How to Assess Diabetic Kidney Disease Progression? From Albuminuria to GFR
Abstract
1. Introduction
2. General Considerations in Diabetic Kidney Disease
2.1. Diagnosis of DKD
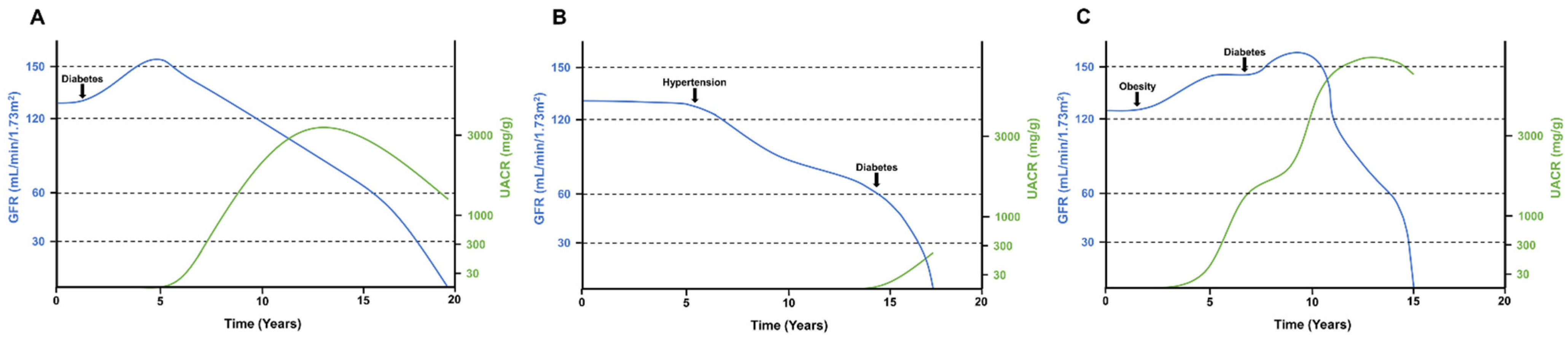
2.2. Progression of DKD
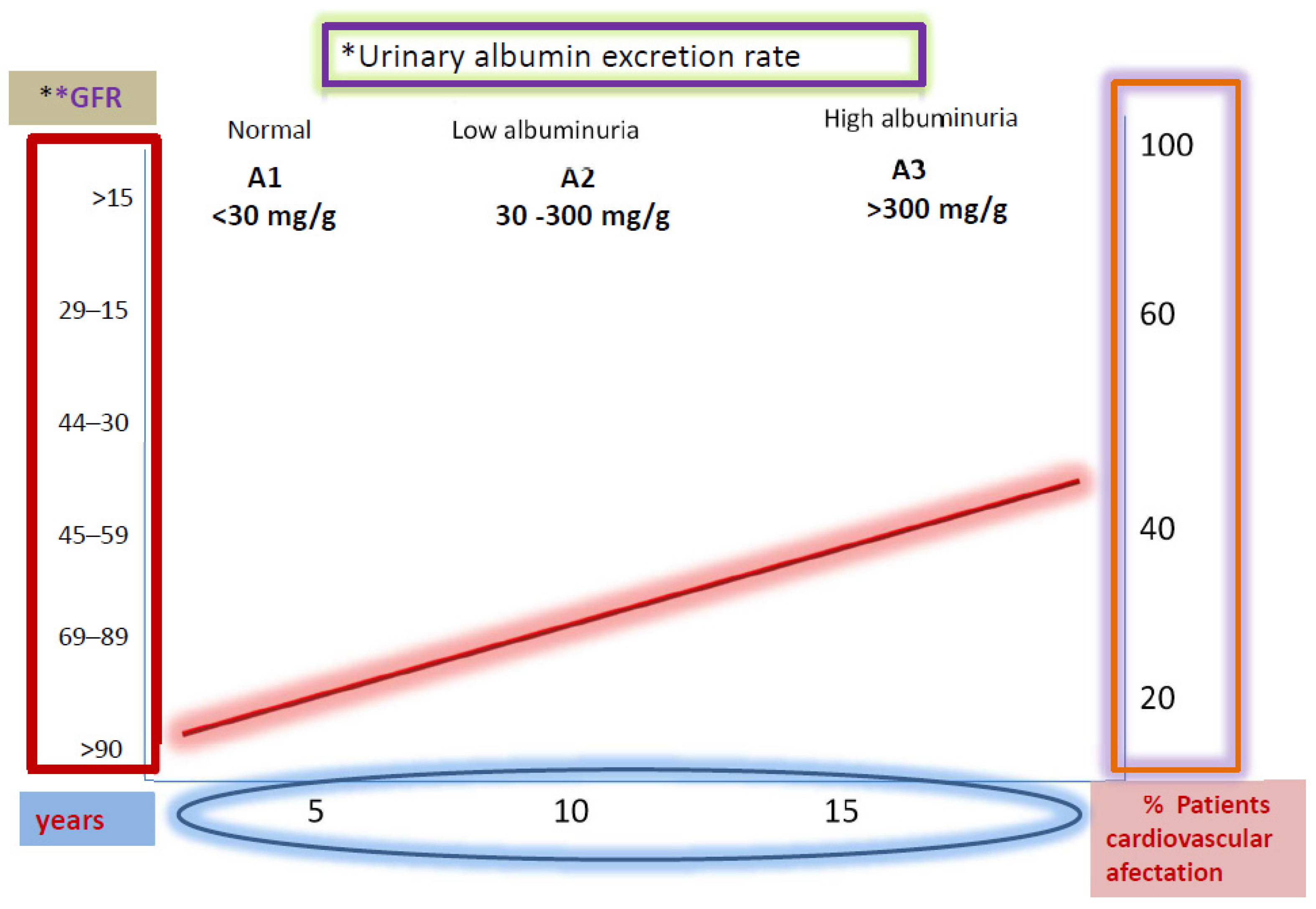
2.3. Albuminuria in DKD
3. Cardiovascular Endpoints in Diabetic Kidney Disease
4. Composite Renal Outcomes
5. GFR Decline in DKD: Ways of Measurement and Threshold
6. Pros and Cons of Renal Endpoints Standardization
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Poncelas, A.; Garre-Olmo, J.; Franch-Nadal, J.; Diez-Espino, J.; Mundet-Tuduri, X.; Barrot-De la Puente, J.; Coll-de Tuero, G.; RedGDPS Study Group. Prevalence of chronic kidney disease in patients with type 2 diabetes in Spain: PERCEDIME2 study. BMC Nephrol. 2013, 14, 46. [Google Scholar] [CrossRef]
- American Diabetes Association 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S103–S123. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Benjamin, I.J.; Burke, G.L.; Chait, A.; Eckel, R.H.; Howard, B.V.; Mitch, W.; Smith, S.C.; Sowers, J.R. Diabetes and cardiovascular disease: A statement for healthcare professionals from the American Heart Association. Circulation 1999, 100, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhao, L.; Zou, Y.; Wang, Y.; Zhang, J.; Wu, Y.; Zhang, R.; Wang, T.; Wang, J.; Zhu, Y.; et al. Association between atherosclerotic cardiovascular diseases risk and renal outcome in patients with type 2 diabetes mellitus. Ren Fail. 2021, 43, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Mann, J.F.E.; Ørsted, D.D.; Brown-Frandsen, K.; Marso, S.P.; Poulter, N.R.; Rasmussen, S.; Tornøe, K.; Zinman, B.; Buse, J.B.; LEADER Steering Committee and Investigators. Liraglutide and Renal Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 839–848. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef]
- White, K.E.; Bilous, R.W. Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J. Am. Soc. Nephrol. 2000, 11, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Caramori, M.L. Should all patients with diabetes have a kidney biopsy? Nephrol. Dial. Transplant. 2017, 32, 3–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bermejo, S.; García-Carro, C.; Soler, M.J. Diabetes and renal disease—Should we biopsy? Nephrol. Dial. Transplant. 2019. [Google Scholar] [CrossRef]
- Bermejo, S.; González, E.; López-Revuelta, K.; Ibernon, M.; López, D.; Martín-Gómez, A.; Garcia-Osuna, R.; Linares, T.; Díaz, M.; Martín, N.; et al. Risk factors for non-diabetic renal disease in diabetic patients. Clin. Kidney J. 2020, 13, 380–388. [Google Scholar] [CrossRef]
- García-Martín, F.; González Monte, E.; Hernández Martínez, E.; Bada Boch, T.; Bustamante Jiménez, N.E.; Praga Terente, M. When to perform renal biopsy in patients with type2 diabetes mellitus? Predictive model of non-diabetic renal disease. Nefrologia 2020, 40, 180–189. [Google Scholar] [CrossRef]
