Impact of Prophylactic Hydroxychloroquine on People at High Risk of COVID-19: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Outcomes
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
3.1. Selection of Studies
3.2. Characteristics of Included Studies
3.3. Risk of Bias of Included Studies
3.4. Prophylactic Effects of Hydroxychloroquine on Primary Outcomes
3.5. Prophylactic Effects of Hydroxychloroquine on Secondary Outcomes
3.6. Cohort Study Description
3.7. Quality of Evidence from RCTs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Worldometer. Worldometer’s COVID-19 Dataset. Available online: https://www.worldometers.info/coronavirus/ (accessed on 9 November 2020).
- Johns Hopkins University. The COVID Tracking Project. Available online: https://covidtracking.com/data/national/hospitalization (accessed on 9 November 2020).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19-Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Pfizer and BionTech Press Release. Pfizer and BionTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase III Study. 11/9/2020. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against (accessed on 17 November 2020).
- National Institutes of Health. Promising Interim Results from Clinical Trial of NIH-Moderna COVID-19 Vaccine. 11/16/2020. Available online: https://www.nih.gov/news-events/news-releases/promising-interim-results-clinical-trial-nih-moderna-covid-19-vaccine (accessed on 17 November 2020).
- Tyson, A.; Johnson, C.; Funk, C.U.S. Public Now Divided Over Whether To Get COVID-19 Vaccine. Concerns about the Safety and Effectiveness of Possible Vaccine, Pace of Approval Process. Pew Research Group. 9/17/2020. Available online: https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/ (accessed on 17 November 2020).
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef] [PubMed]
- COVID Analysis. Early Treatment with Hydroxychloroquine: A Country-Based Analysis. 11/4/2020. Available online: https://hcqtrial.com/ (accessed on 9 November 2020).
- Hernandez, A.V.; Roman, Y.M.; Pasupuleti, V.; Barboza, J.J.; White, C.M. Hydroxychloroquine or Chloroquine for Treatment or Prophylaxis of COVID-19: A Living Systematic Review. Ann. Intern. Med. 2020, 173, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.V.; Roman, Y.M.; Pasupuleti, V.; Barboza, J.J.; White, C.M. Update Alert 3: Hydroxychloroquine or Chloroquine for the Treatment or Prophylaxis of COVID-19. Ann. Intern. Med. 2020, 173, W156–W157. [Google Scholar] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Hartung, J.; Knapp, G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat. Med. 2001, 20, 3875–3889. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2020 (Developed by Evidence Prime, Inc.). Available online: gradepro.org (accessed on 19 November 2020).
- Abella, B.S.; Jolkovsky, E.L.; Biney, B.T.; Uspal, J.E.; Hyman, M.C.; Frank, I.; Hensley, S.E.; Gill, S.; Vogl, D.T.; Maillard, I.; et al. Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Mitjà, O.; Corbacho-Monné, M.; Ubals, M. A; Alemany, A.; Suñer, C.; Tebé, C.; Tobias, A.; Peñafiel, J.; Ballana, E.; Pérez, C.A.; et al. Cluster-Randomized Trial of Hydroxychloroquine for Prevention of Covid-19. N. Engl. J. Med. 2021, 384, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Pullen, M.F.; Bangdiwala, A.S.; Pastick, K.A.; Lofgren, S.M.; Okafor, E.C.; Skipper, C.P.; Nascene, A.A.; Nicol, M.R.; Abassi, M.; et al. Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N. Engl. J. Med. 2020, 383, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Bangdiwala, A.S.; Nicol, M.R; Skipper, C.P.; Pastick, K.A.; Axelrod, M.L.; Pullen, M.F.; Nascene, A.A.; Williams, D.A.; Engen, N.W.; et al. Hydroxychloroquine as pre-exposure prophylaxis for Coronavirus Diseases 2019 (COVID-19) in healthcare workers: A randomized trial. Clin. Infect. Dis. 2021, 72, e835–e843. [Google Scholar] [CrossRef] [PubMed]
- Barnabas, R.V.; Brown, E.R.; Bershteyn, A.; Stankiewicz Karita, H.C.; Johnston, C.; Thorpe, L.E.; Kottkamp, A.; Neuzil, K.M.; Laufer, M.K.; Deming, M.; et al. Hydroxychloroquine as Postexposure Prophylaxis to Prevent Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Randomized Trial. Ann. Intern. Med. 2021, 174, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Chowdhury, S.; Mukherjee, R. Pre Exposure Hydroxychloroquine Prophylaxis for Covid-19 in Healthcare Workers: A Retrospective Cohort. Available online: medRxiv2020.06.09.20116806 (accessed on 1 October 2020).
