Subcutaneous Bupivacaine Infiltration Is Not Effective to Support Control of Postoperative Pain in Paediatric Patients Undergoing Spinal Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Patients
2.2. Procedure
2.3. Statistical Analysis
3. Results
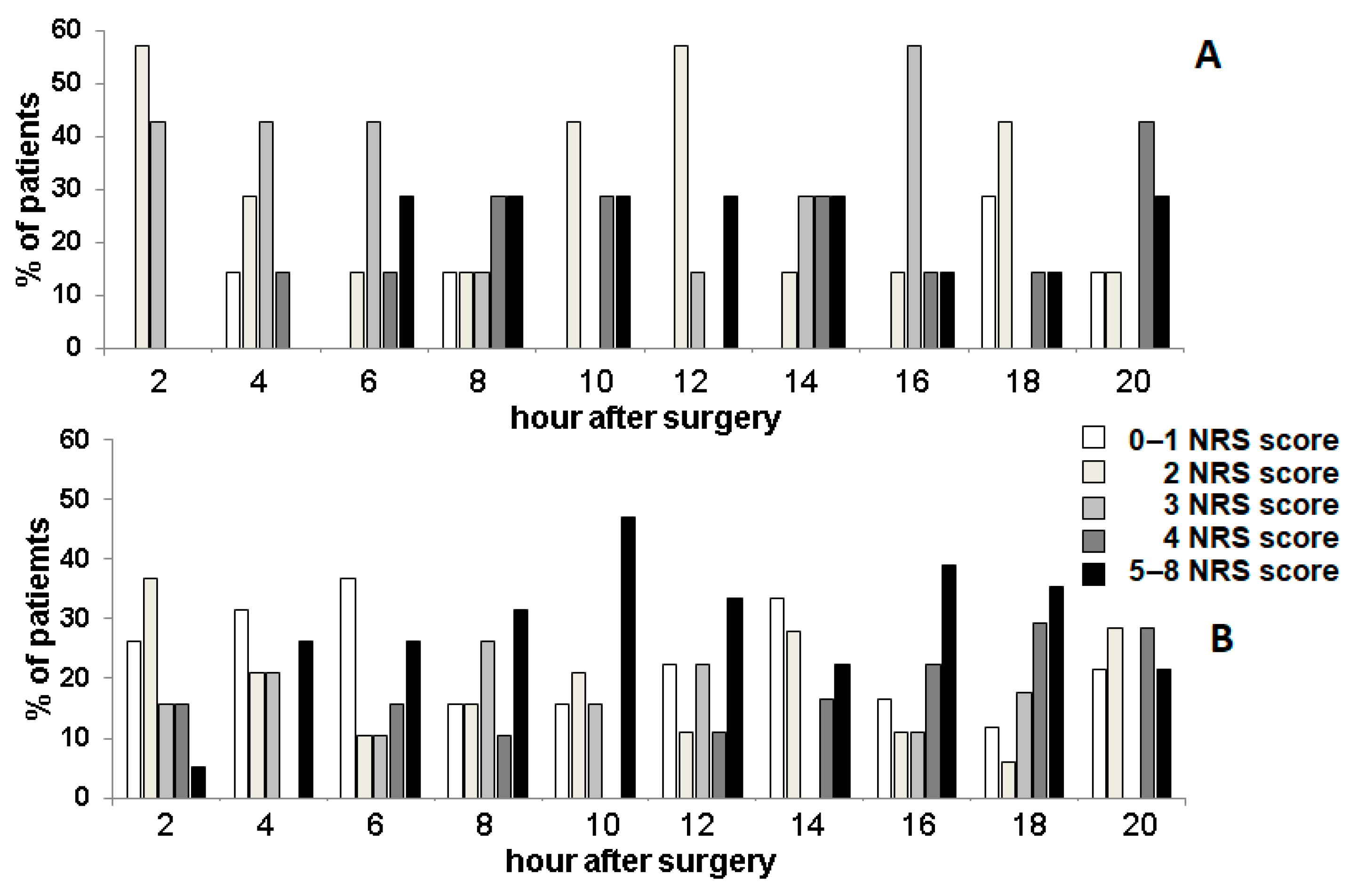
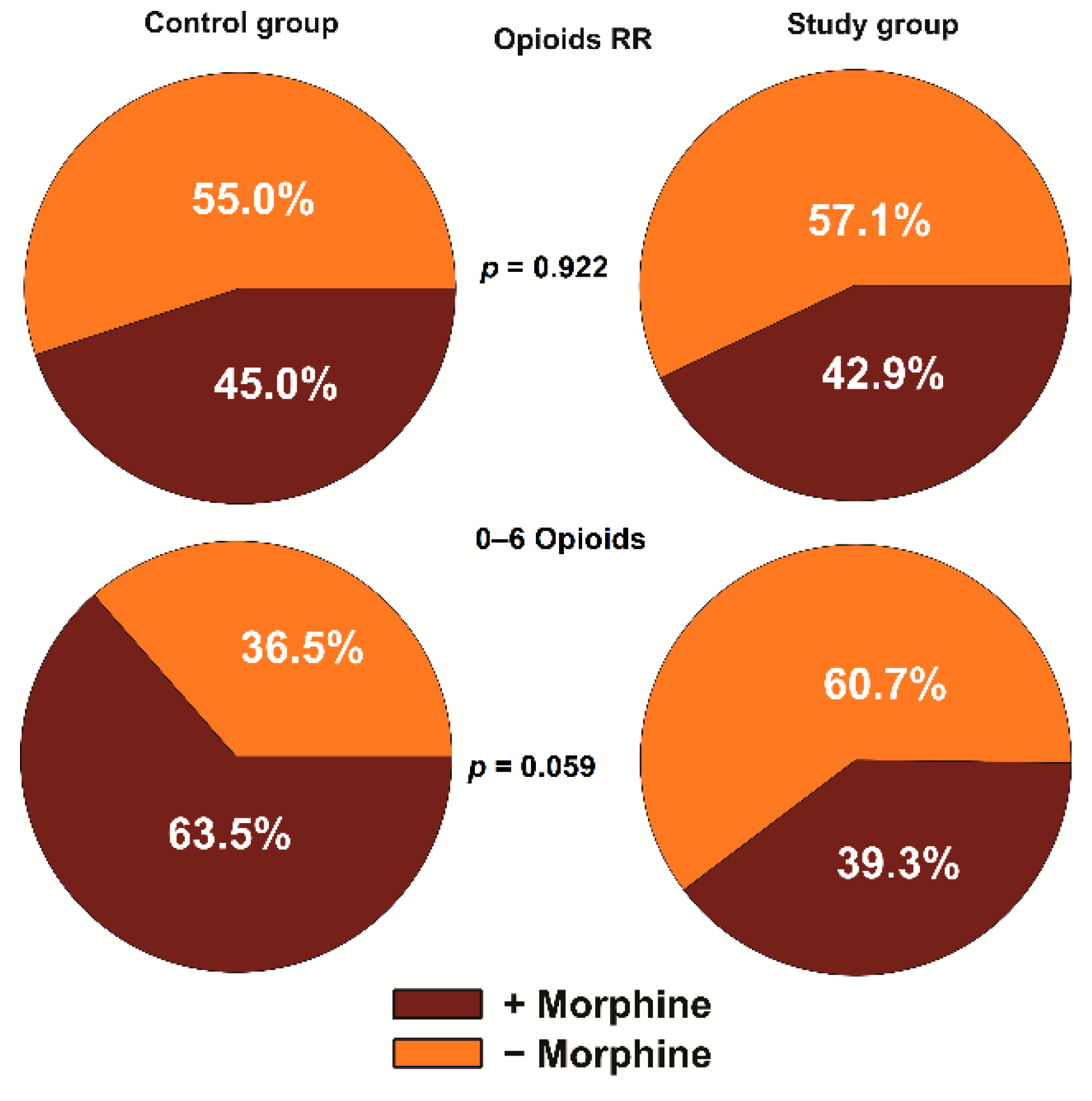
3.1. Analgesic Effect
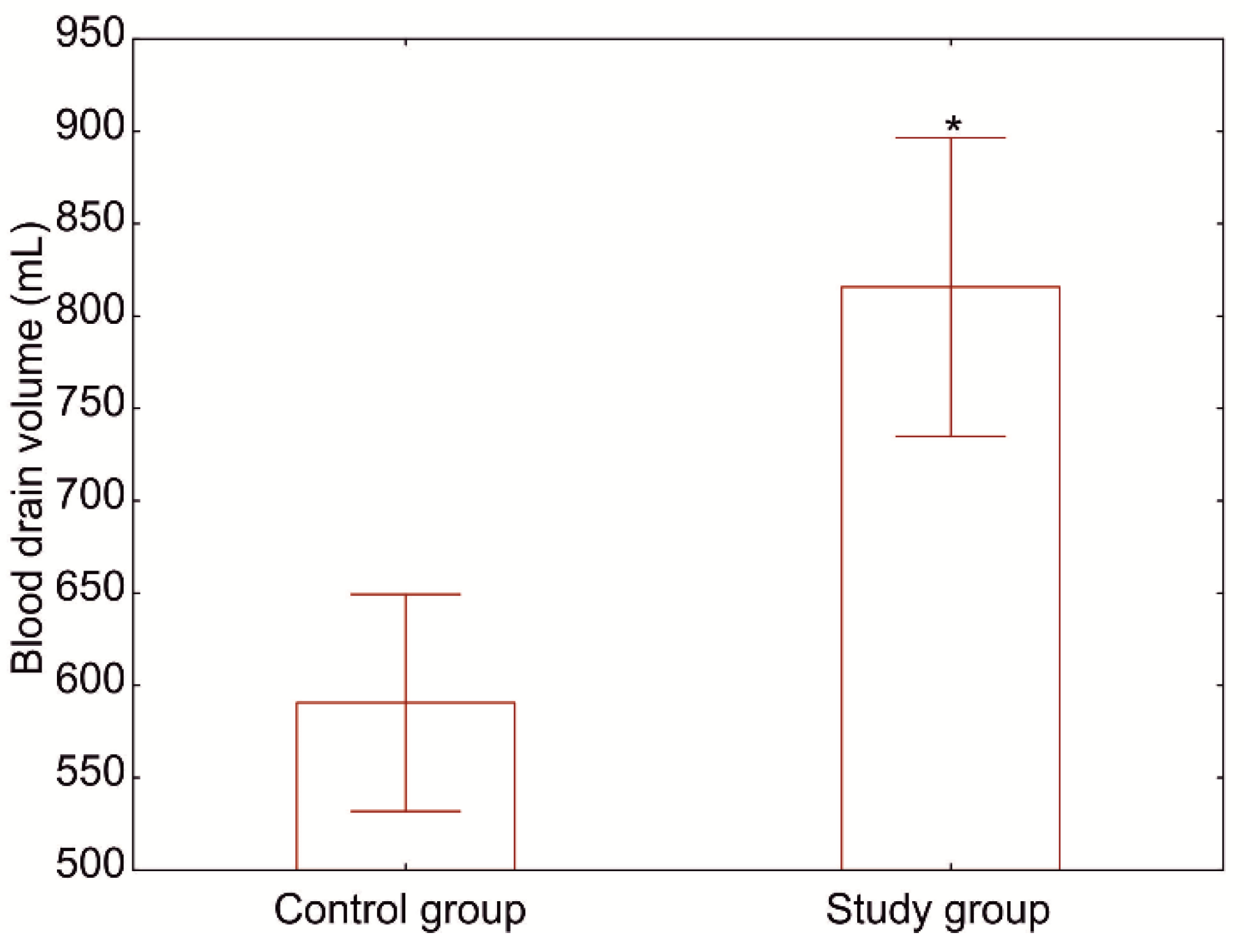
3.2. Effect on Bleeding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choudhry, M.N.; Ahmad, Z.; Verma, R. Adolescent Idiopathic Scoliosis. Open Orthop. J. 2016, 10, 143–154. [Google Scholar] [CrossRef]
- Weinstein, S.L. The Natural History of Adolescent Idiopathic Scoliosis. J. Pediatr. Orthop. 2019, 39, S44–S46. [Google Scholar] [CrossRef]
- Schwab, F.J.; Hawkinson, N.; Lafage, V.; Smith, J.S.; Hart, R.; Mundis, G.; Burton, D.C.; Line, B.; Akbarnia, B.; Boachie-Adjei, O.; et al. Risk factors for major peri-operative complications in adult spinal deformity surgery: A multi-center review of 953 consecutive patients. Eur. Spine J. 2012, 21, 2603–2610. [Google Scholar] [CrossRef]
- Danielewicz, A.; Wójciak, M.; Sawicki, J.; Dresler, S.; Sowa, I.; Latalski, M. Comparison of Different Surgical Systems for Treatment of Early-onset Scoliosis in the Context of Release of Titanium Ions. Spine 2021, 46, E594–E601. [Google Scholar] [CrossRef]
- Ganapathy, S.; Brookes, J.; Bourne, R. Local Infiltration Analgesia. Anesthesiol. Clin. 2011, 29, 329–342. [Google Scholar] [CrossRef]
- Verghese, S.T.; Hannallah, R.S. Acute pain management in children. J. Pain Res. 2010, 3, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Stillwagon, M.R.; Feinstein, S.; Nichols, B.; Andrews, P.N.; Vergun, A.D. Pain control and medication use in children following closed reduction and percutaneous pinning of supracondylar humerus fractures: Are we still overprescribing opioids? J. Pediatr. Orthop. 2020, 40, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.M.; Leão, R.A.; Miranda, L.S.; De Souza, R.O. A brief history behind the most used local anesthetics. Tetrahedron 2020, 76, 131628. [Google Scholar] [CrossRef]
