The Association between Vitamin D and Nonalcoholic Fatty Liver Disease Assessed by Controlled Attenuation Parameter
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Measurement of Anthropometric and Laboratory Parameters
2.3. Measurement of Serum Vitamin D Levels
2.4. Measurement of NAFLD Using CAP and Liver Stiffness
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Study Population
3.2. Parameters Associated with NAFLD
3.3. Association between NAFLD/MAFLD and Vitamin D Level
3.4. Association between Grade of Steatosis and Vitamin D Level
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Marchesini, G.; Marzocchi, R. Metabolic syndrome and NASH. Clin. Liver Dis. 2007, 11, 105–117. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.P.; Pollett, A.; Kirsch, R.; Pomier-Layrargues, G.; Beaton, M.; Levstik, M.; Duarte-Rojo, A.; Wong, D.; Crotty, P.; Elkashab, M. Controlled Attenuation Parameter (CAP): A noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012, 32, 902–910. [Google Scholar] [CrossRef]
- Kwak, M.S.; Chung, G.E.; Yang, J.I.; Yim, J.Y.; Chung, S.J.; Jung, S.Y.; Kim, J.S. Clinical implications of controlled attenuation parameter in a health check-up cohort. Liver Int. 2018, 38, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and chronic diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef]
- Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Shaw, J.E.; Zimmet, P.Z.; Sikaris, K.; Ebeling, P.R.; Daly, R.M. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: Results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab). J. Clin. Endocrinol. Metab. 2012, 97, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Eguchi, M.; Kurotani, K.; Kochi, T.; Kashino, I.; Ito, R.; Kuwahara, K.; Tsuruoka, H.; Kabe, I.; Mizoue, T. Serum 25-hydroxyvitamin D and metabolic syndrome in a Japanese working population: The Furukawa Nutrition and Health Study. Nutrition 2017, 36, 26–32. [Google Scholar] [CrossRef]
- Lim, S.; Shin, H.; Kim, M.J.; Ahn, H.Y.; Kang, S.M.; Yoon, J.W.; Choi, S.H.; Kim, K.W.; Song, J.H.; Choi, S.I.; et al. Vitamin D inadequacy is associated with significant coronary artery stenosis in a community-based elderly cohort: The Korean Longitudinal Study on Health and Aging. J. Clin. Endocrinol. Metab. 2012, 97, 169–178. [Google Scholar] [CrossRef]
- Elangovan, H.; Chahal, S.; Gunton, J.E. Vitamin D in liver disease: Current evidence and potential directions. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.L.; Wang, N.J.; Han, B.; Li, Q.; Chen, Y.; Zhu, C.F.; Chen, Y.C.; Xia, F.Z.; Cang, Z.; Zhu, C.X.; et al. Low vitamin D levels and non-alcoholic fatty liver disease, evidence for their independent association in men in East China: A cross-sectional study (Survey on Prevalence in East China for Metabolic Diseases and Risk Factors (SPECT-China)). Br. J. Nutr. 2016, 115, 1352–1359. [Google Scholar] [CrossRef]
