Clinical Phenotypes and Predictors of Remission in Primary Membranous Nephropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Treatment
2.3. Study Follow-Up and Data Collection
2.4. Study Endpoints
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Shaughnessy, M.M.; Hogan, S.L.; Poulton, C.J.; Falk, R.J.; Singh, H.K.; Nickeleit, V.; Jennette, J.C. Temporal and demographic trends in glomerular disease epidemiology in the southeastern United States, 1986–2015. Clin. J. Am. Soc. Nephrol. 2017, 12, 614–623. [Google Scholar] [CrossRef]
- Obrisca, B.; Ismail, G.; Jurubita, R.; Baston, C.; Andronesi, A.; Mircescu, G. Antiphospholipase A2 Receptor Autoantibodies: A Step Forward in the Management of Primary Membranous Nephropathy. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Beck, L.H., Jr.; Bonegio, R.G.B.; Lambeau, G.; Beck, D.M.; Powell, D.W.; Cummins, T.D.; Klein, J.B.; Salant, D.J. M-Type Phospholipase A2 Receptor as Target Antigen in Idiopathic Membranous Nephropathy. N. Engl. J. Med. 2009, 361, 11–21. [Google Scholar] [CrossRef]
- Tomas, N.M.; Beck, L.H.; Meyer-Schwesinger, C.; Seitz-Polski, B.; Ma, H.; Zahner, G.; Dolla, G.; Hoxha, E.; Helmchen, U.; Dabert-Gay, A.S.; et al. Thrombospondin Type-1 Domain-Containing 7A in Idiopathic Membranous Nephropathy. N. Engl. J. Med. 2014, 371, 2277–2287. [Google Scholar] [CrossRef]
- Sethi, S.; Madden, B.J.; Debiec, H.; Charlesworth, C.M.; Gross, L.; Ravindran, A.; Hummel, A.M.; Specks, U.; Fervenza, F.C.; Ronco, P.; et al. Exostosin 1/exostosin 2–associated membranous nephropathy. J. Am. Soc. Nephrol. 2019, 30, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Caza, T.; Hassen, S.; Dvanajscak, Z.; Kuperman, M.; Edmondson, R.; Herzog, C.; Storey, A.; Arthur, J.; Cossey, L.N.; Sharma, S.; et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021, 99, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Debiec, H.; Madden, B.; Vivarelli, M.; Charlesworth, M.C.; Ravindran, A.; Gross, L.; Ulinski, T.; Buob, D.; Tran, C.L.; et al. Semaphorin 3B–associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int. 2020, 98, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Madden, B.; Debiec, H.; Morelle, J.; Charlesworth, M.C.; Gross, L.; Negron, V.; Buob, D.; Chaudhry, S.; Jadoul, M.; et al. Protocadherin 7—Associated Membranous Nephropathy. J. Am. Soc. Nephrol. 2021, 32, 1249–1261, ASN.2020081165. [Google Scholar] [CrossRef]