- Morrisette, T.; Lodise, T.P.; Scheetz, M.H.; Goswami, S.; Pogue, J.M.; Rybak, M.J. The Pharmacokinetic and Pharmacodynamic Properties of Hydroxychloroquine and Dose Selection for COVID-19: Putting the Cart Before the Horse. Infect. Dis. Ther. 2020, 9, 561–572. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J.E.; Brennan, S.E.; Ryan, R.E.; Thomson, H.J.; Johnston, R.V.; Thomas, J. Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis. In Cochrane Handbook for Systematic Reviews of Interventions, version 6.1 (updated September, 2020); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2020. [Google Scholar]
- Sirotich, E.; Kennedy, K.; Surangiwala, S. Antimalarial Drug Shortages During the COVID-19 Pandemic: Results from the Global Rheumatology Alliance Patient Experience Survey. American College of Neurology Abstracts 2020. Available online: https://acrabstracts.org/abstract/antimalarial-drug-shortages-during-the-covid-19-pandemic-results-from-the-global-rheumatology-alliance-patient-experience-survey/ (accessed on 9 November 2020).
- Sutton, S.; Magagnoli, J.; Hardin, J.W. Impact of Pill Burden on Adherence, Risk of Hospitalization, and Viral Suppression in Patients with HIV Infection and AIDS Receiving Antiretroviral Therapy. Pharmacotherapy. 2016, 36, 385–401. [Google Scholar] [CrossRef] [PubMed]
| Author, Year [ref]/Type of Study/Registration | Objective | Sample Randomized (Arm Sizes), Country(ies), Population | Overall Key Patient Characteristics | Intervention | Comparison | Outcomes | Follow-Up Time |
|---|---|---|---|---|---|---|---|
| Abella, 2020 [19]/Parallel RCT/NCT04329923 | To evaluate the effect of daily HCQ to prevent SARS-CoV-2 infection in hospital-based HCWs over 8 weeks of exposure via RT-PCR testing of NP swabs and serologic antibody testing. | 132 (HCQ = 66, placebo = 66), USA, HCWs who worked > 20 h/week in hospital-based units, had no known history SARS-CoV-2 