPg).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fung, Y.C. The mechanical properties of living tissues. In Biomechanics; Springer: New York, NY, USA, 1981; pp. 221–230. [Google Scholar]
- Ruiz–De-Gopegui, E.; Ascaso, F.; Del Buey, M.; Cristóbal, J. Effects of encircling scleral buckling on the morphology and biomechanical properties of the cornea. Arch. Soc. Española Oftalmol. (Engl. Ed.) 2011, 86, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Del Buey, M.A.; Casas, P.; Caramello, C.; Lopez, N.; de la Rica, M.; Subiron, A.B.; Lanchares, E.; Huerva, V.; Grzybowski, A.; Ascaso, F.J. An Update on Corneal Biomechanics and Architecture in Diabetes. J. Ophthalmol. 2019, 2019, 7645352. [Google Scholar] [CrossRef] [PubMed]
- Del Buey, M.A.; Lavilla, L.; Ascaso, F.J.; Lanchares, E.; Huerva, V.; Cristóbal, J.A. Assessment of corneal biomechanical properties and intraocular pressure in myopic Spanish healthy population. J. Ophthalmol. 2014, 2014, 905129. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Y.; Wei, P.; Jhanji, V. Biomechanics and structure of the cornea: Implications and association with corneal disor-ders. Surv. Ophthalmol. 2018, 63, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, J.A.; del Buey, M.A.; Ascaso, F.J.; Lanchares, E.; Calvo, B.; Doblaré, M. Effect of Limbal Relaxing Incisions During Phacoemulsification Surgery Based on Nomogram Review and Numerical Simulation. Cornea 2009, 28, 1042–1049. [Google Scholar] [CrossRef]
- Lanchares, E.; Calvo, B.; Del Buey, M.A.; Cristóbal, J.A.; Doblaré, M. The Effect of Intraocular Pressure on the Outcome of Myopic Photorefractive Keratectomy: A Numerical Approach. J. Heal. Eng. 2010, 1, 461–476. [Google Scholar] [CrossRef]
- Lanchares, E.; Del Buey, M.A.; Cristóbal, J.A.; Lavilla, L.; Calvo, B. Biomechanical property analysis after corneal collagen cross-linking in relation to ultraviolet A irradiation time. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1223–1227. [Google Scholar] [CrossRef]
- Lanchares, E.; Del Buey, M.A.; Cristóbal, J.A.; Calvo, B.; Ascaso, F.J.; Malvè, M. Computational Simulation of Scleral Buckling Surgery for Rhegmatogenous Retinal Detachment: On the Effect of the Band Size on the Myopization. J. Ophthalmol. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Luce, D.A. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J. Cataract. Refract. Surg. 2005, 31, 156–162. [Google Scholar] [CrossRef]
- Ortiz, D.; Piñero, D.; Shabayek, M.H.; Arnalich-Montiel, F.; Alió, J.L. Corneal biomechanical properties in normal, post-laser in situ keratomileusis, and keratoconic eyes. J. Cataract. Refract. Surg. 2007, 33, 1371–1375. [Google Scholar] [CrossRef]
- Del Buey, M.A.; Cristóbal, J.A.; Ascaso, F.J.; Lavilla, L.; Lanchares, E. Biomechanical properties of the cornea in Fuchs’ corneal dys-trophy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3199–3202. [Google Scholar] [CrossRef]
- Shah, S.; Laiquzzaman, M.; Bhojwani, R.; Mantry, S.; Cunliffe, I. Assessment of the Biomechanical Properties of the Cornea with the Ocular Response Analyzer in Normal and Keratoconic Eyes. Investig. Opthalmology Vis. Sci. 2007, 48, 3026–3031. [Google Scholar] [CrossRef]