- Sethi, S. New “Antigens” in Membranous Nephropathy. J. Am. Soc. Nephrol. 2021, 32, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, Y.; Fu, P. Diagnostic test accuracy of serum anti-PLA2R autoantibodies and glomerular PLA2R antigen for diagnosing idiopathic membranous nephropathy: An updated meta-analysis. Front. Med. 2018, 5, 101. [Google Scholar] [CrossRef]
- Oh, Y.J.; Yang, S.H.; Kim, D.K.; Kang, S.W.; Kim, Y.S. Autoantibodies against phospholipase A2 receptor in Korean patients with membranous nephropathy. PLoS ONE 2013, 8, e62151. [Google Scholar] [CrossRef]
- Bech, A.P.; Hofstra, J.M.; Brenchley, P.E.; Wetzels, J.F.M. Association of anti-PLA2R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2014, 9, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.H., Jr.; Fervenza, F.C.; Beck, D.M.; Bonegio, R.G.B.; Malik, F.A.; Erickson, S.B.; Cosio, F.G.; Cattran, D.C.; Salant, D.J. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Hofstra, J.M.; Beck, L.H.; Beck, D.M.; Wetzels, J.F.; Salant, D.J. Anti-phospholipase a2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2011, 6, 1286–1291. [Google Scholar] [CrossRef]
- Hofstra, J.M.; Debiec, H.; Short, C.D.; Pellé, T.; Kleta, R.; Mathieson, P.W.; Ronco, P.; Brenchley, P.E.; Wetzels, J.F. Antiphospholipase A 2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 2012, 23, 1735–1743. [Google Scholar] [CrossRef]
- Kim, Y.G.; Choi, Y.W.; Kim, S.Y.; Moon, J.Y.; Ihm, C.G.; Lee, T.W.; Jeong, K.-H.; Yang, S.H.; Kim, Y.S.; Oh, Y.J.; et al. Anti-phospholipase A2 receptor antibody as prognostic indicator in idiopathic membranous nephropathy. Am. J. Nephrol. 2015, 42, 250–257. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Debiec, H.; Ruggiero, B.; Chianca, A.; Pellé, T.; Gaspari, F.; Suardi, F.; Gagliardini, E.; Orisio, S.; Benigni, A.; et al. Anti-Phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J. Am. Soc. Nephrol. 2015, 26, 2545–2558. [Google Scholar] [CrossRef]
- Song, E.J.; Jeong, K.H.; Yang, Y.A.; Lim, J.H.; Jung, H.Y.; Choi, J.Y.; Cho, J.H.; Kim, C.D.; Kim, Y.L.; Park, S.H. Anti-phospholipase A2 receptor antibody as a prognostic marker in patients with primary membranous nephropathy. Kidney Res. Clin. Pract. 2018, 37, 426. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.A.M.E.G.; Abdul Hamid, M.A.; Tervaert, J.W.C.; Damoiseaux, J.G.M.C.; Van Paassen, P. Anti-PLA2R antibodies as a prognostic factor in PLA2R-related membranous nephropathy. Am. J. Nephrol. 2015, 42, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Y.; Wang, Y.X.; Li, J.S.; Zhao, S.L.; Diao, T.T.; Waang, Y.; Wang, C.; Qin, Y.; Cao, Y.; Wei, Q.; et al. Serum Anti-PLA2R Antibody Predicts Treatment Outcome in Idiopathic Membranous Nephropathy. Am. J. Nephrol. 2016, 43, 129–140. [Google Scholar] [CrossRef]
