Long-Term Outcomes of Surgical Aortic Valve Replacement in Patients with Rheumatoid Arthritis
Abstract
1. Introduction
2. Patients and Methods
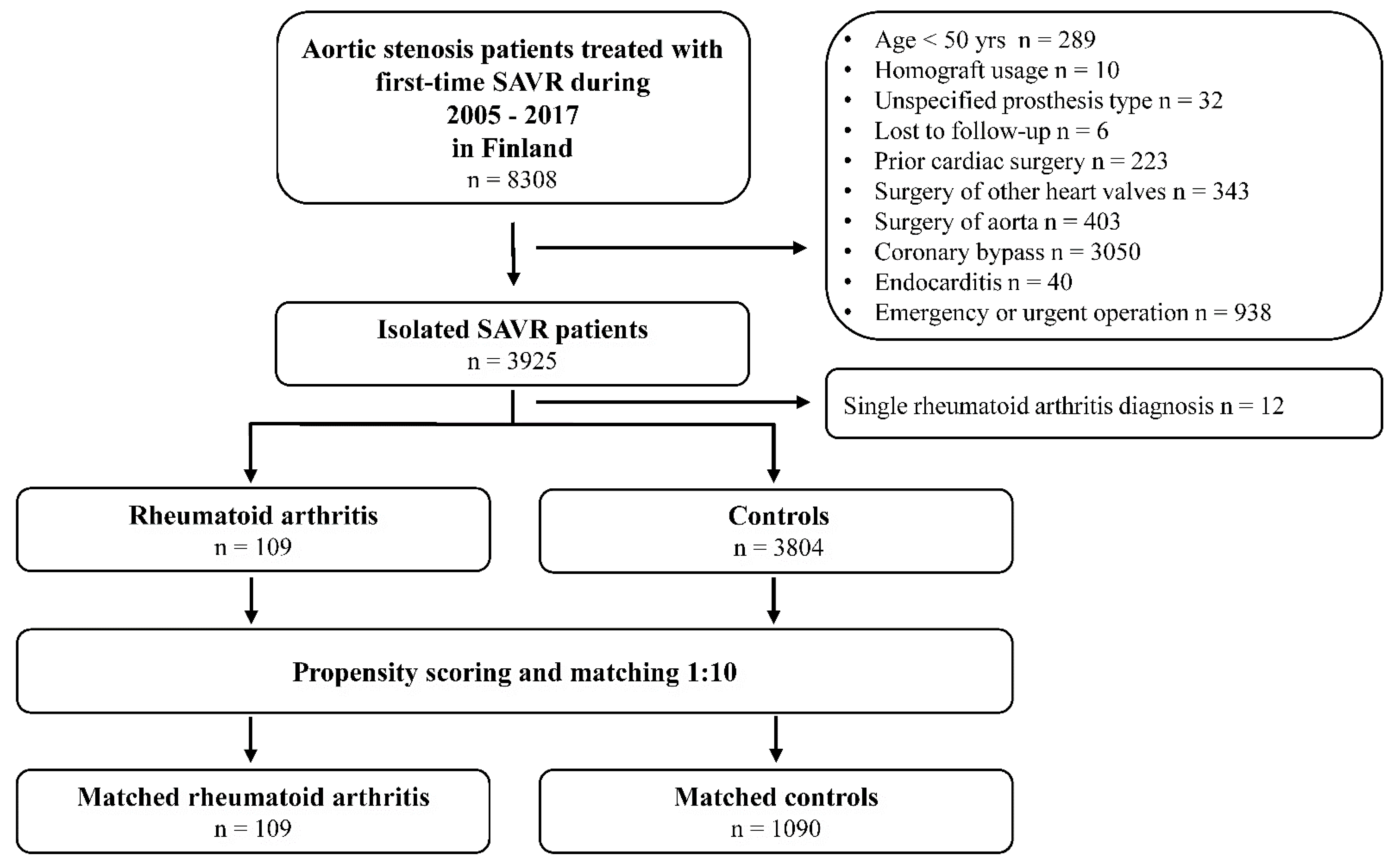
2.1. Study Design and Population
2.2. Definitions
2.3. Matching and Statistical Analysis
3. Results
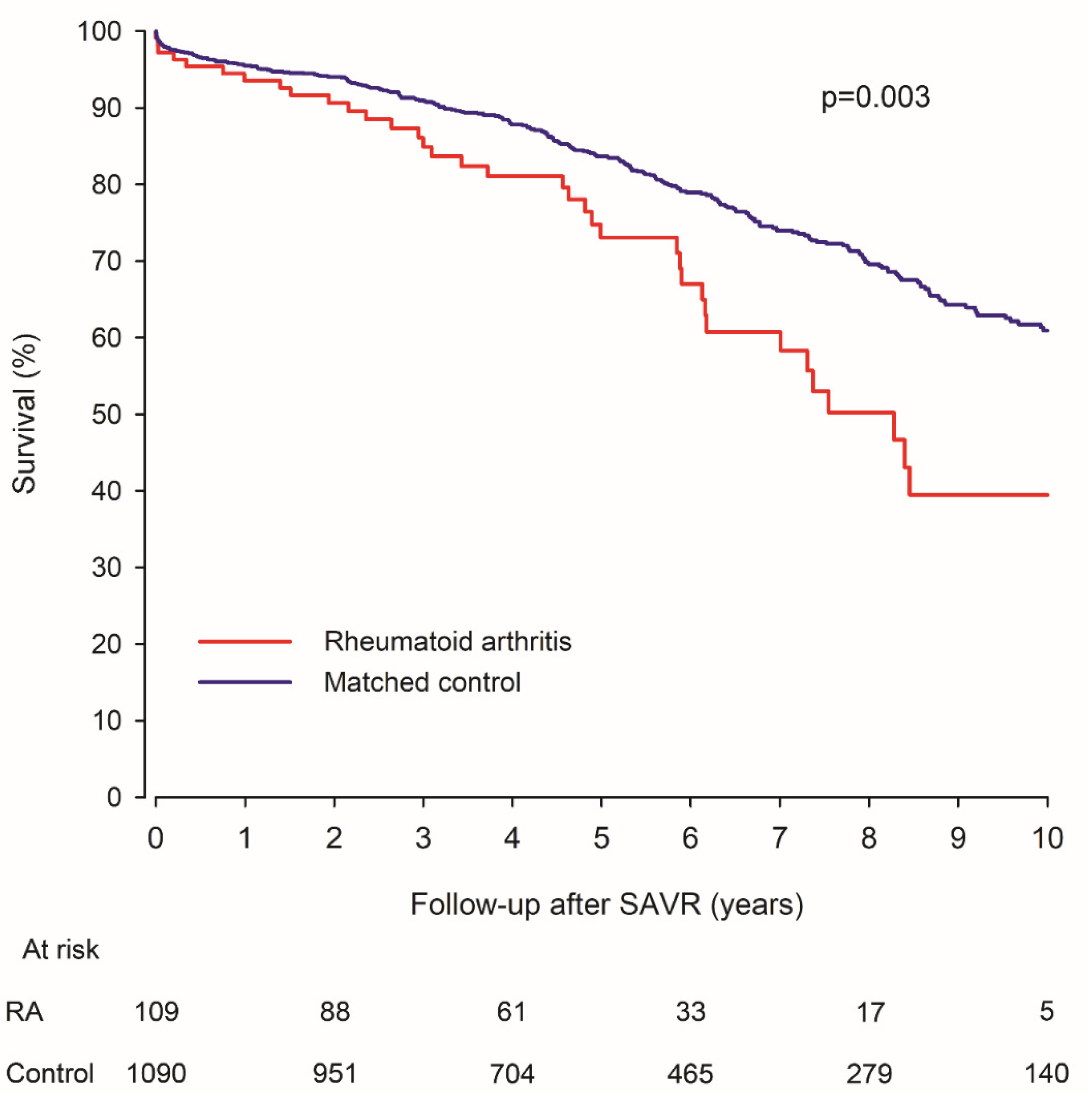
3.1. Mortality
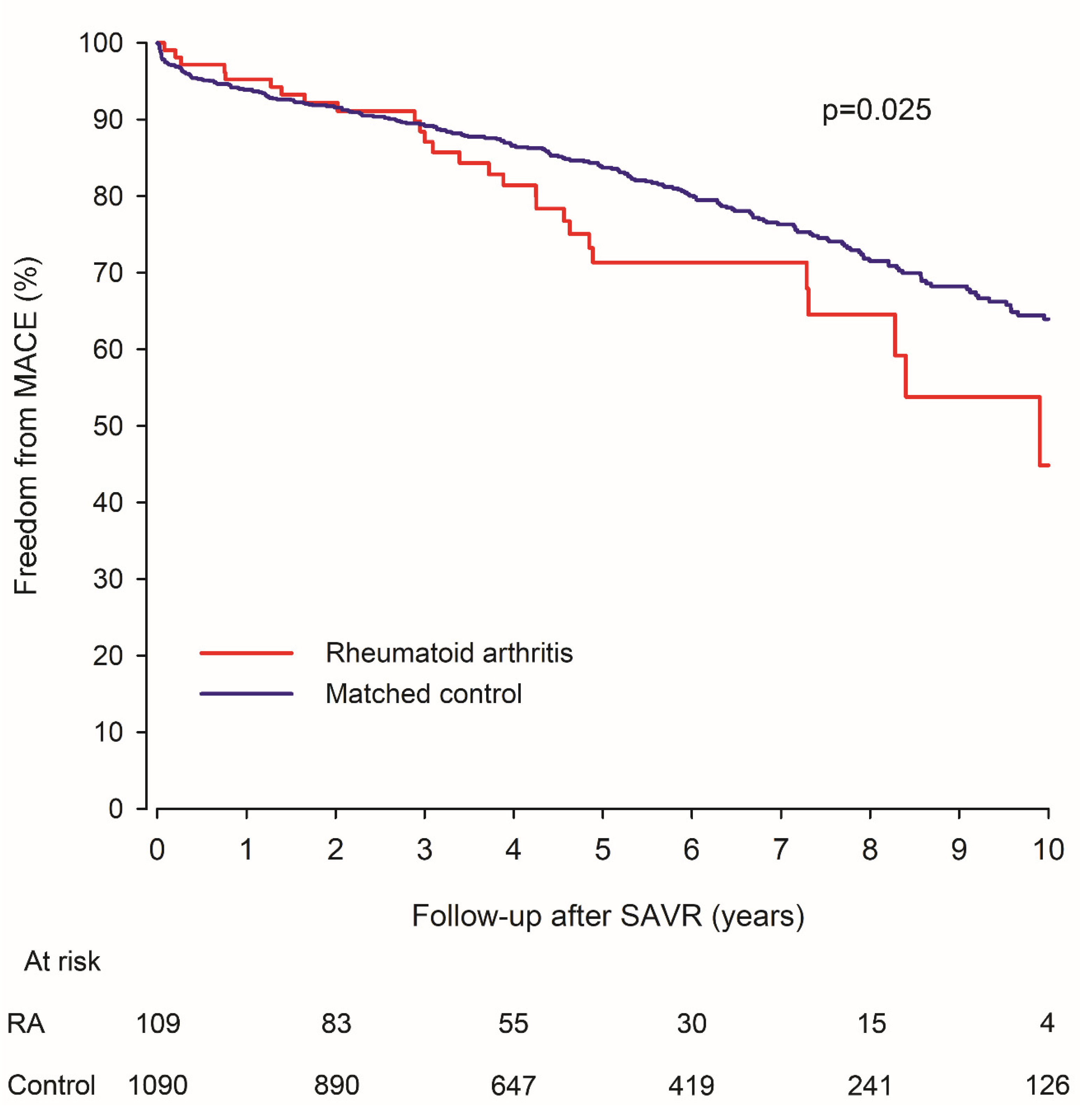
3.2. MACE
3.3. Reoperation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RA | Rheumatoid arthritis |
| CAD | Coronary artery disease |
| TAVR | Transcatheter aortic valve replacement |
| SAVR | Surgical aortic valve replacement |
| MACE | Major adverse cardiovascular event |
| SMD | Standardized mean difference |
| HR | Hazard ratio |
| CI | 95% confidence intervals |
| MI | Myocardial infarction |
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Lee, K.S.; Kronbichler, A.; Eisenhut, M.; Lee, K.H.; Shin, J.I. Cardiovascular involvement in systemic rheumatic diseases: An integrated view for the treating physicians. Autoimmun. Rev. 2018, 17, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Corrao, S.; Messina, S.; Pistone, G.; Galvo, L.; Scaglione, R.; Licata, G. Heart involvement in Rheumatoid Arthritis: Systematic review and meta-analysis. Int. J. Cardiol. 2013, 167, 2031–2038. [Google Scholar] [CrossRef]
- Iveson, J.M.I.; Thadani, U.; Ionescu, M.; Wright, V. Aortic valve incompetence and replacement in rheumatoid arthritis. Ann. Rheum. Dis. 1975, 34, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Corrao, S.; Salli, L.; Arnone, S.; Scaglione, R.; Amato, V.; Cecala, M.; Ligata, A.; Ligata, G. Cardiac