- Touboul, D.; Roberts, C.; Kérautret, J.; Garra, C.; Maurice-Tison, S.; Saubusse, E.; Colin, J. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J. Cataract. Refract. Surg. 2008, 34, 616–622. [Google Scholar] [CrossRef]
- Susanna, C.N.; Diniz-Filho, A.; Daga, F.B.; Susanna, B.N.; Zhu, F.; Ogata, N.G.; Medeiros, F.A. A Prospective Longitudinal Study to Investigate Corneal Hysteresis as a Risk Factor for Predicting Development of Glaucoma. Am. J. Ophthalmol. 2018, 187, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.O.; Beiser, J.A.; Brandt, J.D.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R. The ocular hypertension treatment study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 714–720. [Google Scholar] [CrossRef]
- Murphy, M.L.; Pokrovskaya, O.; Galligan, M.; O’Brien, C. Corneal hysteresis in patients with glaucoma-like optic discs, ocular hypertension and glaucoma. BMC Ophthalmol. 2017, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Susanna, B.N.; Ogata, N.G.; Jammal, A.A.; Susanna, C.N.; Berchuck, S.I.; Medeiros, F.A. Corneal Biomechanics and Visual Field Progression in Eyes with Seemingly Well-Controlled Intraocular Pressure. Ophthalmology 2019, 126, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in Adults—Screening, Diagnosis, and Management. JAMA 2021, 325, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Hagishima, M.; Fujimura, F.; Shimizu, K. Factors affecting corneal hysteresis in normal eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 1491–1494. [Google Scholar] [CrossRef]
- Shen, M.; Fan, F.; Xue, A.; Wang, J.; Zhou, X.; Lu, F. Biomechanical properties of the cornea in high myopia. Vis. Res. 2008, 48, 2167–2171. [Google Scholar] [CrossRef]
- Kirwan, C.; O’Keefe, M.; Lanigan, B. Corneal Hysteresis and Intraocular Pressure Measurement in Children Using the Reichert Ocular Response Analyzer. Am. J. Ophthalmol. 2006, 142, 990–992. [Google Scholar] [CrossRef]
- Haseltine, S.J.; Pae, J.; Ehrlich, J.R.; Shammas, M.; Radcliffe, N.M. Variation in corneal hysteresis and central corneal thickness among black, hispanic and white subjects. Acta Ophthalmol. 2012, 90, 626–631. [Google Scholar] [CrossRef]
- Strobbe, E.; Cellini, M.; Barbaresi, U.; Campos, E.C. Influence of Age and Gender on Corneal Biomechanical Properties in a Healthy Italian Population. Cornea 2014, 33, 968–972. [Google Scholar] [CrossRef]
- Celebi, A.R.C.; Kilavuzoglu, A.E.; Altiparmak, U.E.; Yurteri, C.B.C. Age-related change in corneal biomechanical parameters in a healthy Caucasian population. Ophthalmic Epidemiol. 2017, 25, 55–62. [Google Scholar] [CrossRef]
- Al-Arfaj, K.; Yassin, S.A.; Al-Dairi, W.; Al-Shamlan, F.; Al-Jindan, M. Corneal biomechanics in normal Saudi individuals. Saudi J. Ophthalmol. 2016, 30, 180–184. [Google Scholar] [CrossRef]