- Liang, Y.; Wan, J.; Chen, Y.; Pan, Y. Serum anti-phospholipase A2 receptor (PLA2R) antibody detected at diagnosis as a predictor for clinical remission in patients with primary membranous nephropathy: A meta-analysis. BMC Nephrol. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Alsharhan, L.; Beck, L.H., Jr. Membranous Nephropathy: Core Curriculum. Am. J. Kidney Dis. 2021, 77, 440–453. [Google Scholar] [CrossRef]
- Cattran, D.C.; Feehally, J.; Cook, H.T.; Liu, Z.H.; Fervenza, F.C.; Mezzano, S.A.; Floege, J.; Wetzels, J.F.M.; Troyanov, S.; Jha, V.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. Suppl. 2012, 2, 1–274. [Google Scholar]
- Fervenza, F.C.; Appel, G.B.; Barbour, S.J.; Rovin, B.H.; Lafayette, R.A.; Aslam, N.; Rizk, D.V.; Sedor, J.R.; Simon, J.F.; McCarthy, E.T.; et al. Rituximab or Cyclosporine in the Treatment of Membranous Nephropathy. N. Engl. J. Med. 2019, 381, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Juárez, G.; Rojas-Rivera, J.; van de Logt, A.E.; Justino, J.; Sevillano, A.; Caravaca-Fontán, F. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 2021, 99, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Scolari, F.; Delbarba, E.; Santoro, D.; Gesualdo, L.; Pani, A.; Dallera, N.; Ávila, A.; Rabasco, C.; Cabello, V.; Varela, A.; et al. Rituximab or Cyclophosphamide in the Treatment of Membranous Nephropathy: The RI-CYCLO Randomized Trial. J. Am. Soc. Nephrol. 2021, 32, 972–982, ASN.2020071091. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C.; Altieri, P.; Scolari, F.; Passerini, P.; Roccatello, D.; Cesana, B.; Melis, P.; Valzorio, B.; Sasdelli, M.; Pasquali, S.; et al. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 1998, 9, 444–450. [Google Scholar] [CrossRef]
- Dahan, K.; Debiec, H.; Plaisier, E.; Cachanado, M.; Rousseau, A.; Wakselman, L.; Matignon, M.; Mousson, C.; Simon, T.; Ronco, P.; et al. Rituximab for Severe Membranous Nephropathy: A 6-Month Trial with Extended Follow-Up. J. Am. Soc. Nephrol. 2017, 28, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Rodas, L.M.; Matas-García, A.; Barros, X.; Blasco, M.; Viñas, O.; Llobell, A.; Martin, N.; Quintana, L.F. Antiphospholipase 2 receptor antibody levels to predict complete spontaneous remission in primary membranous nephropathy. Clin. Kidney J. 2019, 12, 36–41. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Joshi, M.; Chaturvedi, A.; Little, D.J.; Thurlow, J.S.; Waldman, M.; Olson, S.W. Detection of PLA2R autoantibodies before the diagnosis of membranous nephropathy. J. Am. Soc. Nephrol. 2020, 31, 208–217. [Google Scholar] [CrossRef]