- Porrini, E.; Ruggenenti, P.; Mogensen, C.E.; Barlovic, D.P.; Praga, M.; Cruzado, J.M.; Hojs, R.; Abbate, M.; de Vries, A.P.J.; ERA-EDTA Diabesity Working Group. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2015, 3, 382–391. [Google Scholar] [CrossRef]
- Akhtar, M.; Taha, N.M.; Nauman, A.; Mujeeb, I.B.; Al-Nabet, A.D.M.H. Diabetic Kidney Disease: Past and Present. Adv. Anat Pathol 2020, 27, 87–97. [Google Scholar] [CrossRef]
- Tervaert, T.W.C.; Mooyaart, A.L.; Amann, K.; Cohen, A.H.; Cook, H.T.; Drachenberg, C.B.; Ferrario, F.; Fogo, A.B.; Haas, M.; de Heer, E.; et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 556–563. [Google Scholar] [CrossRef]
- Mogensen, C.E.; Christensen, C.K.; Vittinghus, E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 1983, 32 (Suppl 2), 64–78. [Google Scholar] [CrossRef]
- Mauer, S.M.; Steffes, M.W.; Ellis, E.N.; Sutherland, D.E.; Brown, D.M.; Goetz, F.C. Structural-functional relationships in diabetic nephropathy. J. Clin. Investig. 1984, 74, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Raptis, A.E.; Viberti, G. Pathogenesis of diabetic nephropathy. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl 2), S424–S437. [Google Scholar] [CrossRef]
- Jiang, H.; Guan, G.; Zhang, R.; Liu, G.; Cheng, J.; Hou, X.; Cui, Y. Identification of urinary soluble E-cadherin as a novel biomarker for diabetic nephropathy. Diabetes Metab. Res. Rev. 2009, 25, 232–241. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am. J. Kidney Dis. 2012, 60, 850–886. [CrossRef] [PubMed]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Diabetes Care 2014, 37, 2864–2883. [Google Scholar] [CrossRef] [PubMed]
- Gaede, P.; Tarnow, L.; Vedel, P.; Parving, H.-H.; Pedersen, O. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol. Dial. Transplant. 2004, 19, 2784–2788. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [CrossRef]
- Spoelstra-de Man, A.M.; Brouwer, C.B.; Stehouwer, C.D.; Smulders, Y.M. Rapid progression of albumin excretion is an independent predictor of cardiovascular mortality in patients with type 2 diabetes and microalbuminuria. Diabetes Care 2001, 24, 2097–2101. [Google Scholar] [CrossRef] [PubMed]
- Viberti, G.C.; Hill, R.D.; Jarrett, R.J.; Argyropoulos, A.; Mahmud, U.; Keen, H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet 1982, 1, 1430–1432. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Hellemons, M.E.; Denig, P.; de Zeeuw, D.; Voorham, J.; Lambers Heerspink, H.J. Is albuminuria screening and treatment optimal in patients with type 2 diabetes in primary care? Observational data of the GIANTT cohort. Nephrol. Dial. Transplant. 2013, 28, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Persson, F.; Rossing, P. Diagnosis of diabetic kidney disease: State of the art and future perspective. Kidney Int. Suppl. 2018, 8, 2–7. [Google Scholar] [CrossRef]