- Kopito, R.; Gaujoux, T.; Montard, R.; Touzeau, O.; Allouch, C.; Borderie, V.; Laroche, L. Reproducibility of viscoelastic property and intraocular pressure measurements obtained with the Ocular Response Analyzer. Acta Ophthalmol. 2010, 89, e225–e230. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, P.-B.; Li, C.-Y.; Zhu, X.-H.; Duan, X.-C. Assessment of intraocular pressure measured by Reichert Ocular Response Analyzer, Goldmann Applanation Tonometry, and Dynamic Contour Tonometry in healthy individuals. Int. J. Ophthalmol. 2012, 5, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, M.; Javadi, M.A.; Yazdani, S.; Ghahari, E.; Behroozi, Z.; Soleimanizad, R.; Moghimi, S.; Nilforoushan, N.; Zarei, R.; Eslami, Y.; et al. Distribution of intraocular pressure, central corneal thickness and vertical cup-to-disc ratio in a healthy Iranian population: The Yazd Eye Study. Acta Ophthalmol. 2016, 95. [Google Scholar] [CrossRef] [PubMed]
- Peyman, M.; Tai, L.Y.; Khaw, K.W.; Ng, C.M.; Win, M.M.; Subrayan, V. Accutome PachPen handheld ultrasonic pachymeter: Intraobserver repeatability and interobserver reproducibility by personnel of different training grades. Int. Ophthalmol. 2015, 35, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Gros-Otero, J.; Arruabarrena-Sánchez, C.; Teus, M. Espesor corneal central en una población sana española [Central corneal thickness in a healthy Spanish population]. Arch. Soc. Esp. Oftalmol. 2011, 86, 73–76. [Google Scholar] [CrossRef]
- Muir, K.W.; Duncan, L.; Enyedi, L.B.; Freedman, S.F. Central Corneal Thickness in Children: Racial Differences (Black vs. White) and Correlation With Measured Intraocular Pressure. J. Glaucoma 2006, 15, 520–523. [Google Scholar] [CrossRef]
- Mansouri, K.; Leite, M.T.; Weinreb, R.N.; Tafreshi, A.; Zangwill, L.M.; Medeiros, F.A. Association between cor-neal biomechanical properties and glaucoma severity. Am. J. Ophthalmol. 2012, 153, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Ang, G.S.; Bochmann, F.; Townend, J.; Azuara-Blanco, A. Corneal Biomechanical Properties in Primary Open Angle Glaucoma and Normal Tension Glaucoma. J. Glaucoma 2008, 17, 259–262. [Google Scholar] [CrossRef]
- Kaushik, S.; Pandav, S.S.; Banger, A.; Aggarwal, K.; Gupta, A. Relationship Between Corneal Biomechanical Properties, Central Corneal Thickness, and Intraocular Pressure Across the Spectrum of Glaucoma. Am. J. Ophthalmol. 2012, 153, 840–849.e2. [Google Scholar] [CrossRef]
- Park, J.H.; Jun, R.M.; Choi, K.-R. Significance of corneal biomechanical properties in patients with progressive normal-tension glaucoma. Br. J. Ophthalmol. 2015, 99, 746–751. [Google Scholar] [CrossRef]
- Pillunat, K.R.; Hermann, C.; Spoerl, E.; Pillunat, L.E. Analyzing biomechanical parameters of the cornea with glaucoma severity in open-angle glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1345–1351. [Google Scholar] [CrossRef]
- Detry-Morel, M.; Jamart, J.; Hautenauven, F.; Pourjavan, S. Comparison of the corneal biomechanical properties with the Ocular Response Analyzer® (ORA) in African and Caucasian normal subjects and patients with glaucoma. Acta Ophthalmol. 2011, 90, e118–e124. [Google Scholar] [CrossRef] [PubMed]
- Del Buey, M.A.; Cristóbal, J.; Lavilla, L.; Palomino, C.; Lanchares, E. The use of the Reichert Ocular Response Analyser to establish the relationship between bomechanical properties and ocular pathology. Investig. Ophthalmol. Vis. Sci. 2008, 49, 653. [Google Scholar]