infection, did not have symptoms suggestive of COVID-19 in the 2 weeks before enrollment, including cough, fever, or shortness of breath Physicians, nurses, certified nursing assistants, emergency technicians, and respiratory therapists were eligible | Median age (range): 33 (20–66) y Male: 31% No prior disease: 71% | 200 mg of HCQ, 3 times a day with food, for 8 weeks. (33,600 mg total) | Custom-molded identically sized and shaped microcrystalline cellulose placebo tablets for 8 weeks | Primary: SAR-CoV-2 positive status via NP swab. Secondary: adverse events, serological antibody positivity for either nucleocapsid or spike protein antigens, ECG changes after 4 weeks of treatment, and clinical outcomes for any participants who became SARS-CoV-2 positive and/or developed COVID-19 symptoms. | 8 weeks |
| Mitjà, 2020 [20]/Cluster RCT/NCT04304053 | To investigate the efficacy/safety of HCQ to prevent secondary PCR-confirmed symptomatic COVID-19 and SARS-CoV-2 infection in contacts exposed to a PCR-positive COVID-19 case. | 2314 (HCQ = 1116, Usual care = 1198), Spain, Age ≥ 18 years, either a healthcare worker, a household contact, a nursing home worker, or a nursing home resident Recent history of close contact exposure to a PCR-confirmed COVID-19 case (i.e., > 15 min within two meters, up to seven days before enrolment) Absence of COVID-19-like symptoms in the two weeks preceding enrollmentInclusion was irrespective of baseline PCR result. | Mean age (SD): 49 (19) yMale: 27% No prior disease: 44% | 800 mg HCQ on day 1 followed by 400 mg once daily for 6 days (3200 mg total) | Usual care (unspecified) | Primary: Confirmed COVID-19 episode, defined as symptomatic illness (> = 1 among: fever, cough, difficulty breathing, myalgia, headache, sore throat, new olfactory and taste disorder(s), or diarrhea) and a positive SARS-CoV-2 RT-PCR test. Secondary: SARS-CoV-2 infection (either RT-PCR detection of SARS-CoV-2 in a NP specimen or the presence of any of the aforementioned symptoms compatible with COVID-19). | 14 days (infection)/ 28 days (adverse events) |