- Black, L.J.; Jacoby, P.; She Ping-Delfos, W.C.; Mori, T.A.; Beilin, L.J.; Olynyk, J.K.; Ayonrinde, O.T.; Huang, R.C.; Holt, P.G.; Hart, P.H.; et al. Low serum 25-hydroxyvitamin D concentrations associate with non-alcoholic fatty liver disease in adolescents independent of adiposity. J. Gastroenterol. Hepatol. 2014, 29, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, J.; Periyalwar, P.; Allampati, S.; Bhinder, V.; Hawkins, C.; Brandt, P.; Khiyami, A.; McCullough, A.J.; Dasarathy, S. Hypovitaminosis D is associated with increased whole body fat mass and greater severity of non-alcoholic fatty liver disease. Liver Int. 2014, 34, e118–e127. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.J.; Kim, M.K.; Park, S.E.; Park, C.Y.; Baek, K.H.; Lee, W.Y.; Kang, M.I.; Park, S.W.; Kim, S.W.; Oh, K.W. High serum vitamin D levels reduce the risk for nonalcoholic fatty liver disease in healthy men independent of metabolic syndrome. Endocr. J. 2013, 60, 743–752. [Google Scholar] [CrossRef]
- Liu, T.; Xu, L.; Chen, F.H.; Zhou, Y.B. Association of serum vitamin D level and nonalcoholic fatty liver disease: A meta-analysis. Eur J. Gastroenterol. Hepatol. 2020, 32, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Jaruvongvanich, V.; Ahuja, W.; Sanguankeo, A.; Wijarnpreecha, K.; Upala, S. Vitamin D and histologic severity of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Dig. Liver Dis. 2017, 49, 618–622. [Google Scholar] [CrossRef]
- Patel, Y.A.; Henao, R.; Moylan, C.A.; Guy, C.D.; Piercy, D.L.; Diehl, A.M.; Abdelmalek, M.F. Vitamin D is not associated with severity in NAFLD: Results of a paired clinical and gene expression profile analysis. Am. J. Gastroenterol. 2016, 111, 1591–1598. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012, 142, 1592–1609. [Google Scholar] [CrossRef]
- Park, H.E.; Lee, H.; Choi, S.Y.; Kwak, M.S.; Yang, J.I.; Yim, J.Y.; Chung, G.E. Clinical significance of hepatic steatosis according to coronary plaque morphology: Assessment using controlled attenuation parameter. J. Gastroenterol. 2019, 54, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Slomski, A. IOM endorses vitamin D, calcium only for bone health, dispels deficiency claims. JAMA 2011, 305, 453–456. [Google Scholar] [CrossRef]