- Hladunewich, M.A.; Troyanov, S.; Calafati, J.; Cattran, D.C. The natural history of the non-nephrotic membranous nephropathy patient. Clin. J. Am. Soc. Nephrol. 2009, 4, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Van de Logt, A.E.; Dahan, K.; Rousseau, A.; van der Molen, R.; Debiec, H.; Ronco, P.; Wetzels, J. Immunological remission in PLA2R-antibody–associated membranous nephropathy: Cyclophosphamide versus rituximab. Kidney Int. 2018, 93, 1016–1017. [Google Scholar] [CrossRef]
- Floege, J.; Barbour, S.J.; Cattran, D.C.; Hogan, J.J.; Nachman, P.H.; Tang, S.C.W.; Wetzels, F.M.; Cheung, M.; Wheeler, D.C.; Winkelmayer, W.C.; et al. Management and treatment of glomerular diseases (part 1): Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 268–280. [Google Scholar] [CrossRef]
- Ravindran, A.; Casal Moura, M.; Fervenza, F.C.; Nasr, S.H.; Alexander, M.P.; Fidler, M.E.; Hernandez, L.P.H.; Zhang, P.; Grande, J.P.; Cornell, L.D.; et al. In Patients with Membranous Lupus Nephritis, Exostosin-Positivity and Exostosin-Negativity Represent Two Different Phenotypes. J. Am. Soc. Nephrol. 2021, 32, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Bobart, S.A.; Tehranian, S.; Sethi, S.; Alexander, M.P.; Nasr, S.H.; Moura Marta, C.; Vrana, J.A.; Said, S.; Giesen, C.D.; Lieske, J.C.; et al. A Target Antigen–Based Approach to the Classification of Membranous Nephropathy. Mayo Clin. Proc. 2021, 96, 577–591. [Google Scholar] [CrossRef]
- Obrișcă, B.; Vornicu, A.; Jurubiță, R.; Achim, C.; Bobeică, R.; Andronesi, A.; Sorohan, B.; Herlea, V.; Procop, A.; Dina, C.; et al. Corticosteroids are the major contributors to the risk for serious infections in autoimmune disorders with severe renal involvement. Clin. Rheumatol. 2021, 1–13. [Google Scholar] [CrossRef]
- Ismail, G.; Mircescu, G.; Ditoiu, A.V.; Tacu, B.D.; Jurubita, R.; Harza, M. Risk factors for predicting venous thromboembolism in patients with nephrotic syndrome: Focus on haemostasis-related parameters. Int. Urol. Nephrol. 2014, 46, 787–792. [Google Scholar] [CrossRef] [PubMed]
| Variable | Negative PLA2R | PLA2R ab <200 RU/mL | PLA2R ab >200 RU/mL | p Value |
|---|---|---|---|---|
| Number of pts | 13 | 27 | 25 | |
| Age | 54 ± 13 | 53 ± 13 | 51 ± 11 | 0.75 |
| Gender (%M) | 76.9% | 63% | 76% | 0.58 |
| BMI (kg/m2) | 31 ± 7.7 | 30.2 ± 4.8 | 30.6 ± 4.7 | 0.9 |
| HTA (%) | 100% | 96.3% | 92% | 0.51 |
| Smoking (%) | 76.9% | 55.6% | 60% | 0.42 |
| Tromboembolic events (%) | 7.7% | 25.9% | 20% | 0.4 |
| Serum creatinine (mg/dL) | 1.15 ± 0.73 | 1.63 ± 0.94 | 1.33 ± 0.5 | 0.27 |