- Beloeil, H.; Gentili, M.; Benhamou, D.; Mazoit, J.-X. The Effect of a Peripheral Block on Inflammation-Induced Prostaglandin E2 and Cyclooxygenase Expression in Rats. Anesth. Analg. 2009, 109, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.M.; Green, T.P.; Esler, C.N. The local infiltration of analgesia following total knee replacement: A review of current literature. J. Bone Jt. Surg. 2012, 94, 1154–1159. [Google Scholar] [CrossRef]
- Busch, C.A.; Shore, B.J.; Bhandari, R.; Ganapathy, S.; MacDonald, S.J.; Bourne, R.B.; Rorabeck, C.H.; McCalden, R.W. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J. Bone Jt. Surg. 2006, 88, 959–963. [Google Scholar] [CrossRef]
- Niemeläinen, M.; Kalliovalkama, J.; Aho, A.J.; Moilanen, T.; Eskelinen, A. Single periarticular local infiltration analgesia reduces opiate consumption until 48 hours after total knee arthroplasty. Acta Orthop. 2014, 85, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.B.; Liu, S.D.; Meng, Q.J.; Qu, H.Z.; Zhang, Z. Single administration of intra-articular bupivacaine in arthroscopic knee surgery: A system-atic review and meta-analysis. BMC Musculoskelet. Disord. 2015, 16, 21. [Google Scholar] [CrossRef]
- Essving, P.; Axelsson, K.; Kjellberg, J.; Wallgren, Ö.; Gupta, A.; Lundin, A. Reduced morphine consumption and pain intensity with local infiltration analgesia (LIA) following total knee arthroplasty. Acta Orthop. 2010, 81, 354–360. [Google Scholar] [CrossRef]
- Barker, J.C.; Joshi, G.P.; Janis, J.E. Basics and best practices of multimodal pain management for the plastic surgeon. Plast. Reconstr. Surg. Glob. Open. 2020, 8, e2833. [Google Scholar] [CrossRef] [PubMed]