| Boulware, 2020 [21]/Parallel RCT/NCT04308668 | To determine the efficacy of HCQ as post-exposure prophylaxis, to prevent symptomatic infection after exposure to COVID-19. | 821 (HCQ = 414), Placebo = 407), USA and Canada. All participants were asymptomatic at enrollment, who had household or occupational exposure to a person with confirmed COVID-19 | Median age (IQR): 40 (33–50) y Male: 48% No prior disease: 73% | HCQ 800 mg (4 tablets) once, then 600 mg 6 to 8 h later, then 600 mg daily for 4 more days (3800 mg total). | Matching placebo folate tablets, identical regimen as HCQ. | Primary: Symptomatic illness confirmed by a positive molecular assay or COVID-19 related symptoms within 14 days. Secondary: hospitalization for COVID-19 or death, PCR-confirmed SARS-CoV-2 infection, COVID-19 symptoms, discontinuation of intervention owing to any cause, and severity of symptoms (if any) at days 5 and 14. | 14 days |
| Rajasingham, 2020 [22]/Parallel RCT / No registration | To explore the potential of HCQ as a pre-exposure prophylaxis for COVID-19 in HCW in a tertiary care hospital. | 1483 (HCQ OW = 494, HCQ TW = 495, Placebo = 494), USA and Canada, HCW > = 18 years with ongoing exposure to persons with COVID-19, working at ER, ICU, hospital wards, and first responders. | Median age (IQR): 41 (34–49) y Male: 49% No prior disease: 66% | HCQ loading dose of 400 mg (two tablets) twice separated by 6–8 h followed by (I) 400 mg OW for 12 w (5600 mg total) or (II) 400 mg TW for 12 w (10,400 mg total). | Matching Placebo: loading dose of two tablets followed by two tablets OW or TW for 12 w. Placebos are combined in analyses (randomization was 2:2:1:1). | Primary: COVID-19 infection confirmed by PCR or probable compatible illness. Secondary: confirmed SARS-CoV-2 infection, possible COVID-19, and hospitalization, death, or other adverse events. | 12 weeks |