| eGFR (mL/min) | 79 ± 36 | 55 ± 27 | 61 ± 23 | 0.05 |
| Serum Albumin (g/dL) | 3.1 ± 0.5 | 2.8 ± 0.5 | 2.5 ± 0.6 | 0.014 |
| Total Proteins (g/dL) | 5.9 ± 0.9 | 5.6 ± 0.8 | 5 ± 0.7 | 0.01 |
| Total cholesterol (mg/dL) | 263 ± 104 | 304 ± 107 | 333 ± 115 | 0.18 |
| Triglycerides (mg/dL) | 187 ± 90 | 185 ± 101 | 223 ± 118 | 0.23 |
| Fibrinogen (mg/dL) | 597 ± 159 | 577 ± 155 | 642 ± 151 | 0.31 |
| Hemoglobin (g/dL) | 13.8 ± 1.8 | 13.8 ± 2.2 | 13.8 ± 1.6 | 0.96 |
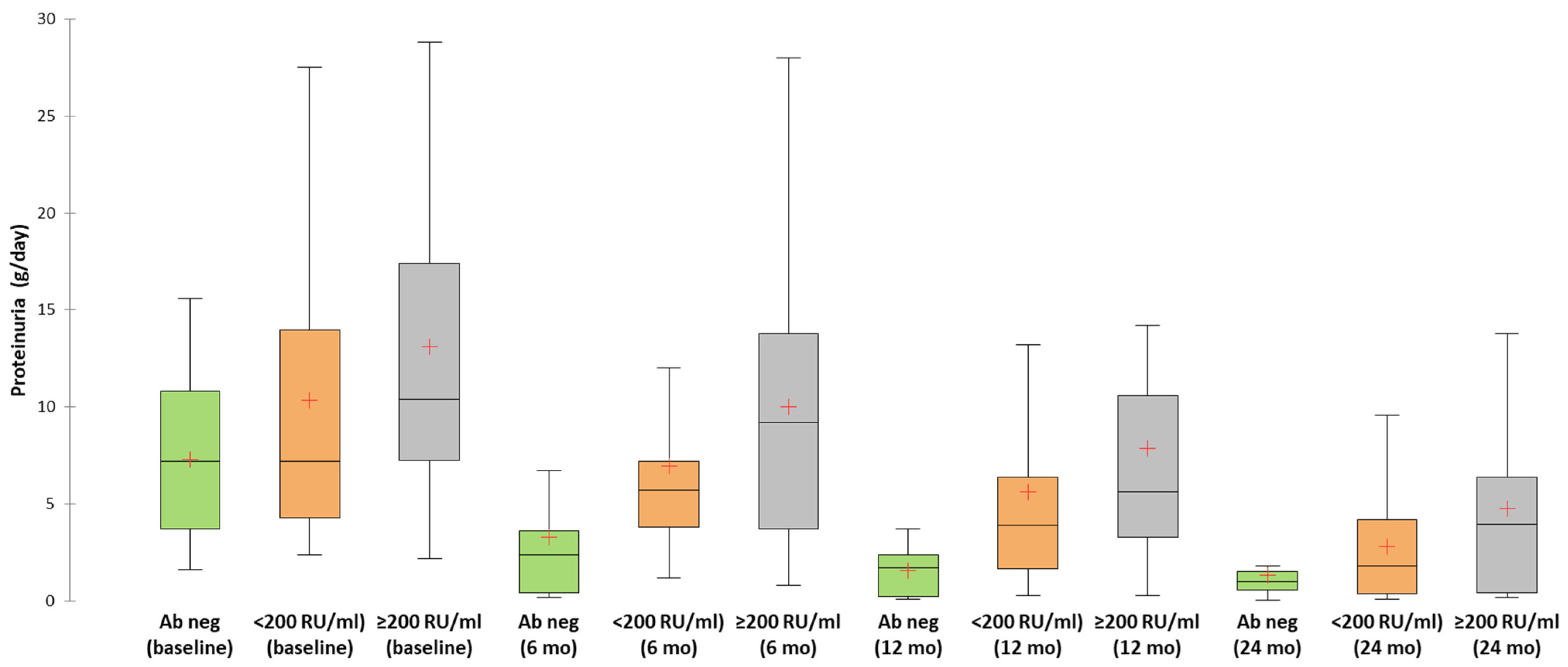
| 24 h proteinuria (g/day) | 7.2 (IQR: 3.4–11.1) | 7.2 (IQR:4.2–14.4) | 10.4 (IQR:7.1–17.4) | 0.04 |
| Hematuria (cells/mmc) | 12 (IQR:5–26) | 17 (IQR:9–40) | 25 (IQR:15–38) | 0.11 |
| Serum IgG level (mg/dL) | 474 (IQR:380–928) | 470 (IQR:340–635) | 470 (IQR:284–645) | 0.42 |
| Median PLAR2R ab titer (RU/mL) | - | 100 (IQR:80–150) | 320 (IQR: 320–467) | <0.001 |
| MN stage (% of patients) | ||||
| ● I | 45.4% | 8.3% | 10.5% | 0.008 |
| ● II | 0% | 33.3% | 36.8% | |
| ● III | 27.3% | 50% | 52.6% | |
| ● IV | 27.3% | 8.3% | 0% | |
| Clinical response (% of patients) | ||||
| ● No response | 7.7% | 25.9% | 36% | 0.4 |
| ● Partial remission | 46.2% | 40.7% | 40% | |
| ● Complete remission | 46.2% | 33.3% | 24% | |
| Time to partial remission (mo) | 18 (IQR:10.5–24) | 12 (IQR:12–24) | 18.5 (IQR:8.25–24) | 0.95 |
| Time to complete remission (mo) | 6 (IQR:6–12.7) | 18 (IQR:12–23.5) | 23 (IQR:15.7–24) | 0.048 |
| Immunological remission (% of patients) | - | 70.4% | 56% | <0.001 |
| Time to immunological remission (mo) | - | 12 (IQR:9–18) | 15 (IQR:8.25–18) | 0.99 |
| Antiproteinuric therapy (% of pts.) | 92.3% | 100% | 96% | 0.39 |
| Immunosuppressive therapy (% of pts.) | ||||
| No IS | 15.4% | 7.4% | 4% | 0.56 |
| Cyclophosphamide-based regimens | 30.8% | 51.9%% | 52% | |
| CNI-based regimens | 46.2% | 22.2% | 28% | |
| Rituximab-based regimes | 7.7% | 18.5% | 16% |
| Period | Negative anti-PLA2R-ab | Anti-PLA2R-ab <200 RU/mL | Anti-PLA2R-ab >200 RU/mL | p | |||
|---|---|---|---|---|---|---|---|
| Anti-PLA2R antibodies | |||||||
| Titer (IQR) (RU/mL) | Immunologic Remission (%) | Titer (IQR) (RU/mL) | Immunologic Remission (%) | Titer (IQR) (RU/mL) | Immunologic Remission (%) | ||
| Baseline | 100 (80–150) | - | 320 (320–467) | - | - | ||
| 3 mo | 85 (52–140) | 0% | 235 (166–320) | 0% | - | ||
| 6 mo | 54 (35–100) | 7.4% | 145 (88–260) | 12% | 0.66 | ||
| 9 mo | 46 (20–100) | 25.9% | 82 (49–213) | 16% | 0.38 | ||
| 12 mo | 32 (10–76) | 40.7% | 75 (13–240) | 28% | 0.33 | ||
| 18 mo | 32 (0–55) | 55.6% | 32 (0–81) | 52% | 0.79 | ||