- Del Buey, M.A.; Lavilla, L.; Cristóbal, J.A.; Lanchares, E.; Palomino, C.; Calvo, B. Corneal resistance factor (CRF) and corneal hyste-resis (CH) associated with glaucoma damage. In Book of Abstract XXVI Congress of the ESCRS; ESCRS: Berlin, Germany, 2008. [Google Scholar]
- Spörl, E.; Terai, N.; Haustein, M.; Böhm, A.; Raiskup-Wolf, F.; Pillunat, L.E. Biomechanical condition of the cornea as a new indica-tor for pathological and structural changes. Ophthalmologe 2009, 106, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Wasielica-Poslednik, J.; Berisha, F.; Aliyeva, S.; Pfeiffer, N.; Hoffmann, E.M. Reproducibility of ocular response analyzer meas-urements and their correlation with central corneal thickness. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Detry-Morel, M.; Jamart, J.; Pourjavan, S. Evaluation of corneal biomechanical properties with the Reichert Ocular Response Analyzer. Eur. J. Ophthalmol. 2011, 21, 138–148. [Google Scholar] [CrossRef]
- Prata, T.S.; Lima, V.C.; De Moraes, C.G.V.; Guedes, L.M.; Magalhães, F.P.; Teixeira, S.H.; Ritch, R.; Paranhos, A. Factors associated with topographic changes of the optic nerve head induced by acute intraocular pressure reduction in glaucoma patients. Eye 2010, 25, 201–207. [Google Scholar] [CrossRef][Green Version]
- Lee, K.M.; Kim, T.W.; Lee, E.J.; Girard, M.J.A.; Mari, J.M.; Weinreb, R.N. Association of corneal hysteresis with lamina cribrosa curva-ture in primary open angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4171–4177. [Google Scholar] [CrossRef]
- Wells, A.P.; Garway-Heath, D.F.; Poostchi, A.; Wong, T.; Chan, K.C.; Sachdev, N. Corneal hysteresis but not corneal thickness cor-relates with optic nerve surface compliance in glaucoma patients. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3262–3268. [Google Scholar] [CrossRef]
- Meda, R.; Wang, Q.; Paoloni, D.; Harasymowycz, P.; Brunette, I. The impact of chronic use of prostaglandin analogues on the biomechanical properties of the cornea in patients with primary open-angle glaucoma. Br. J. Ophthalmol. 2016, 101, 120–125. [Google Scholar] [CrossRef]
- Sun, L.; Shen, M.; Wang, J.; Fang, A.; Xu, A.; Fang, H.; Lu, F. Recovery of Corneal Hysteresis After Reduction of Intraocular Pressure in Chronic Primary Angle-Closure Glaucoma. Am. J. Ophthalmol. 2009, 147, 1061–1066.e2. [Google Scholar] [CrossRef] [PubMed]
- Congdon, N.G.; Broman, A.T.; Bandeen-Roche, K.; Grover, D.; Quigley, H.A. Central corneal thickness and corneal hysteresis associated with glau-coma damage. Am. J. Ophthalmol. 2006, 141, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, F.; Ang, G.S.; Azuara-Blanco, A. Lower corneal hysteresis in glaucoma patients with acquired pit of the optic nerve (APON). Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 735–738. [Google Scholar] [CrossRef]
- Del Buey, M.A.; Cristobal Bescos, J.A.; Lavilla, L.; Ascaso, F.; Mateo-Orobia, A.; Jimenez, B.; Ruiz de Gopegui, E.; Palominio, C. Bio-mechanical properties in healthy subjects with and without refractive errors. A comparative study. Acta Ophthalmol. 2010, 88. [Google Scholar] [CrossRef]