- Ige, O.; Bolaji, B.; Kolawole, I. Effect of wound infiltration with bupivacaine on pulmonary function after elective lower abdominal operations. Afr. Heal. Sci. 2013, 13, 756–761. [Google Scholar] [CrossRef][Green Version]
- Jellish, W.S.; Gamelli, R.L.; Furry, P.A.; McGill, V.L.; Fluder, E.M. Effect of topical local anesthetic application to skin harvest sites for pain manage-ment in burn patients undergoing skin-grafting procedures. Ann. Surg. 1999, 229, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Waite, A.; Gilliver, S.C.; Masterson, G.R.; Hardman, M.J.; Ashcroft, G.S. Clinically relevant doses of lidocaine and bupivacaine do not impair cutaneous wound healing in mice. Br. J. Anaesth. 2010, 104, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Zmora, O.; Stolik-Dollberg, O.; Bar-Zakai, B.; Rosin, D.; Kuriansky, J.; Shabtai, M.; Perel, A.; Ayalon, A. Intraperitoneal Bupivacaine Does Not Attenuate Pain Following Laparoscopic Cholecystectomy. JSLS J. Soc. Laparoendosc. Surg. 2000, 4, 301–304. [Google Scholar]
- Gavrilovska-Brzanov, A.; Kuzmanovska, B.; Kartalov, A.; Donev, L.; Lleshi, A.; Jovanovski-Srceva, M.; Spirovska, T.; Brzanov, N.; Simeonov, R. Evaluation of anesthesia profile in pediatric patients after in-guinal hernia repair with caudal block or local wound infiltration. Maced. J. Med. Sci. 2016, 4, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.K.; Shrivastava, D.; Kurzweil, R.E.; Weiss, D.A.; Long, C.J.; Shukla, A.R. Port site local anesthetic infiltration vs single-dose intrathecal opioid in-jection to control perioperative pain in children undergoing minimal invasive surgery: A comparative analysis. Urology 2016, 97, 179–183. [Google Scholar] [CrossRef]