| 24 mo | 0 (0–35) | 70.4% | 10 (0–61) | 56% | 0.28 | ||
| Proteinuria | |||||||
| Level (g/day) | CR/PR (%) | Level (g/day) | CR/PR (%) | Level (g/day) | CR/PR (%) | p | |
| Baseline | 7.2 (3.45–11.1) | - | 7.2 (4.2–14.4) | - | 10.4 (7.1–17.4) | - | - |
| 3 mo | 5.8 (1.9–8.8) | 0% | 6 (4.2–9.2) | 0% | 9.2 (6–14.9) | 0% | - |
| 6 mo | 2.4 (0.4–5.1) | 30.8% | 5.7 (3.8–7.5) | 3.7% | 9.2 (3.15–14) | 8% | 0.03 |
| 9 mo | 1.55 (0.35–4.05) | 46.2% | 4 (2–8.4) | 11.1% | 8 (5.5–10.8) | 16% | 0.02 |
| 12 mo | 1.7 (0.17–2.5) | 61.5% | 3.9 (1.45–6.9) | 33.3% | 5.6 (2.95–10.7) | 20% | 0.03 |
| 18 mo | 1 (0.15–1.9) | 61.5% | 3.2 (0.43–6.8) | 44.4% | 5 (1.7–10.7) | 24% | 0.06 |
| 24 mo | 1 (0.3–1.8) | 84.6% | 1.8 (0.3–4.8) | 74.1% | 3.95 (0.4–6.52) | 60% | 0.25 |
| Variable | Univariate Analysis | Multivariate Analysis (Model A) | Multivariate Analysis (Model B) | |||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Serum Creatinine (for 1 mg/dL) | 1.16 (0.79–1.7) | 0.44 | 1.21 (0.76–1.93) | 0.41 | 1.22 (0.76–1.95) | 0.4 |
| Serum Albumin (for 1 g/dL) | 1.35 (0.89–2.04) | 0.15 | 0.86 (0.48–1.52) | 0.6 | 0.87 (0.5–1.52) | 0.64 |
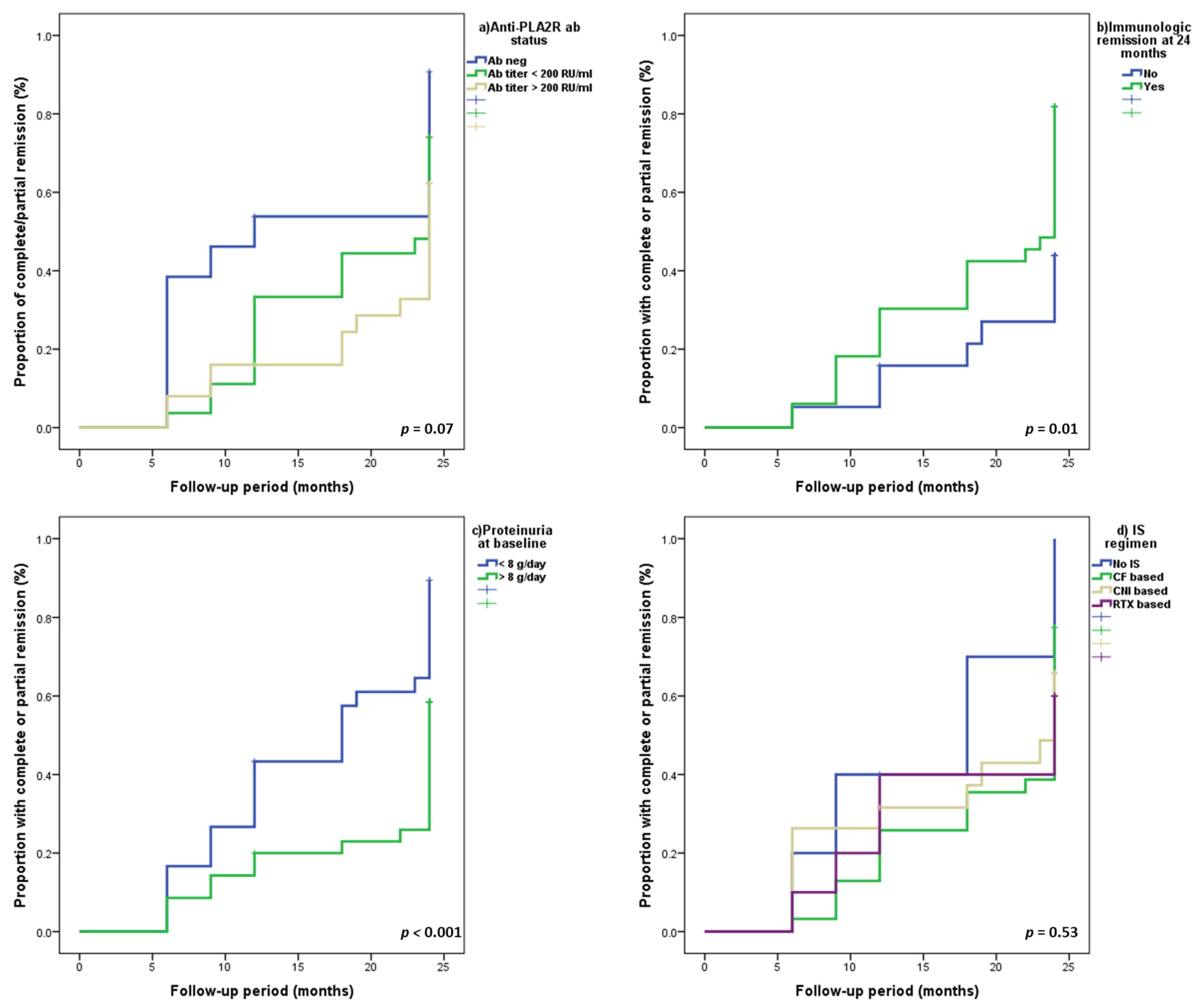
| 24 h Proteinuria (<8 g/d vs. ≥8 g/d) | 2.31 (1.28–4.17) | 0.005 | 2.38 (1.18–4.76) | 0.01 | 2.4 (1.19–4.8) | 0.01 |
| Type of IS (vs. no IS) | - | - | - | - | - | - |
| ● CF-based regimens | 0.54 (0.18–1.57) | 0.26 | 0.89 (0.29–2.73) | 0.83 | 0.86 (0.28–2.62) | 0.79 |
| ● CNI-based regimens | 0.53 (0.17–1.65) | 0.27 | 0.89 (0.26–2.98) | 0.85 | 0.88(0.26–2.93) | 0.83 |
| ● RTX-based regimens | 0.44 (0.12–1.58) | 0.21 | 0.64 (0.17–2.43) | 0.51 | 0.64(0.17–2.43) | 0.51 |
| Immunological remission at 24 mo | 1.46 (0.81–2.65) | 0.2 | 2.25 (1.04–4.85) | 0.04 | 2.26(1.05–4.87) | 0.03 |
| Anti-PLA2R ab titer (vs. >200 RU/mL) | - | - | - | - | - | - |
| ● <200 RU/mL | 1.37 (0.7–2.67) | 0.35 | 1.13 (0.55–2.33) | 0.73 | - | - |
| ● Negative serology | 2.15 (0.98–4.69) | 0.055 | 3.11 (1.13–8.53) | 0.02 | - | - |
| Anti-PLA2R ab (neg vs. pos) | 1.18(0.92–3.58) | 0.08 | - | - | 2.87(1.17–7.02) | 0.02 |
| Variable | No IS | CF-Based Regimens | CNI-Based Regimens | RTX-Based Regimens | p-Value |
|---|---|---|---|---|---|
| Number of pts | 5 | 31 | 19 | 10 | |
| Age (y) | 50 ± 10 | 54 ± 12 | 51 ± 13 | 52 ± 14 | 0.77 |
| Gender (%M) | 100% | 77.4% | 52.6% | 60% | 0.07 |
| BMI (kg/m2) | 33.2 ± 6.9 | 30.5 ± 4.6 | 28.7 ± 6.6 | 32.5 ± 3.6 | 0.21 |
| HTA (%) | 80% | 96.8% | 94.7% | 100% | 0.34 |
| Smoking (%) | 80% | 74.2% | 42.1% | 50% | 0.09 |
| Tromboembolic events (%) | 0% | 29% | 15.8% | 10% | 0.3 |