- Norman, A.W. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 2008, 88, 491s–499s. [Google Scholar] [CrossRef]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Zarski, J.P.; de Ledinghen, V.; Rousselet, M.C.; Sturm, N.; Lebail, B.; Fouchard-Hubert, I.; Gallois, Y.; Oberti, F.; Bertrais, S.; et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013, 57, 1182–1191. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Wang, D.; Lin, H.; Xia, M.; Aleteng, Q.; Li, X.; Ma, H.; Pan, B.; Gao, J.; Gao, X. Vitamin D levels are inversely associated with liver fat content and risk of non-alcoholic fatty liver disease in a Chinese middle-aged and elderly population: The Shanghai Changfeng Study. PLoS ONE 2016, 11, e0157515. [Google Scholar]
- Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Vitamin D and metabolic dysfunction-associated fatty liver disease (MAFLD): An update. Nutrients 2020, 12, 3302. [Google Scholar] [CrossRef] [PubMed]
- Kromrey, M.L.; Ittermann, T.; Berning, M.; Kolb, C.; Hoffmann, R.T.; Lerch, M.M.; Völzke, H.; Kühn, J.P. Accuracy of ultrasonography in the assessment of liver fat compared with MRI. Clin. Radiol. 2019, 74, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Park, H.E.; Lee, H.; Choi, S.Y.; Kwak, M.S.; Yang, J.I.; Yim, J.Y.; Chung, G.E. Usefulness of controlled attenuation parameter for detecting increased arterial stiffness in general population. Dig. Liver Dis. 2018, 50, 1062–1067. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Poropat, G.; Filipec Kanizaj, T.; Skenderevic, N.; Zelic, M.; Matasin, M.; Vranic, L.; Kresovic, A.; Hauser, G. Association between gastroesophageal reflux disease and elastographic parameters of liver steatosis and fibrosis: Controlled attenuation parameter and liver stiffness measurements. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6670065. [Google Scholar]
- Li, L.; Zhang, L.; Pan, S.; Wu, X.; Yin, X. No significant association between vitamin D and nonalcoholic fatty liver disease in a Chinese population. Dig. Dis. Sci. 2013, 58, 2376–2382. [Google Scholar] [CrossRef]
- LeFevre, M.L. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2015, 162, 133–140. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Hwang, Y.C.; Chung, H.Y.; Woo, J.T. Vitamin D and diabetes in Koreans: Analyses based on the Fourth Korea National Health and Nutrition Examination Survey (KNHANES), 2008–2009. Diabet. Med. 2012, 29, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Cimini, F.A.; Barchetta, I.; Carotti, S.; Bertoccini, L.; Baroni, M.G.; Vespasiani-Gentilucci, U.; Cavallo, M.G.; Morini, S. Relationship between adipose tissue dysfunction, vitamin D deficiency and the pathogenesis of non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3407–3417. [Google Scholar] [CrossRef]