- Pedersen, L.K.; Nikolajsen, L.; Rahbek, O.; Duch, B.U.; Møller-Madsen, B. Epidural analgesia is superior to local infiltration analgesia in children with cerebral palsy undergoing unilateral hip reconstruction. Acta Orthop. 2015, 87, 176–182. [Google Scholar] [CrossRef]
- Mattila, I.; Pätilä, T.; Rautiainen, P.; Korpela, R.; Nikander, S.; Puntila, J.; Salminen, J.; Suominen, P.K.; Tynkkynen, P.; Hiller, A. The effect of continuous wound infusion of ropivacaine on postoperative pain after median sternotomy and mediastinal drain in children. Pediatr. Anesth. 2016, 26, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Greze, J.; Vighetti, A.; Incagnoli, P.; Quesada, J.L.; Albaladejo, P.; Palombi, O.; Tonetti, J.; Bosson, J.L.; Payen, J.F. Does continuous wound infiltration enhance baseline intravenous multimodal analgesia after posterior spinal fusion surgery? A randomized, double-blinded, placebo-controlled study. Eur. Spine J. 2017, 26, 832–839. [Google Scholar] [CrossRef]
- Puffer, R.C.; Tou, K.; Winkel, R.E.; Bydon, M.; Currier, B.; Freedman, B.A. Liposomal bupivacaine incisional injection in single-level lumbar spine surgery. Spine J. 2016, 16, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, M.; Sultan, A.A.; Hudson, B.; Goodwin, R.C.; Seif, J.; Khlopas, A.; Bena, J.; Jin, Y.; Gurd, D.P.; Kuivila, T.E.; et al. Liposomal Bupivacaine Is Both Safe and Effective in Controlling Postoperative Pain After Spinal Surgery in Children. Clin. Spine Surg. 2020, 33, E533–E538. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, G.A.; Chojnowski, A. Use of adrenaline and bupivacaine to reduce bleeding and pain following harvesting of bone graft. Ann. R. Coll. Surg. Engl. 2003, 85, 284. [Google Scholar] [CrossRef][Green Version]
- Bameshki, A.R.; Razban, M.; Khadivi, E.; Razavi, M.; Bakhshaee, M. The Effect of Local Injection of Epinephrine and Bupivacaine on Post-Tonsillectomy Pain and Bleeding. Iran. J. Otorhinolaryngol. 2013, 25, 209–214. [Google Scholar]