- Roberts, C.J.; Reinstein, D.Z.; Archer, T.J.; Mahmoud, A.M.; Gobbe, M.; Lee, L. Comparison of ocular biomechanical response pa-rameters in myopic and hyperopic eyes using dynamic bidirectional applanation analysis. J. Cataract Refract. Surg. 2014, 40, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Yagci, R.; Eksioglu, U.; Midillioglu, I.; Yalvac, I.; Altiparmak, E.; Duman, S. Central Corneal Thickness in Primary Open Angle Glaucoma, Pseudoexfoliative Glaucoma, Ocular Hypertension, and Normal Population. Eur. J. Ophthalmol. 2005, 15, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Fabian, I.D.; Barequet, I.S.; Skaat, A.; Rechtman, E.; Goldenfeld, M.; Roberts, C.J.; Melamed, S. Intraocular Pressure Measurements and Biomechanical Properties of the Cornea in Eyes After Penetrating Keratoplasty. Am. J. Ophthalmol. 2011, 151, 774–781. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 574) | DG (n = 147) | FHG (n = 78) | GLD (n = 90) | OHT (n = 176) | Total (n = 1065) | |
|---|---|---|---|---|---|---|
| Female | 291 (50.7%) | 65 (44.2%) | 56 (71.8%) | 71 (78.9%) | 95 (54%) | 578 (54.3%) |
| Male | 283 (49.3%) | 82 (55.8%) | 22 (28.2%) | 19 (21.1%) | 81 (46%) | 487 (45.7%) |
| Age (years) | 39 ± 15 | 56 ± 16 | 47 ± 15 | 48 ± 14 | 51 ± 13 | 46 ± 17 |
| Control (n = 574) | DG (n = 147) | FHG (n = 78) | GLD (n = 90) | OHT (n = 176) | |
|---|---|---|---|---|---|
| IOPg (mmHg) | 15.63 ± 3.1 | 20.07 ± 3.6 | 21.01 ± 4.0 | 18.48 ± 3.6 | 21.76 ± 3.6 |
| IOPcc (mmHg) | 15.72 ± 3.0 | 20.68 ± 3.7 | 20.41 ± 3.8 | 18.30 ± 3.9 | 21.22 ± 3.8 |
| CH (mmHg) | 10.75 ± 1.5 | 9.69 ± 1.9 | 10.70 ± 1.7 | 10.63 ± 1.9 | 10.54 ± 1.8 |
| CRF (mmHg) | 10.75 ± 1.6 | 11.18 ± 2.0 | 12.32 ± 1.9 | 11.50 ± 1.9 | 12.41 ± 1.8 |
| CCT (μ) | 556.8 ± 35.3 | 562.6 ± 39.6 | 576.3 ± 38.3 | 569.5 ± 31.5 | 570.3 ± 34.7 |
| Dependent Variable | (I) Pathology | (J) Pathology | Mean Difference (I–J) | SE | p Value | 95% CI | |
|---|---|---|---|---|---|---|---|
| Upper Limit | Lower Limit | ||||||
| CH | Control | DG | 1.053 (*) | 0.174 | 0.000 * | 0.502 | 1.604 |
| FHG | 0.052 | 0.207 | 1.000 | −0.611 | 0.717 | ||
| GLD | 0.119 | 0.216 | 1.000 | −0.572 | 0.811 | ||
| OHT | 0.210 | 0.153 | 0.995 | −0.272 | 0.693 | ||
| DG | Control | −1.053 (*) | 0.174 | 0.000 * | −1.604 | −0.502 | |
| FHG | −1.000 (*) | 0.254 | 0.003 * | −1.807 | −0.194 | ||
| GLD | −0.934 (*) | 0.262 | 0.013 * | −1.763 | −0.104 | ||
| OHT | −0.842 (*) | 0.213 | 0.003 * | −1.513 | −0.172 | ||
| FHG | Control | −0.052 | 0.207 | 1.000 | −0.717 | 0.611 | |
| DG | 1.000 (*) | 0.254 | 0.003 * | 0.194 | 1.807 | ||
| GLD | 0.066 | 0.285 | 1.000 | −0.836 | 0.970 | ||
| OHT | 0.157 | 0.240 | 1.000 | −0.605 | 0.921 | ||
| GLD | Control | −0.119 | 0.216 | 1.000 | −0.811 | 0.572 | |
| DG | 0.934 (*) | 0.262 | 0.013 * | 0.104 | 1.763 | ||
| FHG | −0.066 | 0.285 | 1.000 | −0.970 | 0.836 | ||
| OHT | 0.091 | 0.248 | 1.000 | −0.696 | 0.879 | ||
| OHT | Control | −0.210 | 0.153 | 0.995 | −0.693 | 0.272 | |
| DG | 0.842 (*) | 0.213 | 0.003 * | 0.172 | 1.513 | ||
| FHG | −0.157 | 0.240 | 1.000 | −0.921 | 0.605 | ||
| GLD | −0.091 | 0.248 | 1.000 | −0.879 | 0.696 | ||
| Dependent Variable | (I) Pathology | (J) Pathology | Mean Difference (I–J) | SE | p-Value | 95% CI | |
|---|---|---|---|---|---|---|---|
| Upper Limit | Lower Limit | ||||||
| CRF | Control | DG | −0.429 | 0.180 | 0.403 | −0.998 | 0.140 |