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf, S.; Sleight, P.; Pogue, J.; Bosch, J.; Davies, R.; Dagenais, G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N. Engl. J. Med. 2000, 342, 145–153. [Google Scholar]
- Ekström, N.; Schiöler, L.; Svensson, A.-M.; Eeg-Olofsson, K.; Miao Jonasson, J.; Zethelius, B.; Cederholm, J.; Eliasson, B.; Gudbjörnsdottir, S. Effectiveness and safety of metformin in 51,675 patients with type 2 diabetes and different levels of renal function: A cohort study from the Swedish National Diabetes Register. BMJ Open 2012, 2, e001076. [Google Scholar] [CrossRef]
- Christiansen, C.F.; Ehrenstein, V.; Heide-Jørgensen, U.; Skovbo, S.; Nørrelund, H.; Sørensen, H.T.; Li, L.; Jick, S. Metformin initiation and renal impairment: A cohort study in Denmark and the UK. BMJ Open 2015, 5, e008531. [Google Scholar] [CrossRef] [PubMed]
- Mariano, F.; Pozzato, M.; Inguaggiato, P.; Guarena, C.; Turello, E.; Manes, M.; David, P.; Berutti, S.; Consiglio, V.; Amore, A.; et al. Metformin-Associated Lactic Acidosis Undergoing Renal Replacement Therapy in Intensive Care Units: A Five-Million Population-Based Study in the North-West of Italy. Blood Purif. 2017, 44, 198–205. [Google Scholar] [CrossRef]
- Bell, S.; Farran, B.; McGurnaghan, S.; McCrimmon, R.J.; Leese, G.P.; Petrie, J.R.; McKeigue, P.; Sattar, N.; Wild, S.; McKnight, J.; et al. Risk of acute kidney injury and survival in patients treated with Metformin: An observational cohort study. BMC Nephrol. 2017, 18, 163. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tarng, D.-C.; Chen, H.-S. Renal Outcomes of Pioglitazone Compared with Acarbose in Diabetic Patients: A Randomized Controlled Study. PLoS ONE 2016, 11, e0165750. [Google Scholar] [CrossRef] [PubMed]
- Van Dalem, J.; Brouwers, M.C.G.J.; Stehouwer, C.D.A.; Krings, A.; Leufkens, H.G.M.; Driessen, J.H.M.; de Vries, F.; Burden, A.M. Risk of hypoglycaemia in users of sulphonylureas compared with metformin in relation to renal function and sulphonylurea metabolite group: Population based cohort study. BMJ 2016, i3625. [Google Scholar] [CrossRef] [PubMed]
- García-Carro, C.; Vergara, A.; Agraz, I.; Jacobs-Cachá, C.; Espinel, E.; Seron, D.; Soler, M.J. The New Era for Reno-Cardiovascular Treatment in Type 2 Diabetes. J. Clin. Med. 2019, 8, 864. [Google Scholar] [CrossRef] [PubMed]
- Górriz, J.L.; Cos Claramunt, F.X.; Duque, N.; Matali, A. Review of the renal endpoints used in cardiovascular safety clinical trials in type 2 diabetes mellitus patients and their importance in primary care. Prim. Care Diabetes 2019, 13, 485–494. [Google Scholar] [CrossRef]
- SOLVD Investigators; Yusuf, S.; Pitt, B.; Davis, C.E.; Hood, W.B.; Cohn, J.N. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med. 1991, 325, 293–302. [Google Scholar] [PubMed]
