Influence of Retinal Microsecond Pulse Laser Treatment in Central Serous Chorioretinopathy: A Short-Term Optical Coherence Tomography Angiography Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Protocol
2.3. Laser Treatment
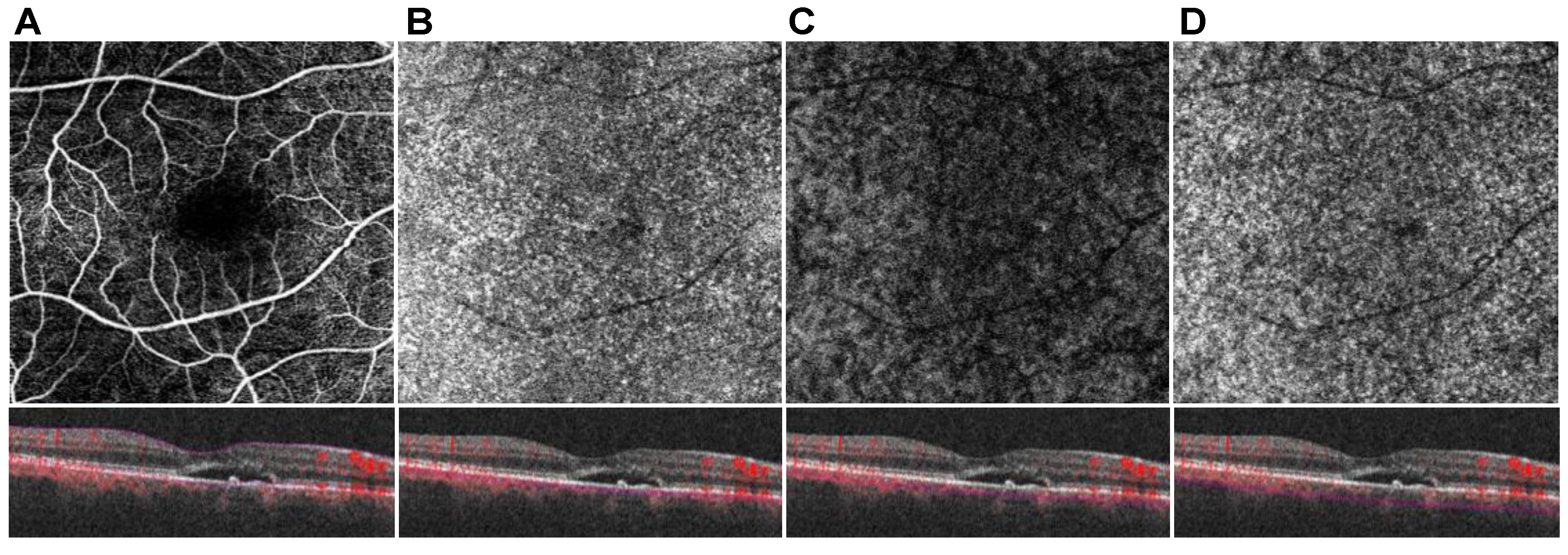
2.4. OCT and OCTA Data Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aSRF | apical subretinal fluid |
| BCVA | best corrected visual acuity |
| CC | choriocapillaris |
| CCP | choriocapillaris perfusion |
| CNV | choroidal neovascularization |
| CSC | central serous chorioretinopathy |
| EDI | enhanced depth of imaging |
| EDTRS | Early Treatment Diabetic Retinopathy Study |
| FA | fluorescein angiography |
| FAF | fundus autofluorescence |
| FR | full retina |
| FRP | full retina perfusion |
| HL | Haller’s layer |
| HLP | Haller’s layer perfusion |
| ICGA | indocyanine green angiography |
| LRT | lesional retinal thickness |
| OCT | optical coherence tomography |
| OCTA | optical coherence tomography angiography |
| PDT | photodynamic therapy |
| PNV | pachychoroid neovasculopathy |
| RPE | retinal pigment epithelium |
| SFCT | subfoveal choroidal thickness |
| SML | subthreshold micropulse laser |
| SL | Sattler’s layer |
| SLP | Sattler’s layer perfusion |
| VEGF | vascular endothelial growth factor |
| SRF | subretinal fluid |
| TMV | total macular volume |
References
- Semeraro, F.; Morescalchi, F.; Russo, A.; Gambicorti, E.; Pilotto, A.; Parmeggiani, F.; Bartollino, S.; Costagliola, C. Central Serous Chorioretinopathy: Pathogenesis and Management. Clin. Ophthalmol. Auckl. NZ 2019, 13, 2341–2352. [Google Scholar] [CrossRef]
- Gallego-Pinazo, R.; Dolz-Marco, R.; Gómez-Ulla, F.; Mrejen, S.; Freund, K.B. Pachychoroid Diseases of the Macula. Med. Hypothesis Discov. Innov. Ophthalmol. 2014, 3, 111–115. [Google Scholar] [PubMed]
- Warrow, D.J.; Hoang, Q.V.; Freund, K.B. Pachychoroid Pigment Epitheliopathy. Retina 2013, 33, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.; Huh, J.; Ahn, S.M.; Lee, B.; Kim, J.T.; Hwang, S.-Y.; Kim, S.-W.; Oh, J. Choriocapillaris Flow Features and Choroidal Vasculature in the Fellow Eyes of Patients with Acute Central Serous Chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 57–70. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid Disease. Eye Lond. Engl. 2019, 33, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, J.; Schworm, B.; Priglinger, S.G. The Pachychoroid Disease Spectrum-and the Need for a Uniform Classification System. Ophthalmol. Retin. 2019, 3, 1013–1015. [Google Scholar] [CrossRef]
- Scholz, P.; Altay, L.; Fauser, S. A Review of Subthreshold Micropulse Laser for Treatment of Macular Disorders; Springer Healthcare: Cham, Switzerland, 2017; Volume 34. [Google Scholar]
- Bousquet, E.; Dhundass, M.; Lejoyeux, R.; Shinojima, A.; Krivosic, V.; Mrejen, S.; Gaudric, A.; Tadayoni, R. Predictive Factors of Response to Mineralocorticoid Receptor Antagonists in Nonresolving Central Serous Chorioretinopathy. Am. J. Ophthalmol. 2019, 198, 80–87. [Google Scholar] [CrossRef]