| Serum creatinine (mg/dL) | 1.26 ± 0.46 | 1.76 ± 0.91 | 0.98 ± 0.25 | 1.27 ± 0.63 | 0.01 |
| eGFR (mL/min) | 64 ± 18 | 51 ± 28 | 77 ± 28 | 67 ± 25 | 0.015 |
| Serum Albumin (g/dL) | 3.26 ± 0.83 | 2.78 ± 0.61 | 2.81 ± 0.55 | 2.58 ± 0.85 | 0.31 |
| Total Proteins (g/dL) | 6.08 ± 0.32 | 5.37 ± 0.81 | 5.34 ± 0.92 | 5.56 ± 1.24 | 0.38 |
| Cholesterol (mg/dL) | 272 ± 91 | 290 ± 102 | 328 ± 113 | 339 ± 142 | 0.44 |
| Triglycerides (mg/dL) | 178 ± 119 | 200 ± 109 | 205 ± 126 | 203 ± 37 | 0.59 |
| Fibrinogen (mg/dL) | 429 ± 88 | 630 ± 160 | 641 ± 151 | 556 ± 106 | 0.02 |
| Hemoglobin (g/dL) | 13.4 ± 2.3 | 13.5 ± 2.2 | 13.9 ± 1.3 | 14.7 ± 1.3 | 0.61 |
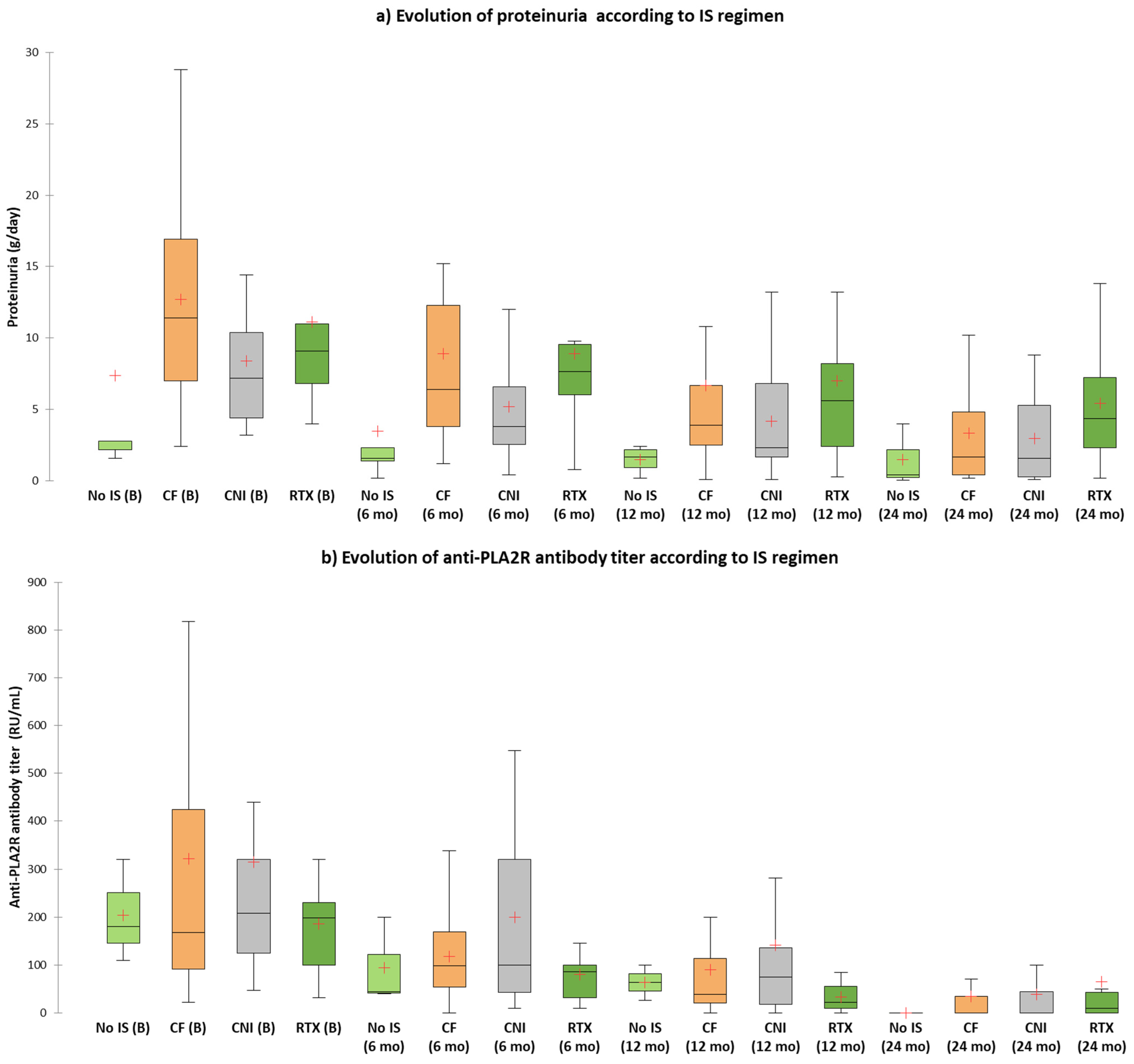
| 24 h proteinuria (g/day) | 2.8 (IQR:1.9–15.4) | 11.4 (IQR:7–17.4) | 7.2 (4.3–10.8) | 9.1 (IQR:6.03–12.8) | 0.02 |
| Hematuria (cells/mmc) | 11 (IQR:3–15) | 17 (IQR:9–43) | 28 (IQR:13–45) | 16 (IQR:7–25) | 0.09 |
| Serum IgG level (mg/dL) | 961 (IQR:708–1160) | 470 (IQR:327–618) | 408 (IQR:305–534) | 703 (IQR:320–850) | 0.009 |
| Anti-PLA2R-ab positive pts (%) | 60% | 87.1% | 68.4% | 90% | 0.21 |
| Anti-PLAR2R-ab titer (RU/mL) | 180 (IQR:110–180) | 168 (IQR:89–448) | 208 (IQR:111–320) | 198 (IQR:100–275) | 0.91 |
| PLA2R ab titer (%) | |||||
| ● <200 RU/mL | 66.7% | 51.9% | 46.2% | 55.6% | 0.68 |
| ● >200 RU/mL | 33.3% | 48.1% | 53.8% | 44.4% | |
| MN stage (% of pts) | |||||
| ● I | 20% | 8% | 25% | 25% | 0.82 |
| ● II | 40% | 28% | 18.8% | 37.5% | |
| ● III | 40% | 52% | 43.8% | 37.5% | |
| ● IV | 0% | 12% | 12.5% | 0% | |
| Evolution characteristics | |||||
| Clinical response (% of pts) | |||||
| ● No response | 0% | 22.6% | 31.6% | 40% | 0.5 |
| ● PR | 60% | 41.9% | 31.6% | 50% | |
| ● CR | 40% | 35.5% | 36.8% | 10% | |
| Time to PR (mo) | 9 (IQR:9–12) | 24 (IQR:12–24) | 15.5 (IQR:6–24) | 12 (IQR:9–24) | 0.43 |
| Time to CR (mo) | 15 (IQR:6–15) | 18 (IQR:12–24) | 12 (IQR:6–23) | 9 (IQR:9–9) | 0.51 |
| IR (% of patients) | 66.7% | 63% | 61.5% | 66.7% | 0.6 |
| Time to IR (mo) | 18 (IQR:9–18) | 18 (IQR:12–24) | 18 (IQR:9–24) | 12 (IQR:7.5–24) | 0.83 |
| Antiproteinuric therapy (%) | 100% | 96.8% | 94.7% | 100% | 0.85 |
| Period | No IS | CF-Based Regimens | CNI-Based Regimens | RTX-Based Regimens | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Anti-PLA2R Antibodies | |||||||||
| Titer (IQR) (RU/mL) | IR (%) | Titer (IQR) (RU/mL) | IR (%) | Titer (IQR) (RU/mL) | IR (%) | Titer (IQR) (RU/mL) | IR (%) | ||
| Baseline | 180 (110–180) | – | 168 (89–448) | – | 208 (111–320) | – | 198 (100–275) | – | – |