| Barnabas, 2020 [23]/Cluster RCT/NCT04328961 | To test HCQ as post-exposure prophylaxis for SARS-CoV-2 infection | 829 (HCQ = 407, Placebo = 422), USA, Individuals able to provide informed consent, were 18 to 80 years of age, had close contact with a person (index) with recent known SARS-CoV-2 infection, had exposure within the prior 96 h, were able to conduct study visits via telehealth, and were not planning to take hydroxychloroquine outside the study. | Median age (IQR): 39 (27–51) y Male: 40% No prior disease (Metabolic disease): 83% | HCQ 400 mg/d orally for 3 days, then 200 mg/d orally for an additional 11 days (3400 mg total) | Ascorbic acid (500 mg/d orally for 3 days, then 250 mg/d orally for 11 days) as a placebo equivalent. | Primary: PCR-confirmed incident SARS-CoV-2 infection through day 14 among persons who were SARS-CoV-2 negative at enrollment. Secondary: PCR-confirmed incident SARS-CoV-2 infection at 28 days, symptomatic COVID-19 disease per CDC definition at 14 days. | 14 days |
| Bhattacharya, 2020 [24]/Cohort/ No registration | To explore the potential of HCQ as a pre-exposure prophylaxis for COVID-19 in health care workers in a tertiary care hospital. | 106 (HCQ = 54, Non-HCQ = 52), India, HCW who worked at Medical College in India dealing with COVID-19 patients in the first two weeks of May 2020. In the given period, a cluster outbreak of cases amongst HCWs in this hospital had occurred—with about 28 HCW testing positive over a period of two weeks. | Mean age (SD): 27.1 (5.8) y Male: 49% No prior disease (comorbidities): 96% | HCQ only. No doses or duration reported. HCW were voluntarily on Pre-exposure HCQ prophylaxis | Non-HCQ; No other details. | Primary: RT-PCR positive COVID-19 infection. Secondary: adverse events. | Not specified |
