Soft Tissue Interface with Various Kinds of Implant Abutment Materials
Abstract
1. Introduction
2. Experimental Section
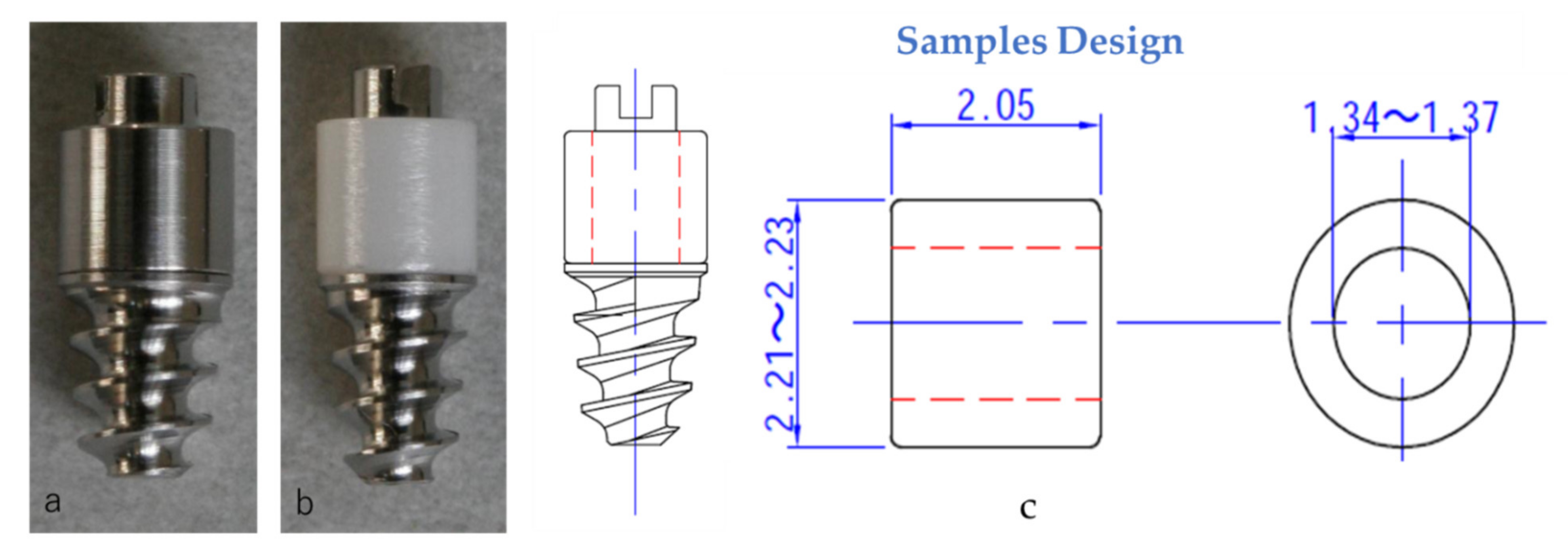
2.1. Sample Plates
2.2. Quantification of Hydroxyl Groups on Specimens
2.3. Quantification of Protein Adsorption on Specimens
2.4. Initial Cell Attachment Assay
2.5. Immunofluorescent Staining
2.6. In Vivo Study and Immunohistochemical Sample Preparation
2.7. Statistical Analysis
3. Results
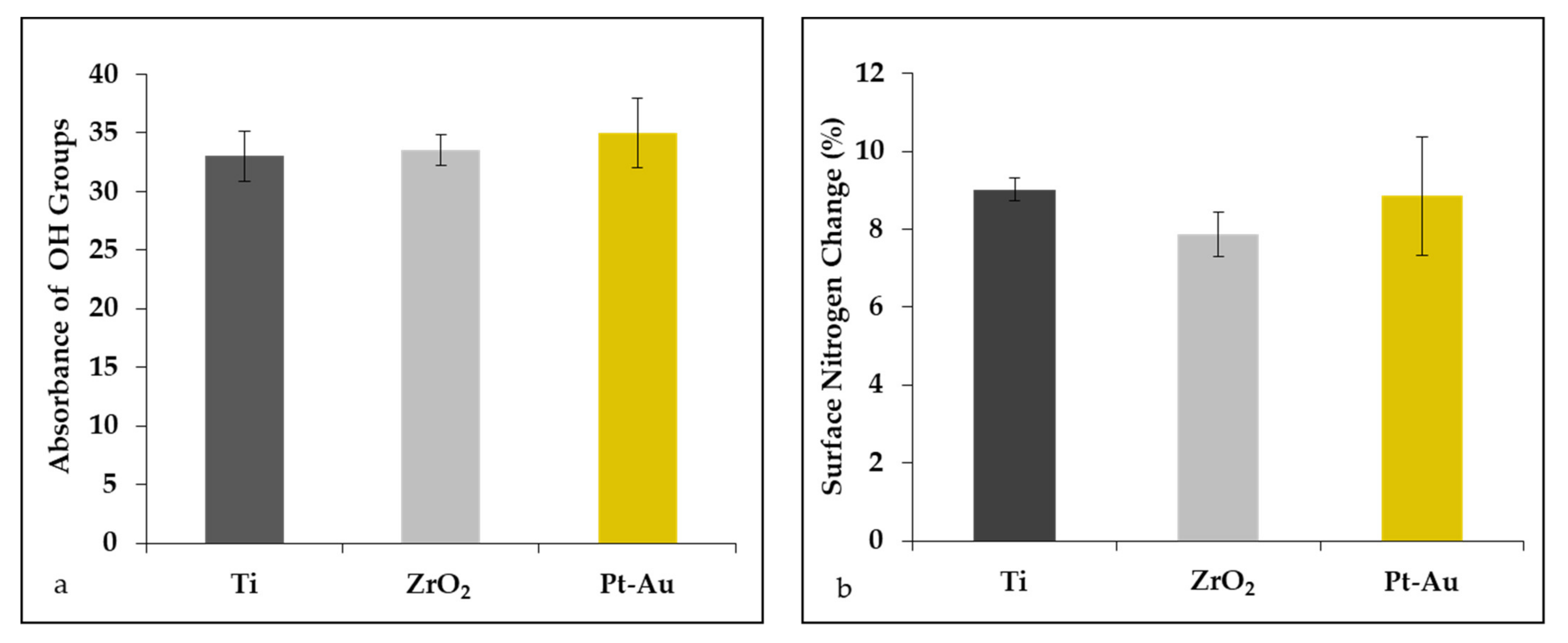
3.1. Presence of Hydroxyl Groups on Specimens
3.2. Protein Adsorption on Specimens
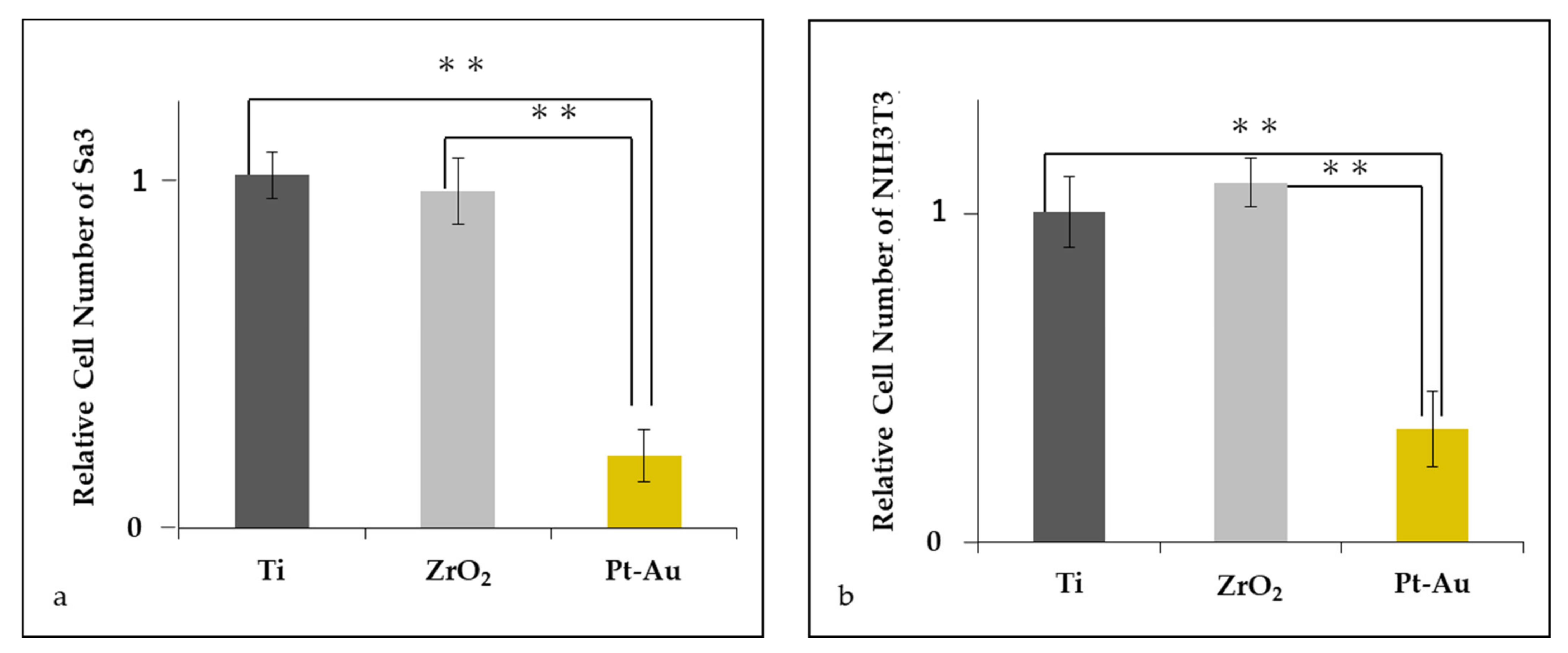
3.3. Initial Cell Attachment
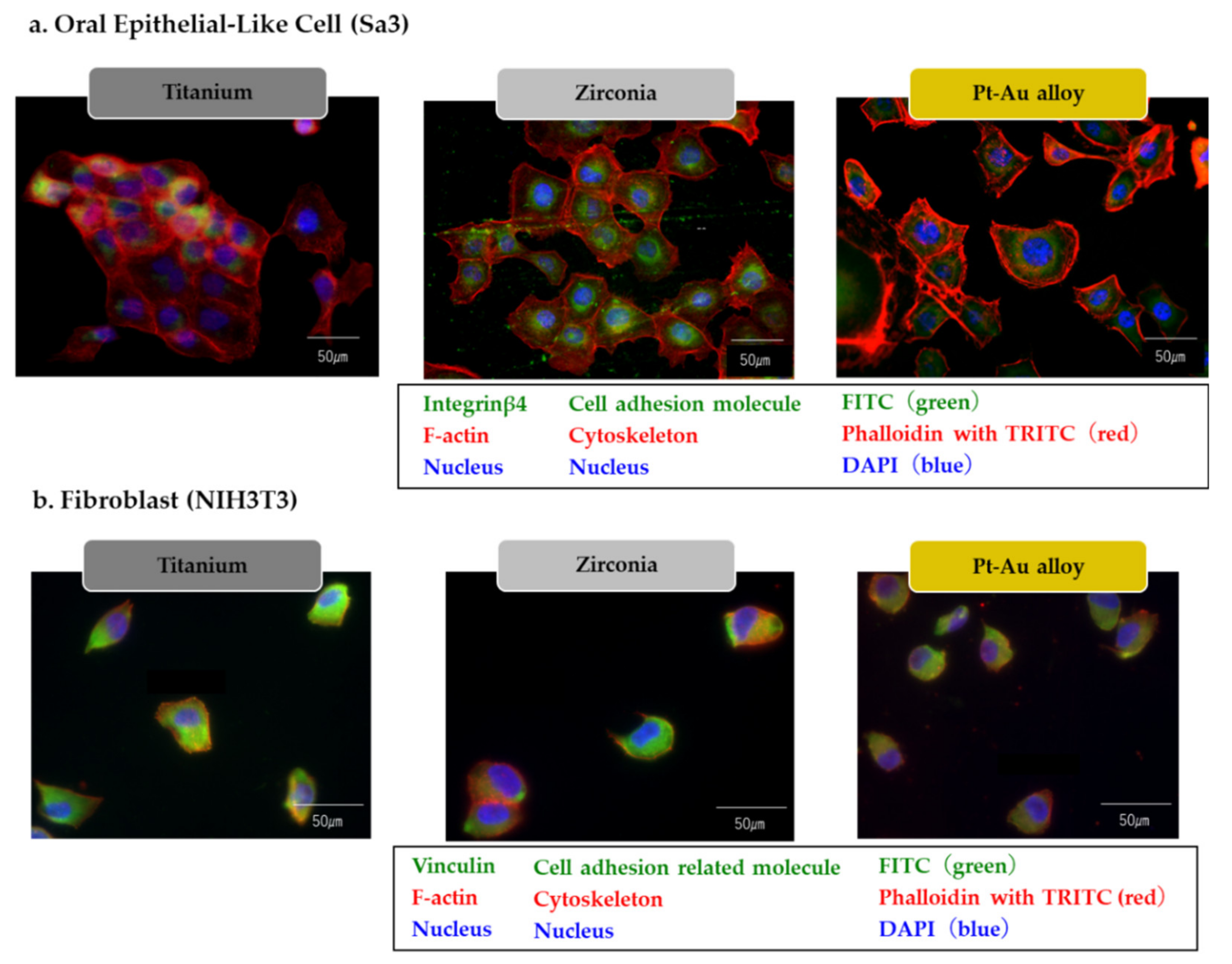
3.4. Immuno-Fluorescent Findings of the Cells
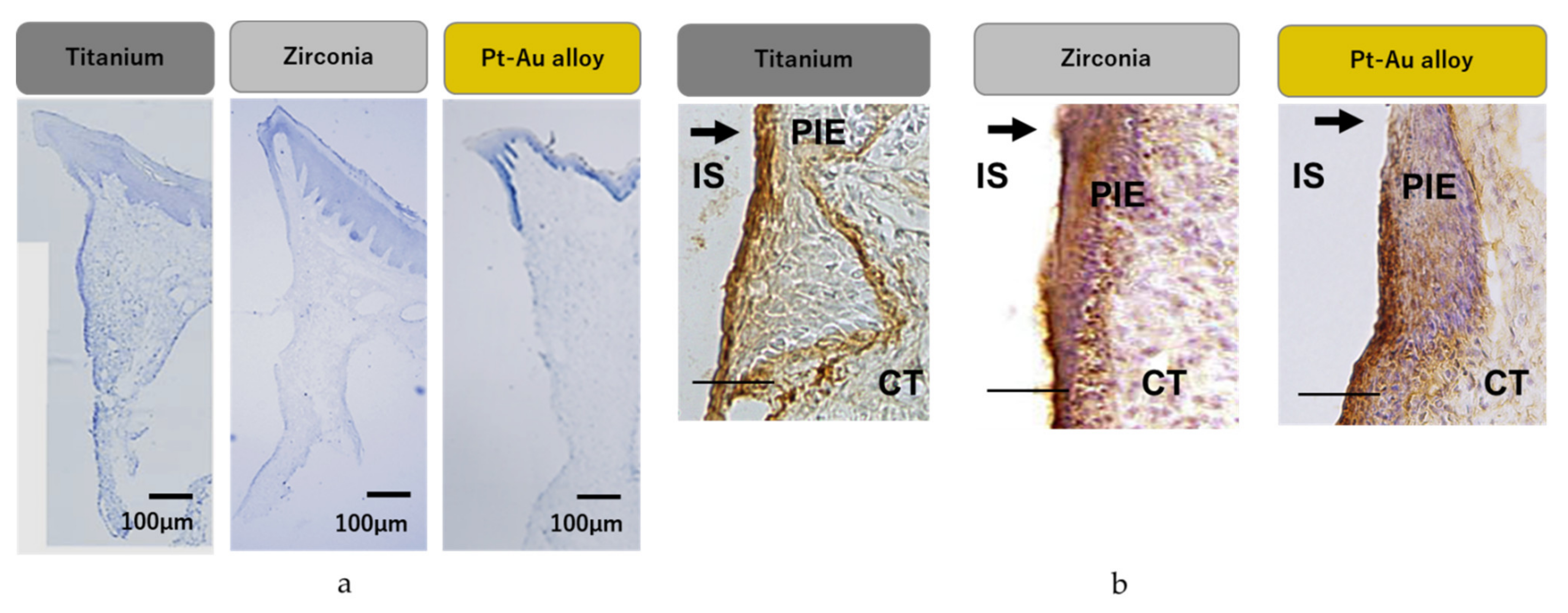
3.5. In Vivo Assessment
3.6. Immuno-Histochemical Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steigenga, J.T.; al-Shammari, K.F.; Nociti, F.H.; Misch, C.E.; Wang, H.L. Dental implant design and its relationship to long-term implant success. Implant. Dent. 2003, 12, 306–317. [Google Scholar] [CrossRef]
- Trullenque-Eriksson, A.; Guisado-Moya, B. Retrospective long-term evaluation of dental implants in totally and partially edentulous patients. Part I: Survival and marginal bone loss. Implant. Dent. 2014, 23, 732–737. [Google Scholar] [CrossRef]
- Palmer, R.; Palmer, P.; Howe, L. Complications and maintenance. Br. Dent. J. 1999, 187, 653–658. [Google Scholar] [CrossRef]