- Gallice, M.; Daruich, A.; Matet, A.; Mouvet, V.; Dirani, A.; Evequoz, G.; Geiser, M.; Behar Cohen, F.; Chiquet, C. Effect of Eplerenone on Choroidal Blood Flow Changes during Isometric Exercise in Patients with Chronic Central Serous Chorioretinopathy. Acta Ophthalmol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sadda, S.R. Lack of Efficacy of Eplerenone for Treatment of Active Central Serous Chorioretinopathy. Eye Lond. Engl. 2020, 34, 1489–1490. [Google Scholar] [CrossRef]
- Lotery, A.; Sivaprasad, S.; O’Connell, A.; Harris, R.A.; Culliford, L.; Cree, A.; Madhusudhan, S.; Griffiths, H.; Ellis, L.; Chakravarthy, U.; et al. Eplerenone versus Placebo for Chronic Central Serous Chorioretinopathy: The VICI RCT. In Efficacy and Mechanism Evaluation; NIHR Journals Library: Southampton, UK, 2021. [Google Scholar]
- van Rijssen, T.J.; van Dijk, E.H.C.; Yzer, S.; Ohno-Matsui, K.; Keunen, J.E.E.; Schlingemann, R.O.; Sivaprasad, S.; Querques, G.; Downes, S.M.; Fauser, S.; et al. Central Serous Chorioretinopathy: Towards an Evidence-Based Treatment Guideline; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 73. [Google Scholar]
- Inagaki, K.; Shuo, T.; Katakura, K.; Ebihara, N.; Murakami, A.; Ohkoshi, K. Sublethal Photothermal Stimulation with a Micropulse Laser Induces Heat Shock Protein Expression in ARPE-19 Cells. J. Ophthalmol. 2015, 2015, 729792. [Google Scholar] [CrossRef]
- Kern, K.; Mertineit, C.L.; Brinkmann, R.; Miura, Y. Expression of Heat Shock Protein 70 and Cell Death Kinetics after Different Thermal Impacts on Cultured Retinal Pigment Epithelial Cells. Exp. Eye Res. 2018, 170, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Lavinsky, D.; Wang, J.; Huie, P.; Dalal, R.; Lee, S.J.; Lee, D.Y.; Palanker, D. Nondamaging Retinal Laser Therapy: Rationale and Applications to the Macula. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2488–2500. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, K.; Kakihara, S.; Tanaka, M.; Shindo, T.; Murata, T. Investigation of the Therapeutic Mechanism of Subthreshold Micropulse Laser Irradiation in Retina. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2020, 258, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Iwami, H.; Pruessner, J.; Shiraki, K.; Brinkmann, R.; Miura, Y. Protective Effect of a Laser-Induced Sub-Lethal Temperature Rise on RPE Cells from Oxidative Stress. Exp. Eye Res. 2014, 124, 37–47. [Google Scholar] [CrossRef]
- De Cillà, S.; Vezzola, D.; Farruggio, S.; Vujosevic, S.; Clemente, N.; Raina, G.; Mary, D.; Casini, G.; Rossetti, L.; Avagliano, L.; et al. The Subthreshold Micropulse Laser Treatment of the Retina Restores the Oxidant/Antioxidant Balance and Counteracts Programmed Forms of Cell Death in the Mice Eyes. Acta Ophthalmol. 2019, 97, e559–e567. [Google Scholar] [CrossRef] [PubMed]