involvement in rheumatoid arthritis: Evidence of silent heart disease. Eur. Heart J. 1995, 16, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Yiu, K.H.; Wang, S.; Mok, M.Y.; Ooi, G.C.; Khong, P.L.; Lau, G.S.; Tse, H.F. Relationship between cardiac valvular and arterial calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. J. Rheumatol. 2011, 38, 621–627. [Google Scholar] [CrossRef]
- Rudasill, S.E.; Sanaiha, Y.; Xing, H.; Mardock, A.L.; Khoury, H.; Jaman, R.; Ebrahimi, R.; Benharash, P. Association of autoimmune connective tissue disease and outcome in patients undergoing transcatheter aortic valve implantation. Am. J. Cardiol. 2019, 123, 1675–1680. [Google Scholar] [CrossRef]
- Elbadawi, A.; Ahmed, H.M.A.; Mahmoud, K.; Mohamed, A.H.; Barssoum, K.; Perez, C.; Mahmoud, A.; Ogunbayo, G.O.; Omer, M.A.; Jneid, H.; et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with rheumatoid arthritis (from the nationwide inpatient database). Am. J. Cardiol. 2019, 124, 1099–1105. [Google Scholar] [CrossRef]
- Kytö, V.; Myllykangas, M.E.; Sipilä, J.; Niiranen, T.J.; Rautava, P.; Gunn, J. Long-term Outcomes of Mechanical Vs Biologic Aortic Valve Prosthesis in Patients Older Than 70 Years. Ann. Thorac. Surg. 2019, 108, 1354–1360. [Google Scholar] [CrossRef]