- Roth, C.L.; Elfers, C.T.; Figlewicz, D.P.; Melhorn, S.J.; Morton, G.J.; Hoofnagle, A.; Yeh, M.M.; Nelson, J.E.; Kowdley, K.V. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology 2012, 55, 1103–1111. [Google Scholar] [CrossRef]
- Barchetta, I.; De Bernardinis, M.; Capoccia, D.; Baroni, M.G.; Fontana, M.; Fraioli, A.; Morini, S.; Leonetti, F.; Cavallo, M.G. Hypovitaminosis D is independently associated with metabolic syndrome in obese patients. PLoS ONE 2013, 8, e68689. [Google Scholar]
- Ock, S.Y.; Ha, K.H.; Kim, B.K.; Kim, H.C.; Shim, J.S.; Lee, M.H.; Yoon, Y.M.; Kim, D.J. Serum 25-hydroxyvitamin d concentration is independently inversely associated with insulin resistance in the healthy, non-obese Korean population. Diabetes Metab. J 2016, 40, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Cefalo, C.M.A.; Conte, C.; Sorice, G.P.; Moffa, S.; Sun, V.A.; Cinti, F.; Salomone, E.; Muscogiuri, G.; Brocchi, A.A.G.; Pontecorvi, A.; et al. Effect of Vitamin D supplementation on obesity-induced insulin resistance: A double-blind, randomized, placebo-controlled trial. Obesity 2018, 26, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Pirgon, O.; Cekmez, F.; Bilgin, H.; Eren, E.; Dundar, B. Low 25-hydroxyvitamin D level is associated with insulin sensitivity in obese adolescents with non-alcoholic fatty liver disease. Obes Res. Clin. Pract. 2013, 7, e275–e283. [Google Scholar] [CrossRef] [PubMed]
- Beilfuss, A.; Sowa, J.P.; Sydor, S.; Beste, M.; Bechmann, L.P.; Schlattjan, M.; Syn, W.K.; Wedemeyer, I.; Mathé, Z.; Jochum, C.; et al. Vitamin D counteracts fibrogenic TGF-β signalling in human hepatic stellate cells both receptor-dependently and independently. Gut 2015, 64, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Chon, Y.E.; Jung, K.S.; Kim, S.U.; Park, J.Y.; Park, Y.N.; Kim, D.Y.; Ahn, S.H.; Chon, C.Y.; Lee, H.W.; Park, Y.; et al. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: A prospective study of a native Korean population. Liver Int. 2014, 34, 102–109. [Google Scholar] [CrossRef] [PubMed]
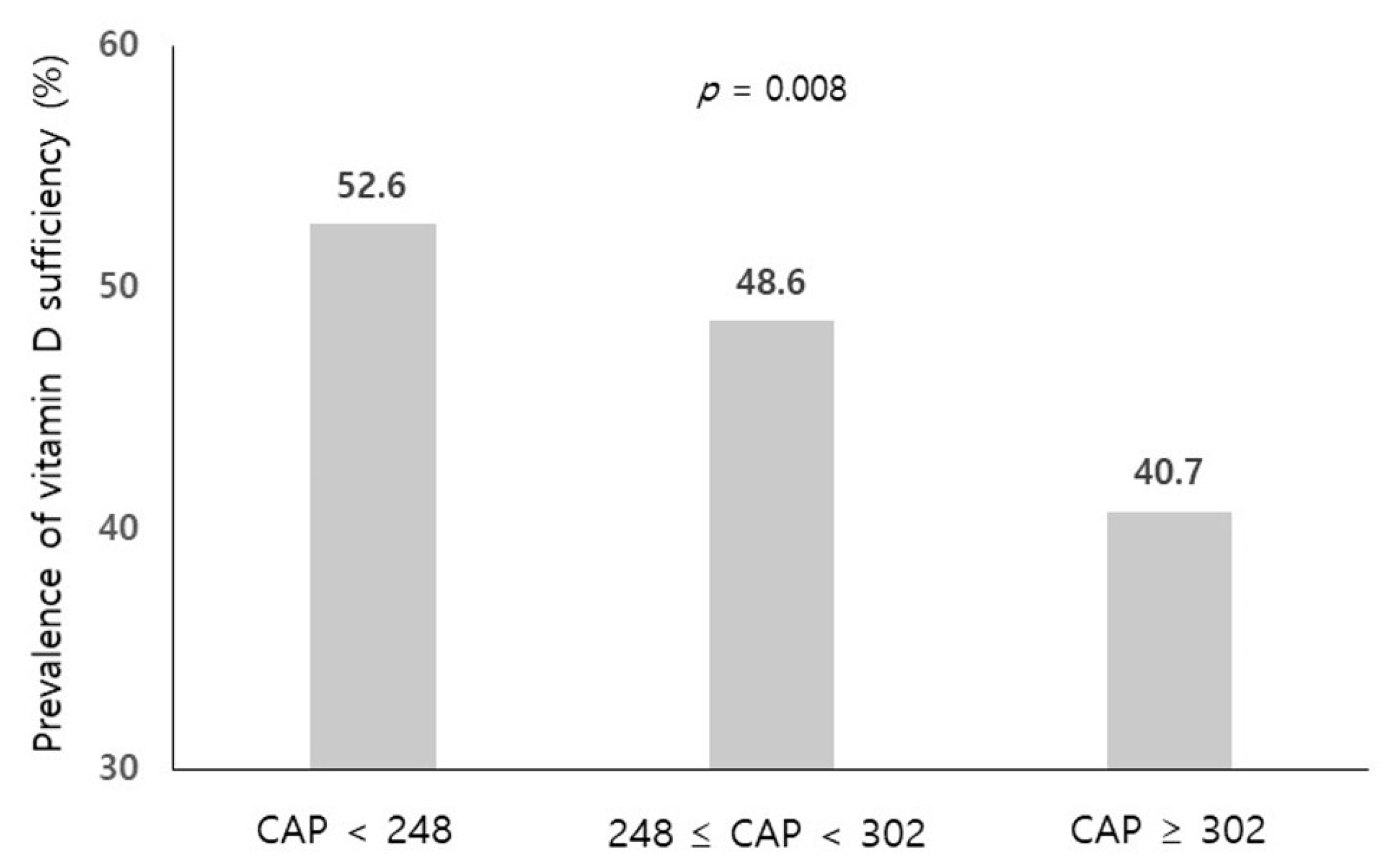
| Vitamin D Sufficiency (≥20 ng/mL) (n = 588) | Vitamin D Deficiency (<20 ng/mL) (n = 614) | p-Value | |
|---|---|---|---|
| Age (years) | 59.0 ± 10.3 | 55.5 ± 10.8 | <0.001 |
| Male, n (%) | 355 (60.4) | 374 (60.9) | 0.849 |
| Current smoking, n (%) | 94 (16.0) | 136 (22.1) | 0.007 |
| BMI (kg/m2) | 24.0 ± 3.3 | 24.7 ± 3.6 | 0.707 |
| BMI ≥ 25 (kg/m2) | 207 (35.2) | 262 (42.7) | 0.008 |