- Valera-Cornejo, D.A.; García-Roa, M.; Quiroz-Mendoza, J.; Arias-Gómez, A.; Ramírez-Neria, P.; Villalpando-Gómez, Y.; Romero-Morales, V.; García-Franco, R. Micropulse Laser in Patients with Refractory and Treatment-Naïve Center-Involved Diabetic Macular Edema: Short Terms Visual and Anatomic Outcomes. Ther. Adv. Ophthalmol. 2021, 13, 2515841420979112. [Google Scholar] [CrossRef] [PubMed]
- Uzlu, D.; Erdöl, H.; Kola, M.; Özbay, A.D. The Efficacy of Subthreshold Micropulse Yellow Laser (577 Nm) in Chronic Central Serous Chorioretinopathy. Lasers Med. Sci. 2020. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Sinclair, S.H.; Elmann, S.; Glaser, B.M. Low Incidence of Choroidal Neovascularization Following Subthreshold Diode Micropulse Laser (SDM) in High-Risk AMD. PLoS ONE 2018, 13, e0202097. [Google Scholar] [CrossRef]
- Tode, J.; Richert, E.; Koinzer, S.; Klettner, A.; von der Burchard, C.; Brinkmann, R.; Lucius, R.; Roider, J. Thermal Stimulation of the Retina Reduces Bruch’s Membrane Thickness in Age Related Macular Degeneration Mouse Models. Transl. Vis. Sci. Technol. 2018, 7, 2. [Google Scholar] [CrossRef]
- Volodin, P.L.; Ivanova, E.V. Clinical Evaluation of Individualized and Navigated Microsecond Pulsing Laser for Acute Central Serous Chorioretinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2020, 51, 512–520. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; An, J. Comparison of the Efficacy and Safety of Subthreshold Micropulse Laser with Photodynamic Therapy for the Treatment of Chronic Central Serous Chorioretinopathy: A Meta-Analysis. Medicine 2021, 100, e25722. [Google Scholar] [CrossRef]
- van Dijk, E.H.C.; Fauser, S.; Breukink, M.B.; Blanco-Garavito, R.; Groenewoud, J.M.M.; Keunen, J.E.E.; Peters, P.J.H.; Dijkman, G.; Souied, E.H.; MacLaren, R.E.; et al. Half-Dose Photodynamic Therapy versus High-Density Subthreshold Micropulse Laser Treatment in Patients with Chronic Central Serous Chorioretinopathy: The PLACE Trial. Ophthalmology 2018, 125, 1547–1555. [Google Scholar] [CrossRef]
- Rommel, F.; Siegfried, F.; Kurz, M.; Brinkmann, M.P.; Rothe, M.; Rudolf, M.; Grisanti, S.; Ranjbar, M. Impact of Correct Anatomical Slab Segmentation on Foveal Avascular Zone Measurements by Optical Coherence Tomography Angiography in Healthy Adults. J. Curr. Ophthalmol. 2018, 30, 156–160. [Google Scholar] [CrossRef]
- Rommel, F.; Rommel, F.; Rothe, M.; Rothe, M.; Kurz, M.; Kurz, M.; Prasuhn, M.; Prasuhn, M.; Grisanti, S.; Ranjbar, M.; et al. Evaluating Diurnal Variations in Retinal Perfusion Using Optical Coherence Tomography Angiography. Int. J. Retin. Vitr. 2020, 6. [Google Scholar] [CrossRef]
- Siegfried, F.; Rommel, F.; Rothe, M.; Brinkmann, M.P.; Sochurek, J.A.M.; Freitag, J.; Grisanti, S.; Ranjbar, M. Evaluating Diurnal Changes in Choroidal Sublayer Perfusion Using Optical Coherence Tomography Angiography. Acta Ophthalmol. 2019, 97, e1062–e1068. [Google Scholar] [CrossRef]
- Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification. ETDRS Report Number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991, 98, 786–806.