| FHG | −1.562 (*) | 0.235 | 0.000 * | −2.317 | −0.808 | ||
| GLD | −0.745 (*) | 0.214 | 0.020 * | −1.430 | −0.060 | ||
| OHT | −1.653 (*) | 0.155 | 0.000 * | −2.144 | −1.163 | ||
| DG | Control | 0.429 | 0.180 | 0.403 | −0.140 | 0.998 | |
| FHG | −1.133 (*) | 0.279 | 0.002 * | −2.018 | −0.249 | ||
| GLD | −0.316 | 0.262 | 0.999 | −1.145 | 0.511 | ||
| OHT | −1.224 (*) | 0.216 | 0.000 * | −1.906 | −0.543 | ||
| FHG | Control | 1.562 (*) | 0.235 | 0.000 * | 0.808 | 2.317 | |
| DG | 1.133 (*) | 0.279 | 0.002 * | 0.249 | 2.018 | ||
| GLD | 0.816 | 0.302 | 0.193 | −0.141 | 1.775 | ||
| OHT | −0.091 | 0.263 | 1.000 | −0.929 | 0.747 | ||
| GLD | Control | 0.745 (*) | 0.214 | 0.020 * | 0.060 | 1.430 | |
| DG | 0.316 | 0.262 | 0.999 | −0.511 | 1.145 | ||
| FHG | −0.816 | 0.302 | 0.193 | −1.775 | 0.141 | ||
| OHT | −0.908 (*) | 0.245 | 0.008 * | −1.686 | −0.129 | ||
| OHT | Control | 1.653 (*) | 0.155 | 0.000 * | 1.163 | 2.144 | |
| DG | 1.224 (*) | 0.216 | 0.000 * | 0.543 | 1.906 | ||
| FHG | 0.091 | 0.263 | 1.000 | −0.747 | 0.929 | ||
| GLD | 0.908 (*) | 0.245 | 0.008 * | 0.129 | 1.686 | ||
| Dependent Variable | (I) Pathology | (J) Pathology | Mean Difference (I–J) | SE | p-Value | 95% CI | |
|---|---|---|---|---|---|---|---|
| Upper Limit | Lower Limit | ||||||
| IOPg | Control | DG | −4.435 (*) | 0.328 | 0.000 * | −5.472 | −3.397 |
| FHG | −5.378 (*) | 0.474 | 0.000 * | −6.902 | −3.854 | ||
| GLD | −2.844 (*) | 0.408 | 0.000 * | −4.149 | −1.539 | ||
| OHT | −6.130 (*) | 0.302 | 0.000 * | −7.083 | −5.177 | ||
| DG | Control | 4.435 (*) | 0.328 | 0.000 * | 3.397 | 5.472 | |
| FHG | −0.943 | 0.546 | 0.921 | −2.680 | 0.792 | ||
| GLD | 1.590 (*) | 0.490 | 0.039 * | 0.038 | 3.142 | ||
| OHT | −1.695 (*) | 0.406 | 0.001 * | −2.974 | −0.415 | ||
| FHG | Control | 5.378 (*) | 0.474 | 0.000 * | 3.854 | 6.902 | |
| DG | 0.943 | 0.546 | 0.921 | −0.792 | 2.680 | ||
| GLD | 2.534 (*) | 0.598 | 0.001 * | 0.636 | 4.432 | ||
| OHT | −0.751 | 0.531 | 0.992 | −2.442 | 0.939 | ||
| GLD | Control | 2.844 (*) | 0.408 | 0.000 * | 1.539 | 4.149 | |
| DG | −1.590 (*) | 0.490 | 0.039 * | −3.142 | −0.038 | ||
| FHG | −2.534 (*) | 0.598 | 0.001 * | −4.432 | −0.636 | ||
| OHT | −3.285 (*) | 0.474 | 0.000 * | −4.785 | −1.785 | ||
| OHT | Control | 6.130 (*) | 0.302 | 0.000 * | 5.177 | 7.083 | |
| DG | 1.695 (*) | 0.406 | 0.001 * | 0.415 | 2.974 | ||
| FHG | 0.751 | 0.531 | 0.992 | −0.939 | 2.442 | ||
| GLD | 3.285 (*) | 0.474 | 0.000 * | 1.785 | 4.785 | ||
| Dependent Variable | (I) Pathology | (J) Pathology | Mean Difference (I–J) | SE | p-Value | 95% CI | |
|---|---|---|---|---|---|---|---|
| Upper Limit | Lower Limit | ||||||
| IOPcc | Control | DG | −4.954 (*) | 0.335 | 0.000 * | −6.014 | −3.895 |
| FHG | −4.686 (*) | 0.450 | 0.000 * | −6.131 | −3.241 | ||
| GLD | −2.577 (*) | 0.434 | 0.000 * | −3.966 | −1.187 | ||
| OHT | −5.503 (*) | 0.314 | 0.000 * | −6.494 | −4.513 | ||
| DG | Control | 4.954 (*) | 0.335 | 0.000 * | 3.895 | 6.014 | |
| FHG | 0.268 | 0.531 | 1.000 | −1.416 | 1.953 | ||
| GLD | 2.377 (*) | 0.518 | 0.000 * | 0.737 | 4.017 | ||
| OHT | −0.548 | 0.422 | 0.998 | −1.877 | 0.779 | ||
| FHG | Control | 4.686 (*) | 0.450 | 0.000 * | 3.241 | 6.131 | |
| DG | −0.268 | 0.531 | 1.000 | −1.953 | 1.416 | ||