- Cefalu, W.T.; Kaul, S.; Gerstein, H.C.; Holman, R.R.; Zinman, B.; Skyler, J.S.; Green, J.B.; Buse, J.B.; Inzucchi, S.E.; Leiter, L.A.; et al. Cardiovascular Outcomes Trials in Type 2 Diabetes: Where Do We Go From Here? Reflections From a Diabetes Care Editors’ Expert Forum. Diabetes Care 2018, 41, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Cornel, J.H.; Bakris, G.L.; Stevens, S.R.; Alvarsson, M.; Bax, W.A.; Chuang, L.-M.; Engel, S.S.; Lopes, R.D.; McGuire, D.K.; Riefflin, A.; et al. Effect of Sitagliptin on Kidney Function and Respective Cardiovascular Outcomes in Type 2 Diabetes: Outcomes From TECOS. Dia Care 2016, 39, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019, 321, 69. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef]
- Wittbrodt, E.T.; Eudicone, J.M.; Bell, K.F.; Enhoffer, D.M.; Latham, K.; Green, J.B. Generalizability of glucagon-like peptide-1 receptor agonist cardiovascular outcome trials enrollment criteria to the US type 2 diabetes population. Am. J. Manag. Care 2018, 24, S146–S155. [Google Scholar] [PubMed]
- Perkovic, V.; de Zeeuw, D.; Mahaffey, K.W.; Fulcher, G.; Erondu, N.; Shaw, W.; Barrett, T.D.; Weidner-Wells, M.; Deng, H.; Matthews, D.R.; et al. Canagliflozin and renal outcomes in type 2 diabetes: Results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018, 6, 691–704. [Google Scholar] [CrossRef]
- Mosenzon, O.; Wiviott, S.D.; Cahn, A.; Rozenberg, A.; Yanuv, I.; Goodrich, E.L.; Murphy, S.A.; Heerspink, H.J.L.; Zelniker, T.A.; Dwyer, J.P.; et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: An analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 606–617. [Google Scholar] [CrossRef]
- Cosentino, F.; Cannon, C.P.; Cherney, D.Z.I.; Masiukiewicz, U.; Pratley, R.; Dagogo-Jack, S.; Frederich, R.; Charbonnel, B.; Mancuso, J.; Shih, W.J.; et al. Efficacy of Ertugliflozin on Heart Failure–Related Events in Patients With Type 2 Diabetes Mellitus and Established Atherosclerotic Cardiovascular Disease: Results of the VERTIS CV Trial. Circulation 2020, 142, 2205–2215. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Mosenzon, O.; Leibowitz, G.; Bhatt, D.L.; Cahn, A.; Hirshberg, B.; Wei, C.; Im, K.; Rozenberg, A.; Yanuv, I.; Stahre, C.; et al. Effect of Saxagliptin on Renal Outcomes in the SAVOR-TIMI 53 Trial. Diabetes Care 2017, 40, 69–76. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Perkovic, V.; Alexander, J.H.; Cooper, M.E.; Marx, N.; Pencina, M.J.; Toto, R.D.; Wanner, C.; Zinman, B.; Baanstra, D.; et al. Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA®): A randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk. Cardiovasc. Diabetol. 2018, 17, 39. [Google Scholar]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Kramer, A.; Pippias, M.; Noordzij, M.; Stel, V.S.; Andrusev, A.M.; Aparicio-Madre, M.I.; Arribas Monzón, F.E.; Åsberg, A.; Barbullushi, M.; Beltrán, P.; et al. The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: A summary. Clin. Kidney J. 2019, 12, 702–720. [Google Scholar] [CrossRef]
- Halimi, J.M. The emerging concept of chronic kidney disease without clinical proteinuria in diabetic patients. Diabetes Metab. 2012, 38, 291–297. [Google Scholar] [CrossRef]