- Otsu, N. Threshold Selection Method From Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, SMC-9, 62–66. [Google Scholar] [CrossRef]
- Nicolò, M.; Rosa, R.; Musetti, D.; Musolino, M.; Saccheggiani, M.; Traverso, C.E. Choroidal Vascular Flow Area in Central Serous Chorioretinopathy Using Swept-Source Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, Y.; Leong, B.C.S.; Parikh, R.; Fragiotta, S.; Freund, K.B. Association between choroidal caverns and choroidal vascular hyperpermeability in eyes with pachychoroid diseases. Retina 2018, 38, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Daruich, A.; Matet, A.; Dirani, A.; Bousquet, E.; Zhao, M.; Farman, N.; Jaisser, F.; Behar-Cohen, F. Central Serous Chorioretinopathy: Recent Findings and New Physiopathology Hypothesis. Prog. Retin. Eye Res. 2015, 48, 82–118. [Google Scholar] [CrossRef] [PubMed]
- Liegl, R.; Ulbig, M.W. Central Serous Chorioretinopathy. Ophthalmol. J. Int. Ophtalmol. Int. J. Ophthalmol. Z. Augenheilkd. 2014, 232, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Maruko, I.; Iida, T.; Sugano, Y.; Ojima, A.; Sekiryu, T. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina 2011, 31, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Kishi, S.; Hagimura, N.; Shimizu, K. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina 1999, 19, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Lai, F.H.P.; Ng, D.S.C.; Iu, L.P.L.; Chen, L.J.; Mak, A.C.Y.; Yip, Y.; Cheung, C.; Young, A.L.; Brelen, M. Analysis of Choriocapillaris Perfusion and Choroidal Layer Changes in Patients with Chronic Central Serous Chorioretinopathy Randomised to Micropulse Laser or Photodynamic Therapy. Br. J. Ophthalmol. 2020, 105, 555–560. [Google Scholar] [CrossRef]
- Costanzo, E.; Cohen, S.Y.; Miere, A.; Querques, G.; Capuano, V.; Semoun, O.; El Ameen, A.; Oubraham, H.; Souied, E.H. Optical Coherence Tomography Angiography in Central Serous Chorioretinopathy. J. Ophthalmol. 2015, 2015. [Google Scholar] [CrossRef]
- Rochepeau, C.; Kodjikian, L.; Garcia, M.-A.; Coulon, C.; Burillon, C.; Denis, P.; Delaunay, B.; Mathis, T. Optical Coherence Tomography Angiography Quantitative Assessment of Choriocapillaris Blood Flow in Central Serous Chorioretinopathy. Am. J. Ophthalmol. 2018, 194, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Lauermann, J.L.; Woetzel, A.K.; Treder, M.; Alnawaiseh, M.; Clemens, C.R.; Eter, N.; Alten, F. Prevalences of Segmentation Errors and Motion Artifacts in OCT-Angiography Differ among Retinal Diseases. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1807–1816. [Google Scholar] [CrossRef] [PubMed]

| Parameter | CSC Eyes | Fellow Eyes |
|---|---|---|