| GLD | 2.109 (*) | 0.599 | 0.015 * | 0.211 | 4.007 | ||
| OHT | −0.817 | 0.518 | 0.969 | −2.462 | 0.828 | ||
| GLD | Control | 2.577 (*) | 0.434 | 0.000 * | 1.187 | 3.966 | |
| DG | −2.377 (*) | 0.518 | 0.000 * | −4.017 | −0.737 | ||
| FHG | −2.109 (*) | 0.599 | 0.015 * | −4.007 | −0.211 | ||
| OHT | −2.926 (*) | 0.505 | 0.000 * | −4.525 | −1.327 | ||
| OHT | Control | 5.503 (*) | 0.314 | 0.000 * | 4.513 | 6.494 | |
| DG | 0.548 | 0.422 | 0.998 | −0.779 | 1.877 | ||
| FHG | 0.817 | 0.518 | 0.969 | −0.828 | 2.462 | ||
| GLD | 2.926 (*) | 0.505 | 0.000 * | 1.327 | 4.525 | ||
| Dependent Variable | (I) Pathology | (J) Pathology | Mean Difference (I–J) | SE | p-Value | 95% CI | |
|---|---|---|---|---|---|---|---|
| Upper Limit | Lower Limit | ||||||
| CCT | Control | DG | −5.80 | 4.36 | 0.997 | −19.71 | 8.10 |
| FHG | −19.45 * | 5.01 | 0.006 * | −35.64 | −3.27 | ||
| GLD | −12.64 | 4.34 | 0.122 | −26.63 | 1.35 | ||
| OHT | −13.47 * | 3.52 | 0.005 * | −24.62 | −2.33 | ||
| DG | Control | 5.80 | 4.36 | 0.997 | −8.10 | 19.71 | |
| FHG | −13.64 | 6.26 | 0.585 | −33.54 | 6.24 | ||
| GLD | −6.83 | 5.73 | 0.999 | −25.02 | 11.35 | ||
| OHT | −7.67 | 5.14 | 0.984 | −23.92 | 8.58 | ||
| FHG | Control | 19.45 * | 5.01 | 0.006 * | 3.27 | 35.64 | |
| DG | 13.64 | 6.26 | 0.585 | −6.24 | 33.54 | ||
| GLD | 6.81 | 6.24 | 1.000 | −13.07 | 26.70 | ||
| OHT | 5.97 | 5.70 | 1.000 | −12.21 | 24.16 | ||
| GLD | Control | 12.64 | 4.34 | 0.122 | −1.35 | 26.63 | |
| DG | 6.83 | 5.73 | 0.999 | −11.35 | 25.02 | ||
| FHG | −6.81 | 6.24 | 1.000 | −26.70 | 13.07 | ||
| OHT | −0.83 | 5.11 | 1.000 | −17.12 | 15.44 | ||
| OHT | Control | 13.47 * | 3.52 | 0.005 * | 2.33 | 24.62 | |
| DG | 7.67 | 5.14 | 0.984 | −8.58 | 23.92 | ||
| FHG | −5.97 | 5.70 | 1.000 | −24.16 | 12.21 | ||
| GLD | 0.83 | 5.11 | 1.000 | −15.44 | 17.12 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Buey-Sayas, M.Á.; Lanchares-Sancho, E.; Campins-Falcó, P.; Pinazo-Durán, M.D.; Peris-Martínez, C. Corneal Biomechanical Parameters and Central Corneal Thickness in Glaucoma Patients, Glaucoma Suspects, and a Healthy Population. J. Clin. Med. 2021, 10, 2637. https://doi.org/10.3390/jcm10122637
del Buey-Sayas MÁ, Lanchares-Sancho E, Campins-Falcó P, Pinazo-Durán MD, Peris-Martínez C. Corneal Biomechanical Parameters and Central Corneal Thickness in Glaucoma Patients, Glaucoma Suspects, and a Healthy Population. Journal of Clinical Medicine. 2021; 10(12):2637. https://doi.org/10.3390/jcm10122637
Chicago/Turabian Styledel Buey-Sayas, Mª. Ángeles, Elena Lanchares-Sancho, Pilar Campins-Falcó, María Dolores Pinazo-Durán, and Cristina Peris-Martínez. 2021. "Corneal Biomechanical Parameters and Central Corneal Thickness in Glaucoma Patients, Glaucoma Suspects, and a Healthy Population" Journal of Clinical Medicine 10, no. 12: 2637. https://doi.org/10.3390/jcm10122637
APA Styledel Buey-Sayas, M. Á., Lanchares-Sancho, E., Campins-Falcó, P., Pinazo-Durán, M. D., & Peris-Martínez, C. (2021). Corneal Biomechanical Parameters and Central Corneal Thickness in Glaucoma Patients, Glaucoma Suspects, and a Healthy Population. Journal of Clinical Medicine, 10(12), 2637. https://doi.org/10.3390/jcm10122637