| Study Group | Control Group | |
|---|---|---|
| Number of patients (F/M) | 17 (13/4) | 13 (11/2) |
| Age | 15.1 (12.6–17.5) | 14.0 (8.5–17.0) |
| Weight (kg) | 49.2 ± 9.3 | 49.5 ± 8.7 |
| Duration of surgery (min.) | 269.5 ± 33.2 | 280 ± 31.4 |
| Number of segments | 11 (10–11) | 12 (11–12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danielewicz, A.; Fatyga, M.; Starobrat, G.; Różańska-Boczula, M.; Wójciak, M.; Sowa, I.; Dresler, S.; Latalski, M. Subcutaneous Bupivacaine Infiltration Is Not Effective to Support Control of Postoperative Pain in Paediatric Patients Undergoing Spinal Surgery. J. Clin. Med. 2021, 10, 2407. https://doi.org/10.3390/jcm10112407
Danielewicz A, Fatyga M, Starobrat G, Różańska-Boczula M, Wójciak M, Sowa I, Dresler S, Latalski M. Subcutaneous Bupivacaine Infiltration Is Not Effective to Support Control of Postoperative Pain in Paediatric Patients Undergoing Spinal Surgery. Journal of Clinical Medicine. 2021; 10(11):2407. https://doi.org/10.3390/jcm10112407
Chicago/Turabian StyleDanielewicz, Anna, Marek Fatyga, Grzegorz Starobrat, Monika Różańska-Boczula, Magdalena Wójciak, Ireneusz Sowa, Sławomir Dresler, and Michał Latalski. 2021. "Subcutaneous Bupivacaine Infiltration Is Not Effective to Support Control of Postoperative Pain in Paediatric Patients Undergoing Spinal Surgery" Journal of Clinical Medicine 10, no. 11: 2407. https://doi.org/10.3390/jcm10112407
APA StyleDanielewicz, A., Fatyga, M., Starobrat, G., Różańska-Boczula, M., Wójciak, M., Sowa, I., Dresler, S., & Latalski, M. (2021). Subcutaneous Bupivacaine Infiltration Is Not Effective to Support Control of Postoperative Pain in Paediatric Patients Undergoing Spinal Surgery. Journal of Clinical Medicine, 10(11), 2407. https://doi.org/10.3390/jcm10112407