| Waist circumference (cm) | 87.7 ± 9.2 | 88.9 ± 9.9 | 0.036 |
| Systolic blood pressure, mmHg | 122.1 ± 16.3 | 122.1 ± 16.5 | 0.972 |
| Diastolic blood pressure, mmHg | 80.4 ± 10.7 | 81.3 ± 10.9 | 0.149 |
| Comorbidities | |||
| Diabetes mellitus, n (%) | 110 (18.7) | 102 (16.6) | 0.341 |
| Hypertension, n (%) | 183 (31.1) | 169 (27.5) | 0.171 |
| Hypercholesterolemia, n (%) | 190 (32.3) | 145 (23.5) | 0.002 |
| Laboratory parameters | |||
| Total cholesterol (mg/dL) | 188.5 ± 40.1 | 194.5 ± 40.9 | 0.010 |
| Triglyceride (mg/dL) + | 97 (68–137) | 97 (70–149) | 0.041 |
| HDL-cholesterol (mg/dL) | 55.8 ± 15.1 | 53.9 ± 15.0 | 0.031 |
| Fasting glucose (mg/dL) | 104.8 ± 22.1 | 105.9 ± 24.4 | 0.412 |
| Creatinine (mg/dL) | 0.85 ± 0.2 | 0.84 ± 0.2 | 0.786 |
| HS-CRP (mg/dL) | 0.13 ± 0.3 | 0.15 ± 0.4 | 0.297 |
| Transient elastography | |||
| Controlled attenuation parameter, dB/m + | 246 (215–285) | 258 (217–300) | 0.005 |
| CAP ≥ 248 | 287 (48.8) | 343 (55.9) | 0.014 |
| CAP ≥ 302 | 99 (16.8) | 144 (23.5) | 0.004 |
| Liver stiffness measurement, kPa + | 3.6 (3.1–4.4) | 4.2 (3.5–4.8) | 0.851 |
| Tertile 1 (<3.4) | 195 (33.2) | 194 (31.6) | 0.359 |
| Tertile 2 (3.4–4.2) | 208 (35.4) | 203 (33.1) | |
| Tertile 3 (≥4.3) | 185 (31.5) | 217 (35.3) | |
| Variables | Odds Ratio | 95% Confidence Interval | p-Value * |
|---|---|---|---|
| Age, years | 1.01 | 1.00–1.02 | 0.220 |
| Male | 2.30 | 1.82–2.92 | <0.001 |
| Hypertension | 1.62 | 1.26–2.08 | <0.001 |
| Diabetes mellitus | 1.52 | 1.07–2.15 | 0.020 |
| Hypercholesterolemia | 1.50 | 1.16–1.93 | 0.002 |
| Smoking | 1.79 | 1.33–2.41 | <0.001 |
| Body mass index, kg/m2 | 1.29 | 1.24–1.34 | <0.001 |
| BMI ≥ 25, kg/m2 | 5.66 | 4.36–7.36 | <0.001 |
| Fasting glucose, mg/dL | 1.02 | 1.01–1.03 | <0.001 |
| Total cholesterol, mg/dL | 1.00 | 1.00–1.00 | 0.420 |
| Triglyceride, mg/dL + | 5.03 | 3.85–6.58 | <0.001 |
| HDL cholesterol, mg/dL | 0.96 | 0.95–0.97 | <0.001 |
| HS-CRP (mg/dL) | 1.29 | 0.89–1.87 | 0.176 |
| Vitamin D ≥ 20 (vs. <20)(ng/mL) | 0.75 | 0.60–0.95 | 0.014 |
| Vitamin D Quartile | |||
| 1st (–14.6) | 1 (reference) | 0.019 ** | |
| 2nd (14.7–19.5) | 0.82 | 0.59–1.13 | 0.218 |
| 3rd (19.6–26.7) | 0.73 | 0.53–1.01 | 0.056 |
| 4th (26.8–) | 0.60 | 0.44–0.83 | 0.002 |
| Vitamin D | Age, Sex Adjusted | Multivariate Model I | Multivariate Model II | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| ≥20 (vs. <20 ng/mL) | 0.72 (0.57–0.91) | 0.007 | 0.79 (0.60–1.00) | 0.051 | 0.78 (0.60–1.02) | 0.069 |
| Quartile | ||||||
| 1st (–14.6) | 1 (reference) | 0.006 * | 1 (reference) | 0.037 * | 1 (reference) | 0.065 * |