| Gender (male/female), n (%) | 17 (63.0)/10 (37.0) | 11 (64.7)/6 (53.3) |
| Age (years) | 54 (48.0; 63.0) | 54 (49.5; 64.5) |
| Disease duration, months | 20 (7; 48) | - |
| Previous treatment (conservative/Anti-VEGF), n (%) | 15 (55.6)/12 (44.4) | - |
| Parameter | CSC Eyes | Fellow Eyes | p-Value |
|---|---|---|---|
| BCVA (logMAR) | 0.4 (0.1; 0.14) | 0.0 (0.0; 0.0) | <0.001 |
| CRT (µm) | 306 (254; 389) | 271 (246; 295) | 0.047 |
| TMV (mm³) | 8.7 (8.2; 9.4) | 8,65 (8.3; 9.08) | 0.697 |
| SFCT (µm) | 310 (286; 356) | 275 (237; 312.8) | 0.022 |
| FRP (%) | 26.44 (23.61; 29.52) | 28.77 (23.49; 30.14) | 0.540 |
| CCP (%) | 43.12 (38.79; 44.79) | 46.35 (42.23; 47.08) | 0.159 |
| SLP (%) | 60.43 (58.37; 63.11) | 58.19 (57.04; 60.36) | 0.126 |
| HLP (%) | 64.27 (61.24; 67.08) | 61.88 (58.36; 67.33) | 0.391 |
| CCP/HLP | 0.68 (0.58; 0.71) | 0.74 (0.65; 0.79) | 0.142 |
| SLP/HLP | 0.95 (0.92; 0.98) | 0.93 (0.91; 0.99) | 0.742 |
| Parameter | Baseline | 4 Weeks | p-Value |
|---|---|---|---|
| BCVA (logMAR) | 0.4 (0.1; 0.14) | 0.3 (0.1; 0.5) | 0.348 |
| CRT (µm) | 306 (254; 389) | 266 (236; 324) | 0.024 |
| TMV (mm3) | 8.7 (8.2; 9.4) | 8.5 (7.9; 9.0) | 0.62 |
| SFCT (µm) | 310 (286; 356) | 299 (273; 337) | 0.135 |
| aSRF (µm2) SRF area (µm2) | 0.17 (0.07; 0.43) 5.4 (1.4; 8.25) | 0.075 (0.05; 0.33) 1.8 (0.7; 7.6) | 0.115 0.123 |
| LRT (µm) | 364 (317; 465) | 333 (272; 391) | 0.044 |
| CCP (%) | 43.12 (38.79; 44.79) | 42.35 (40.97; 43.94) | 0.918 |
| SLP (%) | 60.43 (58.37; 63.11) | 61.61 (59.9; 62.81) | 0.469 |
| HLP (%) | 64.27 (61.24; 67.08) | 63.63 (60.99; 66.71) | 0.642 |
| CCP/HLP | 0.68 (0.58; 0.71) | 0.65 (0.61; 0.71) | 0.877 |
| SLP/HLP | 0.95 (0.92; 0.98) | 0.95 (0.92; 1.01) | 0.215 |
| Lesional CCP (%) | 20.45 (13.58; 38.39) | 29.84 (17.06; 35,94) | 0.352 |
| Lesional SLP (%) | 69.1 (57.57; 80.39) | 65.33 (55.95; 74.0) | 0.352 |
| Lesional HLP (%) | 72.58 (57.6; 82.8) | 70.97 (53.96; 78.83) | 0.352 |
| Lesional CCP/HLP | 0.29 (0.17; 0.61) | 0.41 (0.24; 0.68) | 0.326 |
| Lesional SLP/HLP | 0.98 (0.88; 1.02) | 0.96 (0.83; 1.15) | 0.959 |
| Parameter | CSC without CNV | PNV |
|---|---|---|
| Gender (male/female), n (%) | 9 (64.3); 5 (35.7) | 8 (61.5); 5 (38.5) |
| Age (years) | 52 (45.75; 55.75) | 60 (52.5; 65) |
| Disease duration, months | 15 (7; 32.5) | 36 (9; 78) |
| Parameter | CSC without CNV | PNV | ||||
|---|---|---|---|---|---|---|
| Before SML | After SML | p-Value | Before SML | After SML | p-Value | |
| BCVA (logMAR) | 0.1 (0.1; 0.4) | 0.15 (0.075; 0.4) | 0.660 | 0.4 (0.35; 0.6) | 0.4 (0.3; 0.55) | 0.366 |
| CRT (µm) | 279 (246; 385) | 260 (245; 326) | 0.594 | 325 (279; 411) | 267 (211; 325) | 0.009 |
| TMV | 8.45 (7.9; 9.73) | 8.25 (7.88; 9.53) | 0.365 | 8.8 (8.35; 9.15) | 8.6 (7.95; 8.9) | 0.090 |