- Petrie, C.S.; Williams, J.L. Comparative evaluation of implant designs: Influence of diameter, length, and taper on strains in the alveolar crest. A three-dimensional finite-element analysis. Clin. Oral Implant. Res. 2005, 16, 486–494. [Google Scholar] [CrossRef]
- Vinhas, A.S.; Aroso, C.; Salazar, F.; Lopez-Jarana, P.; Rios-Santos, J.V.; Herrero-Climent, M. Review of the Mechanical Behavior of Different Implant-Abutment Connections. Int. J. Environ. Res. Public Health 2020, 17, 8685. [Google Scholar] [CrossRef] [PubMed]
- Sailer, I.; Zembic, A.; Jung, R.E.; Siegenthaler, D.; Holderegger, C.; Hammerle, C.H. Randomized controlled clinical trial of customized zirconia and titanium implant abutments for canine and posterior single-tooth implant reconstructions: Preliminary results at 1 year of function. Clin. Oral Implant. Res. 2009, 20, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Zembic, A.; Sailer, I.; Jung, R.E.; Hammerle, C.H. Randomized-controlled clinical trial of customized zirconia and titanium implant abutments for single-tooth implants in canine and posterior regions: 3-year results. Clin. Oral Implant. Res. 2009, 20, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.G.; Llamas, D.; Avera, S. The UCLA abutment: A four-year review. J. Prosthet Dent. 1992, 67, 509–515. [Google Scholar] [CrossRef]
- Conejo, J.; Kobayashi, T.; Anadioti, E.; Blatz, M.B. Performance of CAD/CAM monolithic ceramic Implant-supported restorations bonded to titanium inserts: A systematic review. Eur. J. Oral Implant. 2017, 10 (Suppl. 1), 139–146. [Google Scholar]