- Malmberg, M.; Sipilä, J.; Rautava, P.; Gunn, J.; Kytö, V. Outcomes After ST-Segment Versus Non-ST-Segment Elevation Myocardial Infarction Revascularized by Coronary Artery Bypass Grafting. Am. J. Cardiol. 2020, 135, 17–23. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Vander Weele, T.J.; Ding, P. Sensitivity analysis in observational research: Introducing the e-value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Stulak, J.M.; Suri, R.M.; Matteson, E.L.; Dearani, J.A.; Connolly, H.M.; Schaff, H.V. Mitral valve repair is durable in patients with rheumatoid arthritis. Ann. Thorac. Surg. 2012, 94, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, C.M.; Kwedar, K.; Boley, T.; Markwell, S.; Hazelrigg, S. Mitral valve procedure selection and outcomes in patients with rheumatoid arthritis. J. Heart Valve. Dis. 2013, 22, 14–19. [Google Scholar]
- Grimaldi, A.; de Gennaro, L.; Vermi, A.C.; Pappalardo, F.; Brunetti, N.D.; Biase, M.D.; la Canna, G.; Alfieri, O. Cardiac valve involvement in systemic diseases: A review. Clin. Cardiol. 2013, 36, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Bois, J.P.; Crowson, C.S.; Khullar, T.; Achenbach, S.J.; Krause, M.L.; Mankad, R. Progression rate of severity of aortic stenosis in patients with rheumatoid arthritis. Echocardiography 2017, 34, 1410–1416. [Google Scholar] [CrossRef]
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. the Tromsø Study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef]
- Shetty, R.; Pibarot, P.; Audet, R.; Janvier, R.; Dagenais, F.; Perron, J.; Couture, C.; Voisine, P.; Després, J.P.; Mathieu, P. Lipid-mediated inflammation and degeneration of bioprosthetic heart valves. Eur. J. Clin. Investig. 2009, 39, 471–480. [Google Scholar] [CrossRef]