| SFCT (µm) | 329 (295; 373.75) | 299 (284; 352) | 0.044 | 299 (265; 332) | 301 (272; 337) | 0.929 |
| aSRF (µm2) SRF area (µm2) | 0.12 (0.06; 0.55) 4.2 (1.35; 7.65) | 0.065 (0.033; 0.196) 1.4 (0.8; 6.33) | 0.208 0.208 | 0.26 (0.09; 0.39) 5.9 (1.55; 9.38) | 0.17 (0.05; 0.41) 5.05 (0.375; 7.75) | 0.326 0.406 |
| LRT (µm) | 370.5 (310.5; 499.5) | 347 (274.25; 405.75) | 0.300 | 364 (310; 426.5) | 326 (240.5; 405) | 0.055 |
| FRP (%) | 25.85 (23.27; 28.39) | 24.88 (22.75; 28.43) | 0.646 | 26.44 (23.91; 31.02) | 24.91 (23.37; 29.12) | 0.753 |
| CCP (%) | 43.39 (38.32; 46.51) | 42.54 (40.9; 44.47) | 0.959 | 41.92 (39.26; 44.48) | 41.77 (40.27; 44.23) | 0.600 |
| SLP (%) | 60.43 (58.02; 63.16) | 61.88 (59.66; 62.86) | 0.575 | 60.86 (58.66; 63.57) | 61.22 (59.99; 63.47) | 0.600 |
| HLP (%) | 64.19 (60.66; 68.01) | 64.27 (60.63; 67.39) | 0.878 | 65.02 (61.28; 66.57) | 63.14 (61.15; 65.91) | 0.463 |
| CCP/HLP | 0.68 (0.57; 0.74) | 0.66 (0.61; 0.71) | 0.878 | 0.65 (0.59; 0.71) | 0.66 (0.60; 0.74) | 0.463 |
| SLP/HLP | 0.95 (0.92; 0.97) | 0.95 (0.92; 0.98) | 0.575 | 0.95 (0.91; 0.99) | 0.97 (0.92; 1.0) | 0.075 |
| Lesion CCP (%) | 22.44 (18.55; 42.67) | 30.77 (19.51; 33.97) | 0.799 | 12.86 (10.11; 38.2) | 24.85 (10.14; 49.78) | 0.294 |
| Lesion SLP (%) | 67.47 (56.65; 75.99) | 64.37 (59.68; 71.06) | 0.445 | 74.25 (55.47; 86.53) | 73.07 (48.67; 81.99) | 0.463 |
| Lesion HLP (%) | 73.39 (58.46; 82.55) | 73.64 (52.19; 81.38) | 0.575 | 72.58 (50.66; 86.01) | 69.34 (51.53; 80.15) | 0.249 |
| Lesion CCP/HLP | 0.29 (0.27; 0.71) | 0.41 (0.32; 0.54) | 0.799 | 0.18 (0.13; 0.65) | 0.46 (0.13; 0.94) | 0.249 |
| Lesion SLP/HLP | 0.99 (0.84; 1.0) | 0.95 (0.79; 1.11) | 0.799 | 0.96 (0.91; 1.14) | 1.04 (0.88; 1.27) | 0.917 |
| Parameter | Before SML | After SML | ||||
|---|---|---|---|---|---|---|
| CSC without CNV | PNV | p-Value | CSC without CNV | PNV | p-Value | |
| BCVA (logMAR) | 0.1 (0.1; 0.4) | 0.4 (0.35; 0.6) | 0.007 | 0.15 (0.075; 0.4) | 0.4 (0.3; 0.55) | 0.014 |
| CRT (µm) | 278 (246; 385) | 325 (279; 411) | 0.259 | 260 (244.75; 325.75) | 267 (213; 325 | 0.793 |
| TMV (mm3) | 8.45 (7.9; 9.7) | 8.8 (8.35; 9.15) | 0.519 | 8.25 (7.88; 9.53) | 8.6 (7.95; 8.9) | 0.685 |
| SFCT (µm) | 329 (295; 373) | 299 (265; 333) | 0.141 | 299 (284; 352) | 301 (273; 337) | 0.720 |
| aSRF (µm2) | 0.12 (0.06; 0.51) | 0.26 (0.09; 0.39) | 0.705 | 0.07 (0.033; 0.19) | 0.17 (0.05; 0.41) | 0.274 |
| SRF area (µm2) | 4.2 (1.35; 7.65) | 5.9 (1.55; 9.38) | 0.689 | 1.4 (0.8; 6.35) | 5.05 (0.375; 7.57) | 0.852 |
| LRT (µm) | 371 (311; 500) | 364 (310; 427) | 0.981 | 347 (274.25; 405.75) | 326 (241; 405) | 0.583 |