| Outcomes | Anticipated Absolute Effects * (95% CI) | Relative Effect (95% CI) | № of Participants (Studies) | Certainty of the Evidence(GRADE) | |
|---|---|---|---|---|---|
| Risk with Control | Risk with Hydroxychloroquine | ||||
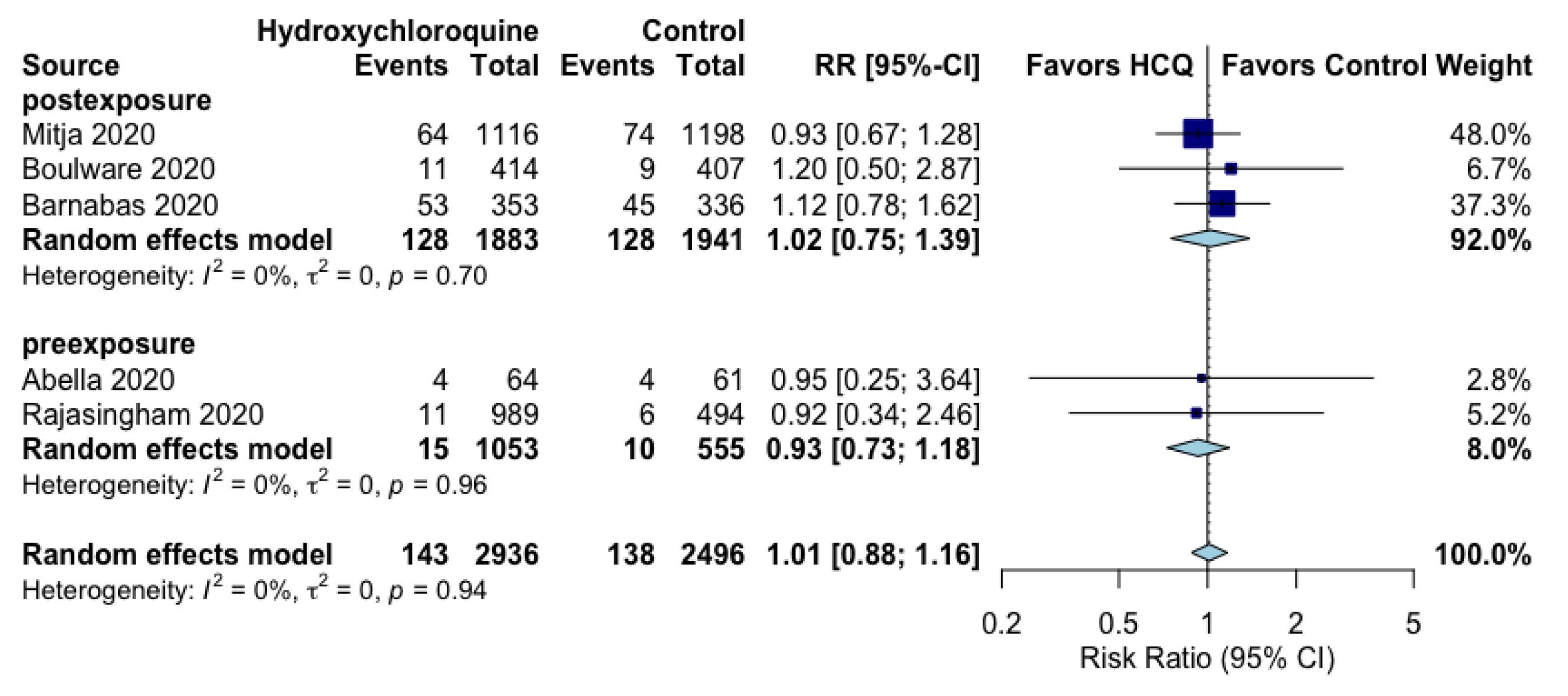
| SARS-CoV-2 Positivity assessed with: RT-PCR follow up: range 2 weeks to 12 weeks | 6 per 100 | 6 per 100 (5 to 6) | RR 1.01 (0.88 to 1.16) | 5432 (5 RCTs) | ⨁⨁◯◯ LOW a |
| SARS-CoV-2 positivity (pre-exposure) assessed with: RT-PCR follow up: range 8 weeks to 12 weeks | 2 per 100 | 2 per 100 (1 to 2) | RR 0.93 (0.73 to 1.18) | 1608 (2 RCTs) | ⨁⨁◯◯ LOW b |
| SARS-CoV-2 positivity (post-exposure) assessed with: RT-PCR follow up: mean 14 days | 7 per 100 | 7 per 100 (5 to 9) | RR 1.02 (0.75 to 1.39) | 3824 (3 RCTs) | ⨁⨁◯◯ LOW c |
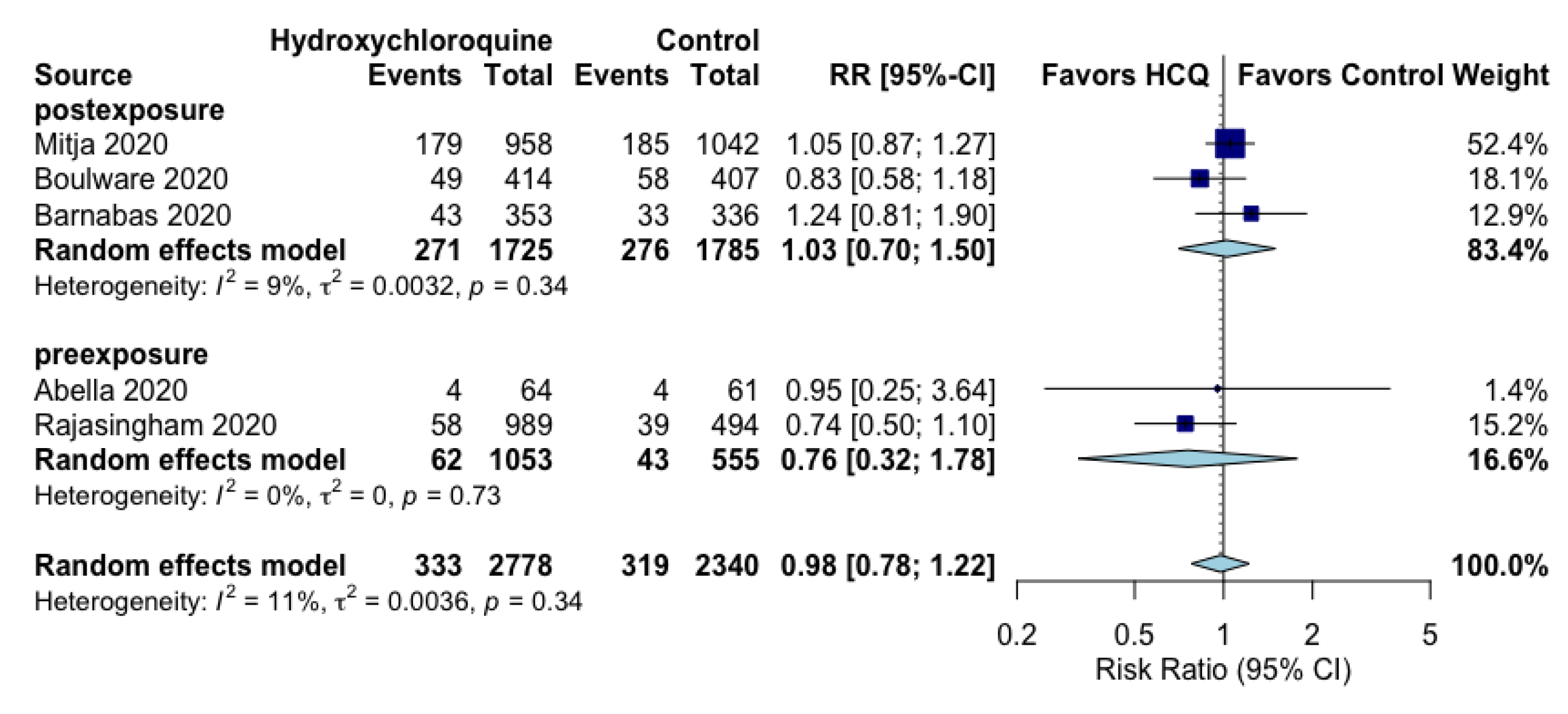
| Composite COVID-19 Infection assessed with: RT-PCR positivity or symptoms compatible with COVID-19 follow up: range 2 weeks to 12 weeks | 14 per 100 | 13 per 100 (11 to 17) | RR 0.98 (0.78 to 1.22) | 5118 (5 RCTs) | ⨁⨁◯◯ LOW a |
| Composite COVID-19 infection (pre-exposure) assessed with: RT-PCR positivity or symptoms compatible with COVID-19 follow up: range 8 weeks to 12 weeks | 8 per 100 | 6 per 100 (2 to 14) | RR 0.76 (0.32 to 1.78) | 1608 (2 RCTs) | ⨁◯◯◯ VERY LOW b,d |
| Composite COVID-19 infection (post-exposure) assessed with: RT-PCR positivity or symptoms compatible with COVID-19 follow up: mean 14 days | 15 per 100 | 16 per 100 (11 to 23) | RR 1.03 (0.70 to 1.50) | 3510 (3 RCTs) | ⨁⨁◯◯ LOW c,e |
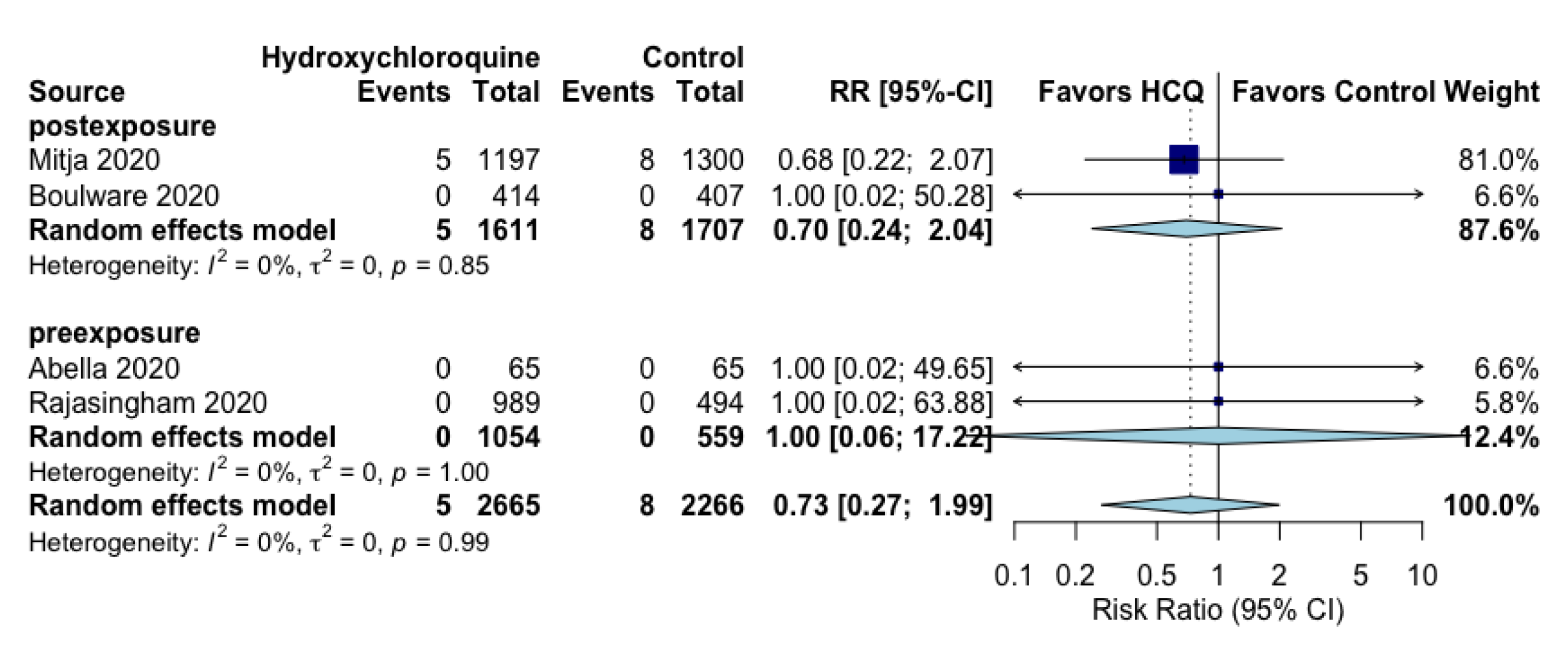
| All-cause mortalityfollow up: range 2 weeks to 12 weeks | 0 per 100 | 0 per 100 (0 to 1) | RR 0.73 (0.27 to 1.99) | 4931 (4 RCTs) | ⨁◯◯◯ VERY LOW f,g |
| Clinical worseningassessed with: hospitalization or ICU admissionfollow up: range 2 weeks to 12 weeks | 0 per 100 | 0 per 100 (0 to 1) | RR 1.01 (0.17 to 5.92) | 3133 (3 RCTs) | ⨁◯◯◯ VERY LOW f,h |