- Levine, A.J.; Dimitri, W.R.; Bonser, R.S. Aortic regurgitation in rheumatoid arthritis necessitating aortic valve replacement. Eur. J. Cardiothoracic. Surg. 1999, 15, 213–214. [Google Scholar] [CrossRef]
- Ferguson, L.D.; Siebert, S.; McInnes, I.B.; Sattar, N. Cardiometabolic comorbidities in RA and PsA: Lessons learned and future directions. Nat. Rev. Rheumatol. 2019, 15, 461–474. [Google Scholar] [CrossRef]
- Aviña-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Care Res. 2008, 59, 1690–1697. [Google Scholar] [CrossRef]
- Semb, A.G.; Ikdahl, E.; Wibetoe, G.; Crowson, C.; Rollefstad, S. Atherosclerotic cardiovascular disease prevention in rheumatoid arthritis. Nat. Rev. Rheumatol. 2020, 16, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, E.; Gabriel, S.E.; Matteson, E.L.; Davis, J.M., 3rd; Therneau, T.M.; Crowson, C.S. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J. Rheumatol. 2017, 44, 732–739. [Google Scholar] [CrossRef]
- Kerola, A.M.; Nieminen, T.V.M.; Virta, L.J.; Kautiainen, H.; Kerola, T.; Pohjolainen, T. No increased cardiovascular mortality among early rheumatoid arthritis patients: A nationwide register study in 2000-2008. Clin. Exp. Rheumatol. 2015, 33, 391–398. [Google Scholar] [PubMed]
- Agca, R.; Heslinga, S.C.; van Halm, V.P.; Nurmohamed, M.T. Atherosclerotic cardiovascular disease in patients with chronic inflammatory joint disorders. Heart 2016, 102, 790–795. [Google Scholar] [CrossRef]