| FRP (%) | 25.85 (23.26; 28.39) | 26.44 (23.91; 31.02) | 0.635 | 24.88 (22.75; 28.43) | 24.91 (23.37; 29.12) | 0.792 |
| CCP (%) | 43.39 (38.32; 46.51) | 41.92 (39.26; 44.48) | 0.792 | 42.54 (40.92; 44.47) | 41.77 (40.27; 44.23) | 0.562 |
| SLP (%) | 60.43 (58.02; 63.17) | 60.86 (58.66; 63.57) | 0.792 | 61.89 (59.66; 62.86) | 61.22 (59.99; 63.47) | 0.958 |
| HLP (%) | 64.19 (60.66; 68.01) | 65.02 (61.28; 66.56) | 0.958 | 64.27 (60.63; 67.39) | 63.14 (61.15; 65.91) | 0.562 |
| CCP/HLP | 0.68 (0.57; 0.74) | 0.65 (0.59; 0.71) | 0.875 | 0.66 (0.61; 0.71) | 0.66 (0.60; 0.74) | 0.958 |
| SLP/HLP | 0.95 (0.92; 0.99) | 0.95 (0.92; 0.99) | 0.958 | 0.95 (0.92; 0.98) | 0.97 (0.92; 1.02) | 0.713 |
| Lesion CCP (%) | 22.44 (18.55; 42.67) | 12.85 (10.11; 38.20) | 0.118 | 30.77 (19.51; 33.97) | 24.85 (10.14; 49.78) | 0.635 |
| Lesion SLP (%) | 67.47 (56.65; 75.99) | 74.25 (55.47; 86.53) | 0.428 | 64.37 (59.68; 71.06) | 73.07 (48.67; 81.99) | 0.428 |
| Lesion HLP (%) | 73.39 (58.46; 82.55) | 72.58 (58.46; 82.55) | 0.958 | 73.0 (52.19; 81.38) | 69.34 (51.53; 80.15) | 0.713 |
| Lesion CCP/HLP | 0.29 (0.27; 0.72) | 0.18 (0.13; 0.65) | 0.220 | 0.41 (0.32; 0.54) | 0.46 (0.13; 0.95) | 1.0 |
| Lesion SLP/HLP | 0.99 (1.03; 0.85) | 0.96 (0.91; 1.16) | 1.000 | 0.95 (0.79; 1.11) | 1.04 (0.88; 1.27) | 0.368 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasuhn, M.; Miura, Y.; Tura, A.; Rommel, F.; Kakkassery, V.; Sonntag, S.; Grisanti, S.; Ranjbar, M. Influence of Retinal Microsecond Pulse Laser Treatment in Central Serous Chorioretinopathy: A Short-Term Optical Coherence Tomography Angiography Study. J. Clin. Med. 2021, 10, 2418. https://doi.org/10.3390/jcm10112418
Prasuhn M, Miura Y, Tura A, Rommel F, Kakkassery V, Sonntag S, Grisanti S, Ranjbar M. Influence of Retinal Microsecond Pulse Laser Treatment in Central Serous Chorioretinopathy: A Short-Term Optical Coherence Tomography Angiography Study. Journal of Clinical Medicine. 2021; 10(11):2418. https://doi.org/10.3390/jcm10112418
Chicago/Turabian StylePrasuhn, Michelle, Yoko Miura, Aysegül Tura, Felix Rommel, Vinodh Kakkassery, Svenja Sonntag, Salvatore Grisanti, and Mahdy Ranjbar. 2021. "Influence of Retinal Microsecond Pulse Laser Treatment in Central Serous Chorioretinopathy: A Short-Term Optical Coherence Tomography Angiography Study" Journal of Clinical Medicine 10, no. 11: 2418. https://doi.org/10.3390/jcm10112418
APA StylePrasuhn, M., Miura, Y., Tura, A., Rommel, F., Kakkassery, V., Sonntag, S., Grisanti, S., & Ranjbar, M. (2021). Influence of Retinal Microsecond Pulse Laser Treatment in Central Serous Chorioretinopathy: A Short-Term Optical Coherence Tomography Angiography Study. Journal of Clinical Medicine, 10(11), 2418. https://doi.org/10.3390/jcm10112418