- Martínez-Castelao, A.; Górriz, J.L.; Segura-de la Morena, J.; Cebollada, J.; Escalada, J.; Esmatjes, E.; Fácila, L.; Gamarra, J.; Gràcia, S.; Hernánd-Moreno, J.; et al. Consensus document for the detection and management of chronic kidney disease. Nefrologia 2014, 34, 243–262. [Google Scholar]
- Espinel, E.; Agraz, I.; Ibernon, M.; Ramos, N.; Fort, J.; Serón, D. Renal Biopsy in Type 2 Diabetic Patients. J. Clin. Med. 2015, 4, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Turin, T.C.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Appel, L.J.; Arima, H.; Chadban, S.J.; Cirillo, M.; Djurdjev, O.; et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014, 311, 2518–2531. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Koitka-Weber, A.; Cooper, M.E.; Schernthaner, G.; Pfarr, E.; Woerle, H.J.; von Eynatten, M.; Wanner, C. Choice of endpoint in kidney outcome trials: Considerations from the EMPA-REG OUTCOME® trial. Nephrol. Dial. Transplant. 2020, 35, 2103–2111. [Google Scholar] [CrossRef]
- Nistor, I.; Bolignano, D.; Haller, M.C.; Nagler, E.; van der Veer, S.N.; Jager, K.; Covic, A.; Webster, A.; Van Biesen, W. Why creating standardized core outcome sets for chronic kidney disease will improve clinical practice. Nephrol. Dial. Transplant. 2017, 32, 1268–1273. [Google Scholar] [CrossRef][Green Version]
- Strippoli, G.F.M.; Craig, J.C.; Schena, F.P. The number, quality, and coverage of randomized controlled trials in nephrology. J. Am. Soc. Nephrol. 2004, 15, 411–419. [Google Scholar] [CrossRef]
- Zoccali, C.; Blankestijn, P.J.; Bruchfeld, A.; Capasso, G.; Fliser, D.; Fouque, D.; Goumenos, D.; Ketteler, M.; Massy, Z.; Rychlık, I.; et al. Children of a lesser god: Exclusion of chronic kidney disease patients from clinical trials. Nephrol. Dial. Transplant. 2019, 34, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Prischl, F.C.; Wanner, C. Renal Outcomes of Antidiabetic Treatment Options for Type 2 Diabetes—A Proposed MARE Definition. Kidney Int. Rep. 2018, 3, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, K.W.; Jardine, M.J.; Bompoint, S.; Cannon, C.P.; Neal, B.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; et al. Canagliflozin and Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus and Chronic Kidney Disease in Primary and Secondary Cardiovascular Prevention Groups. Circulation 2019, 140, 739–750. [Google Scholar] [CrossRef] [PubMed]
| Predictive Risk Factors for DKD | Predictive Risk Factors for NDKD |
|---|---|
| -Diabetic retinopathy | -Microhaematuria |
| -Longer duration of DM (≥10 years) | -Shorter duration of DM (<5 years) |
| -Chronic lower limb ischemia | -Overweight grade II (BMI: 35–39.9 kg/m2). |
| -Nephrotic range proteinuria | -Older age |
| -Insulin treatment | -History of CKD prior to diabetes development |
| Category | AER | ACR | ACR | Renal Composite Outcome |
|---|---|---|---|---|