- Otto, C.M.; Prendergast, B. Aortic-valve stenosis—From patients at risk to severe valve obstruction. N. Eng. J. Med. 2014, 371, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; PARTNER Trial Investigators; et al. Transcatheter aortic-valve implantation for aortic valve in patients who cannot undergo surgery. N. Eng. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Sund, R. Quality of the Finnish Hospital Discharge Register: A systematic review. Scand. J. Public Health 2012, 40, 505–515. [Google Scholar] [CrossRef]
| Original Cohort | Matched Cohort | |||||
|---|---|---|---|---|---|---|
| Rheumatoid Arthritis | Control | Rheumatoid Arthritis | Control | |||
| Variable | n = 109 | n = 3804 | |SMD| | n = 109 | n = 1090 | |SMD| |
| Age, years (SD) | 71.7 (8.0) | 70.7 (8.7) | 0.13 | 71.7 (8.0) | 71.8 (8.4) | 0.02 |
| Female sex | 70 (64.2%) | 1835 (48.2%) | 0.33 | 70 (64.2%) | 685 (62.8%) | 0.07 |
| Comorbidities | ||||||
| Atrial fibrillation | 22 (20.2%) | 863 (22.7%) | 0.06 | 22 (20.2%) | 235 (21.6%) | 0.08 |
| Cerebrovascular disease | 13 (11.9%) | 391 (10.3%) | 0.05 | 13 (11.9%) | 128 (11.7%) | 0.01 |
| Chronic pulmonary disease | 22 (20.2%) | 439 (11.5%) | 0.24 | 22 (20.2%) | 209 (19.2%) | 0.03 |
| Diabetes | 12 (11.0%) | 640 (16.8%) | 0.17 | 12 (11.0%) | 120 (11.0%) | 0.01 |
| Heart failure | 27 (24.8%) | 890 (23.4%) | 0.03 | 27 (24.8%) | 235 (21.6%) | 0.08 |
| Hypertension | 45 (41.3%) | 1683 (44.2%) | 0.06 | 45 (41.3%) | 507 (46.5%) | 0.09 |
| Malignancy | 17 (15.6%) | 451 (11.9%) | 0.11 | 17 (15.6%) | 158 (14.5%) | 0.01 |
| Peripheral vascular disease | 6 (5.5%) | 210 (5.5%) | 0.001 | 6 (5.5%) | 64 (5.9%) | 0.004 |
| Psychotic disorder | 1 (0.9%) | 32 (0.8%) | 0.01 | 1 (0.9%) | 6 (0.6%) | 0.02 |