- Ikeda, H.; Yamaza, T.; Yoshinari, M.; Ohsaki, Y.; Ayukawa, Y.; Kido, M.A.; Inoue, T.; Shimono, M.; Koyano, K.; Tanaka, T. Ultrastructural and immunoelectron microscopic studies of the peri-implant epithelium-implant (Ti-6Al-4V) interface of rat maxilla. J. Periodontol. 2000, 71, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Mino, S.; Goto, T.; Kido, M.A.; Terada, Y.; Tanaka, T. Changes in the distribution of laminin-5 during peri-implant epithelium formation after immediate titanium implantation in rats. Biomaterials 2005, 26, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, I.; Atsuta, I.; Ayukawa, Y.; Oshiro, W.; Yasunami, N.; Furuhashi, A.; Koyano, K. Epithelial and Connective Tissue Sealing around Titanium Implants with Various Typical Surface Finishes. ACS Biomater. Sci. Eng. 2019, 5, 4976–4984. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T.; Kon, M.; Doi, H.; Ukai, H.; Murakami, K.; Hamanaka, H.; Asaoka, K. Amount of hydroxyl radical on calcium-ion-implanted titanium and point of zero charge of constituent oxide of the surface-modified layer. J. Mater. Sci. Mater. Med. 1998, 9, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Chen, J.; Zhang, X. Interaction of calcium and phosphate in apatite coating on titanium with serum albumin. Biomaterials 2002, 23, 2499–2507. [Google Scholar] [CrossRef]