| 2nd (14.7–19.5) | 0.73 (0.52–1.02) | 0.062 | 0.70 (0.49–0.99) | 0.046 | 0.68 (0.47–1.00) | 0.049 |
| 3rd (19.6–26.7) | 0.62 (0.45–0.87) | 0.005 | 0.66 (0.46–0.95) | 0.024 | 0.65 (0.44–0.94) | 0.022 |
| 4th (26.8–) | 0.57 (0.41–0.80) | 0.001 | 0.61 (0.43–0.87) | 0.007 | 0.64 (0.44–0.94) | 0.021 |
| Vitamin D | Unadjusted | Age, Sex and Smoking Adjusted | Multivariate Model | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| ≥20 (vs. <20 ng/mL) | 0.73 (0.59–0.91) | 0.005 | 0.72 (0.57–0.90) | 0.005 | 0.78 (0.59–1.03) | 0.079 |
| Quartile | ||||||
| 1st (–14.6) | 1 (reference) | 0.006 * | 1 (reference) | 0.015 * | 1 (reference) | 0.167 * |
| 2nd (14.7–19.5) | 0.97 (0.71–1.31) | 0.829 | 0.87 (0.64–1.20) | 0.403 | 0.74 (0.50–1.09) | 0.123 |
| 3rd (19.6–26.7) | 0.85 (0.63–1.16) | 0.31 | 0.74 (0.54–1.02) | 0.069 | 0.69 (0.47–1.03) | 0.066 |
| 4th (26.8–) | 0.60 (0.44–0.82) | 0.001 | 0.60 (0.43–0.83) | 0.002 | 0.67 (0.45–0.99) | 0.046 |
| Reference (CAP < 248) | 248 ≤ CAP < 302 | CAP ≥ 302 | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Vitamin D Quartile | ||||
| 1st (–14.6) | 1 (reference) | 1 (reference) | ||
| 2nd (14.7–19.5) | 0.68 (0.45–1.03) | 0.069 | 0.69 (0.42–1.11) | 0.127 |
| 3rd (19.6–26.7) | 0.66 (0.44–0.99) | 0.044 | 0.63 (0.38–1.02) | 0.059 |
| 4th (26.8–) | 0.71 (0.47–1.06) | 0.091 | 0.52 (0.31–0.87) | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, N.J.; Park, H.E.; Yoon, J.W.; Kwak, M.-S.; Yang, J.I.; Chung, S.J.; Yim, J.Y.; Chung, G.E. The Association between Vitamin D and Nonalcoholic Fatty Liver Disease Assessed by Controlled Attenuation Parameter. J. Clin. Med. 2021, 10, 2611. https://doi.org/10.3390/jcm10122611
Heo NJ, Park HE, Yoon JW, Kwak M-S, Yang JI, Chung SJ, Yim JY, Chung GE. The Association between Vitamin D and Nonalcoholic Fatty Liver Disease Assessed by Controlled Attenuation Parameter. Journal of Clinical Medicine. 2021; 10(12):2611. https://doi.org/10.3390/jcm10122611
Chicago/Turabian StyleHeo, Nam Ju, Hyo Eun Park, Ji Won Yoon, Min-Sun Kwak, Jong In Yang, Su Jin Chung, Jeong Yoon Yim, and Goh Eun Chung. 2021. "The Association between Vitamin D and Nonalcoholic Fatty Liver Disease Assessed by Controlled Attenuation Parameter" Journal of Clinical Medicine 10, no. 12: 2611. https://doi.org/10.3390/jcm10122611
APA StyleHeo, N. J., Park, H. E., Yoon, J. W., Kwak, M.-S., Yang, J. I., Chung, S. J., Yim, J. Y., & Chung, G. E. (2021). The Association between Vitamin D and Nonalcoholic Fatty Liver Disease Assessed by Controlled Attenuation Parameter. Journal of Clinical Medicine, 10(12), 2611. https://doi.org/10.3390/jcm10122611