| Severe adverse vents follow up: range 2 weeks to 8 weeks | 1 per 100 | 1 per 100 (1 to 2) | RR 0.91 (0.48 to 1.75) | 3456 (3 RCTs) | ⨁⨁◯◯ LOW i,j |
| Adverse events assessed with: Any type of adverse event follow up: range 2 weeks to 8 weeks | 9 per 100 | 26 per 100 (7 to 100) | RR 2.79 (0.72 to 10.82) | 4156 (4 RCTs) | ⨁◯◯◯ VERY LOW k,l,m |
| Diarrhea, abdominal pain, or vomiting follow up: range 2 weeks to 12 weeks | 4 per 100 | 17 per 100 (6 to 49) | RR 4.56 (1.58 to 13.19) | 5639 (5 RCTs) | ⨁◯◯◯ VERY LOW a,n,o |
| Headache follow up: range 2 weeks to 12 weeks | 2 per 100 | 3 per 100 (1 to 11) | RR 1.38 (0.39 to 4.80) | 5639 (5 RCTs) | ⨁◯◯◯ VERY LOW a,p,q |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, A.V.; Ingemi, J., III; Sherman, M.; Pasupuleti, V.; Barboza, J.J.; Piscoya, A.; Roman, Y.M.; White, C.M. Impact of Prophylactic Hydroxychloroquine on People at High Risk of COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2609. https://doi.org/10.3390/jcm10122609
Hernandez AV, Ingemi J III, Sherman M, Pasupuleti V, Barboza JJ, Piscoya A, Roman YM, White CM. Impact of Prophylactic Hydroxychloroquine on People at High Risk of COVID-19: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(12):2609. https://doi.org/10.3390/jcm10122609
Chicago/Turabian StyleHernandez, Adrian V., John Ingemi, III, Michael Sherman, Vinay Pasupuleti, Joshuan J. Barboza, Alejandro Piscoya, Yuani M. Roman, and Charles M. White. 2021. "Impact of Prophylactic Hydroxychloroquine on People at High Risk of COVID-19: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 12: 2609. https://doi.org/10.3390/jcm10122609
APA StyleHernandez, A. V., Ingemi, J., III, Sherman, M., Pasupuleti, V., Barboza, J. J., Piscoya, A., Roman, Y. M., & White, C. M. (2021). Impact of Prophylactic Hydroxychloroquine on People at High Risk of COVID-19: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(12), 2609. https://doi.org/10.3390/jcm10122609