| 3 mo | 85 (51–85) | 0% | 140 (75–243) | 0% | 170 (73–317) | 0% | 160 (87–205) | 0% | – |
| 6 mo | 44 (40–44) | 0% | 98 (53–170) | 11.% | 100 (34–320) | 7.7% | 86 (25–122) | 11.1% | 0.92 |
| 9 mo | 48 (22–48) | 33.3% | 50 (38–100) | 14.8% | 110 (27–293) | 30.8% | 26 (10–148) | 22.2% | 0.65 |
| 12 mo | 63 (27–63) | 33.3% | 39 (18–127) | 33.3% | 75 (0–160) | 30.8% | 22 (10–55) | 44.4% | 0.92 |
| 18 mo | 24 (16–24) | 33.3% | 32 (0–74) | 59.3% | 42 (0–85) | 46.2% | 26 (0–60) | 55.6% | 0.76 |
| 24 mo | 15 (10–25) | 66.7% | 0 (0–45) | 63% | 0 (0–56) | 61.5% | 10 (0–117) | 66.7% | 0.99 |
| Proteinuria | p-Value | ||||||||
| Level (g/day) | CR/PR (%) | Level (g/day) | CR/PR (%) | Level (g/day) | CR/PR (%) | Level (g/day) | CR/PR (%) | ||
| Baseline | 2.8 (1.9–15.4) | – | 11.4 (7–17.4) | – | 7.2 (4.3–10.8) | – | 9.1 (6.03–12.8) | – | – |
| 3 mo | 1.9 (0.65–17.1) | 0% | 7.4 (5.2–13.6) | 0% | 6.4 (3.3–9.6) | 0% | 9 (5.67–12.3) | 0% | – |
| 6 mo | 1.6 (0.8–7.1) | 20% | 6.4 (3.8–12.4) | 3.2% | 3.8 (2.4–6.6) | 21.1% | 7.65 (5–12.05) | 10% | 0.2 |
| 9 mo | 1.8 (0.9–3.15) | 40% | 6 (2–8.4) | 12.9% | 4.85 (2.07–9.8) | 26.3% | 8.2 (2.8–14.5) | 20% | 0.44 |
| 12 mo | 1.65 (0.45–2.32) | 60% | 3.9 (2.37–7.14) | 25.8% | 2.3 (1.22–7.6) | 36.8% | 5.6 (2.4–10.7) | 40% | 0.44 |
| 18 mo | 1.7 (0.47–2.02) | 80% | 3.06 (1–9.5) | 35.5% | 2.2 (0.6–6.1) | 36.8% | 5.8 (3.65–11.5) | 40% | 0.29 |
| 24 mo | 0.4 (0.03–0.4) | 100% | 1.65 (0.4–4.8) | 77.4% | 1.6 (0.3–5.75) | 63.2% | 4.35 (1.4–9.45) | 60% | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurubiță, R.; Obrișcă, B.; Sorohan, B.; Achim, C.; Micu, G.E.; Mircescu, G.; Ismail, G. Clinical Phenotypes and Predictors of Remission in Primary Membranous Nephropathy. J. Clin. Med. 2021, 10, 2624. https://doi.org/10.3390/jcm10122624
Jurubiță R, Obrișcă B, Sorohan B, Achim C, Micu GE, Mircescu G, Ismail G. Clinical Phenotypes and Predictors of Remission in Primary Membranous Nephropathy. Journal of Clinical Medicine. 2021; 10(12):2624. https://doi.org/10.3390/jcm10122624
Chicago/Turabian StyleJurubiță, Roxana, Bogdan Obrișcă, Bogdan Sorohan, Camelia Achim, Georgia Elena Micu, Gabriel Mircescu, and Gener Ismail. 2021. "Clinical Phenotypes and Predictors of Remission in Primary Membranous Nephropathy" Journal of Clinical Medicine 10, no. 12: 2624. https://doi.org/10.3390/jcm10122624
APA StyleJurubiță, R., Obrișcă, B., Sorohan, B., Achim, C., Micu, G. E., Mircescu, G., & Ismail, G. (2021). Clinical Phenotypes and Predictors of Remission in Primary Membranous Nephropathy. Journal of Clinical Medicine, 10(12), 2624. https://doi.org/10.3390/jcm10122624