- Kieswetter, K.; Schwartz, Z.; Hummert, T.W.; Cochran, D.L.; Simpson, J.; Dean, D.D.; Boyan, B.D. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J. Biomed. Mater. Res. 1996, 32, 55–63. [Google Scholar] [CrossRef]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The difference of fibroblast behavior on titanium substrata with different surface characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Ogino, Y.; Moriyama, Y.; Tsukiyama, Y.; Koyano, K. In vivo and in vitro studies of epithelial cell behavior around titanium implants with machined and rough surfaces. Clin. Implant. Dent. Relat. Res. 2014, 16, 772–781. [Google Scholar] [CrossRef]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Rakhmatia, Y.D.; Yasunami, N.; Koyano, K. Influence of titanium surface topography on peri-implant soft tissue integration. Key Eng. Mater. 2013, 529–530, 559–564. [Google Scholar] [CrossRef]
- Sawase, T.; Wennerberg, A.; Hallgren, C.; Albrektsson, T.; Baba, K. Chemical and topographical surface analysis of five different implant abutments. Clin. Oral Implant. Res. 2000, 11, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Saito, H.; Tsutsumi, Y.; Doi, H.; Imai, H.; Hanawa, T. Active hydroxyl groups on surface oxide film of tita-nium, 316L stainless steel, and cobalt-chromium-molybdenum alloy and its effect on the immobilization of poly(ethylene glycol). Mater. Trans. 2008, 49, 805–811. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Belkin, A.M.; Stepp, M.A. Integrins as receptors for laminins. Microsc. Res. Tech. 2000, 51, 280–301. [Google Scholar] [CrossRef]