| (mg/24 h) | (mg/mmol) | (mg/g) | Terms | |
| A1 | <30 | <3 | <30 | Normal to mildly increased |
| A2 | 30–300 | 3–30 | 30–300 | Moderately increased |
| A3 | >300 | >30 | >300 | Severely increased |
| Trial | Drug | Population | Baseline Renal Characteristics | Renal Composite Outcome | Albuminuria | GFR/Creatinine | ESRD |
|---|---|---|---|---|---|---|---|
| EMPA-REG OUTCOME [5] | Empagliflozin | 7020 patients with T2D with GFR > 30 mL/min | 25.9% of patients had GFR <60 mL/min | Progression to macroalbuminuria, doubling of serum creatinine with GFR ≤ 45 mL/min, ESRD, renal death | Incident microalbuminuria (UACR 30–300 mg/g) Incident macroalbuminuria (UACR > 300 mg/gr) | Doubling of serum creatinine and GFR < 45 mL/min | Need of RRT |
| CANVAS program [51] | Canagliflozin | 10142 patients with T2D and cardiovascular disease. | 20.1% of patients had GFR < 60 mL/min | Sustained ≥ 40% decrease in GFR, ESRD or renal death | New microalbuminuria or new macroalbuminuria with ≥ 30% increased UACR | Sustained 40% reduction in GFR for ≥ 30 days | Sustained GFR < 15 mL/min for > 30 days, dyalisis ≥ 30 days or renal transplant. |
| CREDENCE trial [54] | Canagliflozin | 4401 patients with T2D, GFR 30–89 mL/min and UACR 300–5000 mg/gr | All patients had GFR of 30–89 mL/min and UACR 300–5000 mg/g | Doubling of serum creatinine, ESRD, or death from renal or cardiovascular disease | Comparison of UACR versus placebo | Sustained doubling of serum creatinine | Sutained GFR < 15 mL/min for > 30 days or need for dialysis or renal transplant |
| DECLARE-TIMI 58 [52] | Dapagliflozin | 17160 patients with T2D and cardiovascular disease | 7.4% of patients had GFR ≤ 60 mL/min | Sustained ≥ 40% decrease in GFR to ≤ 60 mL/min, ESRD, renal or cardiovascular death | Comparison of UACR versus placebo | Sustained ≥ 40% decrease in FGR to ≤ 60 mL/min | Sustained GFR < 15 mL/min, or dialysis for ≥ 90 days or renal transplant |
| DAPA-HF [55] | Dapagliflozin | 4744 patients with T2D and non-T2D, HF with EF < 40% | All patients had GFR > 30 mL/min | Sustained ≥ 50% decrease in GFR, ESRD, renal death | Not reported | Sustained ≥ 50% decrease in GFR | Sustained GFR < 15 mL/min ≥ 28 days, or need for continuous RRT |
| SAVOR-TIMI [56] | Saxagliptin | 16492 patients with T2D and cardiovascular disease | 15.6% of patients had GFR < 50 mL/min | Doubling serum creatinine or ESRD | Categorical change in UACR from baseline | Doubling of serum creatinine | Need for renal dialysis, transplant or serum creatinine > 530 µmol/L |
| CARMELINA trial [43] | Linagliptin | 6979 patients with T2D and high cardiovascular and renal risk | 74% of patients had GFR of 30–59 mL/min and 15.2% had GFR < 30 mL/min | Sustained ≥ 40% decrease in GFR and GFR ≤ 60 mL/min, ESRD, renal death | Microalbuminuria (ACR 30–300 mg/gr) or macroalbuminuria (UACR ≥ 300 mg/g) | Sustained ≥ 40% decrease in GFR and GFR ≤ 60 mL/min | Need for renal dialysis ≥ 30 days or renal transplant |