| Prior myocardial infarction | 8 (7.3%) | 259 (6.8%) | 0.02 | 8 (7.3%) | 69 (6.3%) | 0.04 |
| Renal failure | 1 (0.9%) | 73 (1.9%) | 0.09 | 1 (0.9%) | 10 (0.9%) | 0.05 |
| Type of aortic valve prosthesis | 0.33 | 0.06 | ||||
| Biological | 87 (79.8%) | 2727 (71.7%) | 87 (79.8%) | 902 (82.8%) | ||
| Mechanical | 22 (20.2%) | 1077 (28.3%) | 22 (20.2%) | 188 (17.3%) | ||
| Surgical center (n = 8) | 0.10 | 0.03 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malmberg, M.; Palomäki, A.; Sipilä, J.O.T.; Rautava, P.; Gunn, J.; Kytö, V. Long-Term Outcomes of Surgical Aortic Valve Replacement in Patients with Rheumatoid Arthritis. J. Clin. Med. 2021, 10, 2492. https://doi.org/10.3390/jcm10112492
Malmberg M, Palomäki A, Sipilä JOT, Rautava P, Gunn J, Kytö V. Long-Term Outcomes of Surgical Aortic Valve Replacement in Patients with Rheumatoid Arthritis. Journal of Clinical Medicine. 2021; 10(11):2492. https://doi.org/10.3390/jcm10112492
Chicago/Turabian StyleMalmberg, Markus, Antti Palomäki, Jussi O. T. Sipilä, Päivi Rautava, Jarmo Gunn, and Ville Kytö. 2021. "Long-Term Outcomes of Surgical Aortic Valve Replacement in Patients with Rheumatoid Arthritis" Journal of Clinical Medicine 10, no. 11: 2492. https://doi.org/10.3390/jcm10112492
APA StyleMalmberg, M., Palomäki, A., Sipilä, J. O. T., Rautava, P., Gunn, J., & Kytö, V. (2021). Long-Term Outcomes of Surgical Aortic Valve Replacement in Patients with Rheumatoid Arthritis. Journal of Clinical Medicine, 10(11), 2492. https://doi.org/10.3390/jcm10112492