- Barberi, J.; Spriano, S. Titanium and protein adsorption: An overview of mechanisms and effects of surface features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef]
- Elshahawya, W.; Watanabe, I. Biocompatibility of dental alloys used in dental fixed prosthodontics. Tanta Dent. J. 2014, 11, 150–159. [Google Scholar] [CrossRef]
- Smukler, H.; Chaibi, M. Periodontal and dental considerations in clinical crown extension: A rational basis for treatment. Int. J. Periodontics Restor. Dent. 1997, 17, 464–477. [Google Scholar]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions–Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, J.; Pei, X.; Han, J.; Wang, J. Network meta-analysis of survival rate and complications in implant-supported single crowns with different abutment materials. J. Dent. 2019, 88, 103115. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Narimatsu, I.; Kondo, R.; Oshiro, W.; Koyano, K. Epithelial sealing effective-ness against titanium or zirconia implants surface. J. Biomed. Mater. Res. A 2019, 107, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Ayukawa, Y.; Atsuta, I.; Moriyama, Y.; Jinno, Y.; Koyano, K. Localization of Integrin Beta-4 Subunit at Soft Tis-sue-Titanium or Zirconia Interface. J. Clin. Med. 2020, 9, 3331. [Google Scholar] [CrossRef]
- Sampatanukul, T.; Serichetaphongse, P.; Pimkhaokham, A. Histological evaluations and inflammatory responses of different dental implant abutment materials: A human histology pilot study. Clin. Implant. Dent. Relat. Res. 2018, 20, 160–169. [Google Scholar] [CrossRef]
- Nievers, M.G.; Schaapveld, R.Q.; Sonnenberg, A. Biology and function of hemidesmosomes. Matrix Biol. 1999, 18, 5–17. [Google Scholar] [CrossRef]
- Welander, M.; Abrahamsson, I.; Berglundh, T. The mucosal barrier at implant abutments of different materials. Clin. Oral Implant. Res. 2008, 19, 635–641. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Rakhmatia, Y.D.; Koyano, K. Soft Tissue Interface with Various Kinds of Implant Abutment Materials. J. Clin. Med. 2021, 10, 2386. https://doi.org/10.3390/jcm10112386
Furuhashi A, Ayukawa Y, Atsuta I, Rakhmatia YD, Koyano K. Soft Tissue Interface with Various Kinds of Implant Abutment Materials. Journal of Clinical Medicine. 2021; 10(11):2386. https://doi.org/10.3390/jcm10112386
Chicago/Turabian StyleFuruhashi, Akihiro, Yasunori Ayukawa, Ikiru Atsuta, Yunia Dwi Rakhmatia, and Kiyoshi Koyano. 2021. "Soft Tissue Interface with Various Kinds of Implant Abutment Materials" Journal of Clinical Medicine 10, no. 11: 2386. https://doi.org/10.3390/jcm10112386
APA StyleFuruhashi, A., Ayukawa, Y., Atsuta, I., Rakhmatia, Y. D., & Koyano, K. (2021). Soft Tissue Interface with Various Kinds of Implant Abutment Materials. Journal of Clinical Medicine, 10(11), 2386. https://doi.org/10.3390/jcm10112386