| LEADER trial [6] | Liraglutide | 9340 patients with T2D | 23.1% of patients had GFR < 60 mL/min | New macroalbuminuria, doubling of serum creatinine with GFR ≤ 45 mL/min, need for continuous RRT or renal death | New macroalbuminuria (UACR > 300 mg/gr or urinary albumin > 300 mg/24 h) | Doubling of the serum creatinine with GFR ≤ 45 mL/min | Need for continuous RRT |
| SUSTAIN-6 trial [44] | Semaglutide | 3297 patients with T2D and Cardiovascular disease | 28.5% of patients had FGR < 60 mL/min | New macroalbuminuria, doubling of serum creatinine with GFR ≤ 45 mL/min, need for continuous RRT or renal death | New macroalbuminuria (UACR > 300 mg/gr or urinary albumin > 300 mg/24 h) | Doubling of the serum creatinine with GFR ≤ 45 mL/min | Need for continuous RRT |
| EXSCEL trial [47] | Exenatide | 14752 patients with T2D | 21.6% of patients had GFR < 60 mL/min | New macroalbuminuria sustained ≥ 40% decrease in GFR or RRT or renal death | New macroalbuminuria | Sustained ≥ 40% decrease in GFR | Need for continuous RRT |
| REWIND study [46] | Dulaglutide | 9901 patients with T2D | 22.2% of patients had GFR < 60 mL/min | New macroalbuminuria, sustained ≥ 30% decrease in GFR or chronic RRT | New macroalbuminuria (UACR > 33.9 mg/mmol) | Sustained ≥ 30% decrease in GFR | Need for continuous RRT |
| FIDELIO-DKD [57] | Finerenone | 5734 patients with CKD and T2D | All patients had GFR of 25–60 mL/min and UACR of 300–5000 mg/g | Kidney failure, sustained ≥ 40% decrease in GFR or death from renal causes | Change in UACR from baseline to month 4 | Sustained ≥ 40% decrease in GFR | GFR < 15 mL/min or initiation of RRT (≥ 90 days) or kidney transplantation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Carro, C.; Vergara, A.; Bermejo, S.; Azancot, M.A.; Sánchez-Fructuoso, A.I.; Sánchez de la Nieta, M.D.; Agraz, I.; Soler, M.J. How to Assess Diabetic Kidney Disease Progression? From Albuminuria to GFR. J. Clin. Med. 2021, 10, 2505. https://doi.org/10.3390/jcm10112505
García-Carro C, Vergara A, Bermejo S, Azancot MA, Sánchez-Fructuoso AI, Sánchez de la Nieta MD, Agraz I, Soler MJ. How to Assess Diabetic Kidney Disease Progression? From Albuminuria to GFR. Journal of Clinical Medicine. 2021; 10(11):2505. https://doi.org/10.3390/jcm10112505
Chicago/Turabian StyleGarcía-Carro, Clara, Ander Vergara, Sheila Bermejo, María A. Azancot, Ana I. Sánchez-Fructuoso, M. Dolores Sánchez de la Nieta, Irene Agraz, and María José Soler. 2021. "How to Assess Diabetic Kidney Disease Progression? From Albuminuria to GFR" Journal of Clinical Medicine 10, no. 11: 2505. https://doi.org/10.3390/jcm10112505
APA StyleGarcía-Carro, C., Vergara, A., Bermejo, S., Azancot, M. A., Sánchez-Fructuoso, A. I., Sánchez de la Nieta, M. D., Agraz, I., & Soler, M. J. (2021). How to Assess Diabetic Kidney Disease Progression? From Albuminuria to GFR. Journal of Clinical Medicine, 10(11), 2505. https://doi.org/10.3390/jcm10112505






