Outcomes Related to Percutaneous Nephrostomies (PCN) in Malignancy-Associated Ureteric Obstruction: A Systematic Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Search Strategy and Study Selection
2.5. Data Extraction and Analysis
3. Results
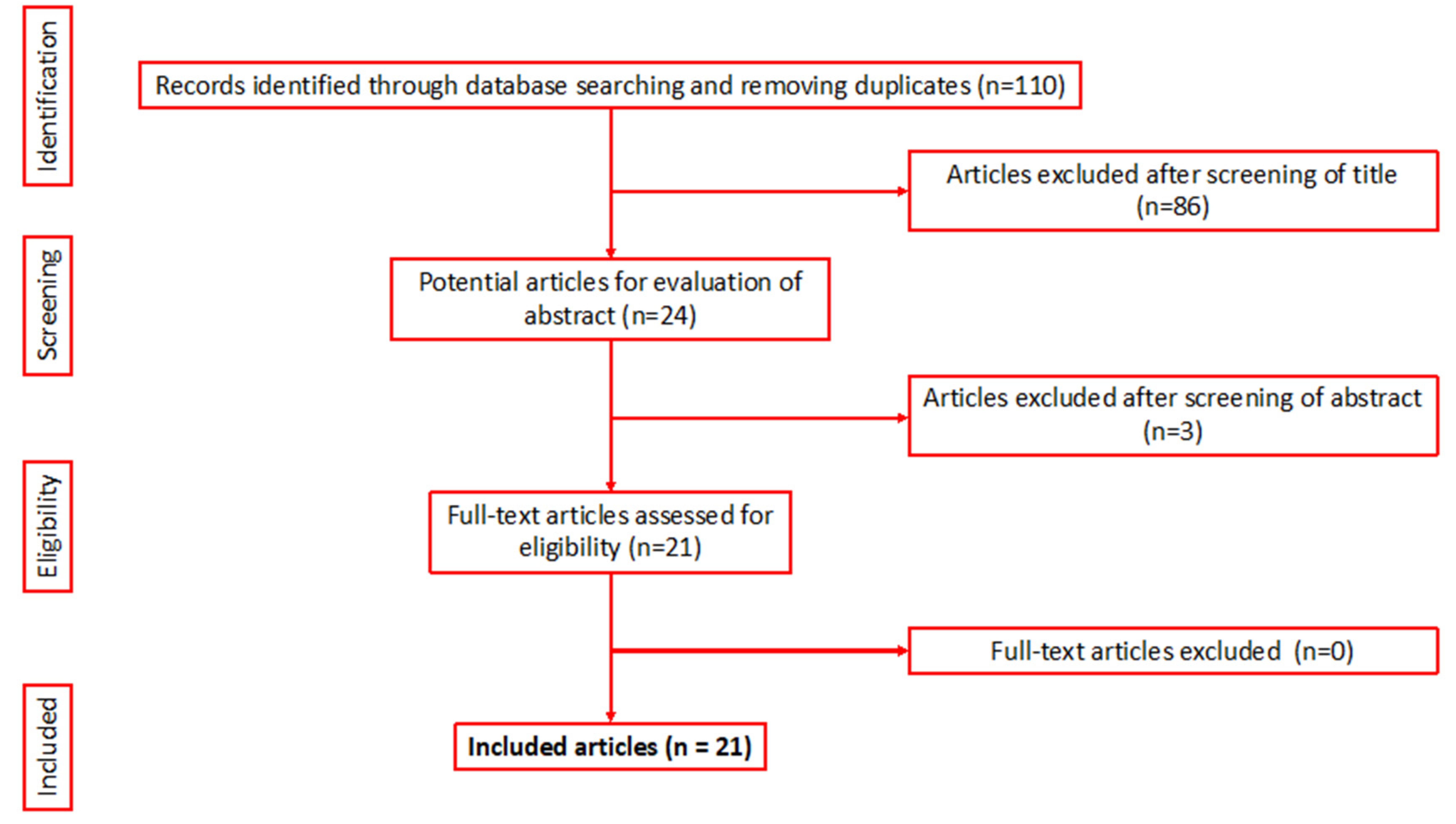
3.1. Literature Search and Included Studies
3.2. Patient Characteristics
3.3. Primary Outcomes
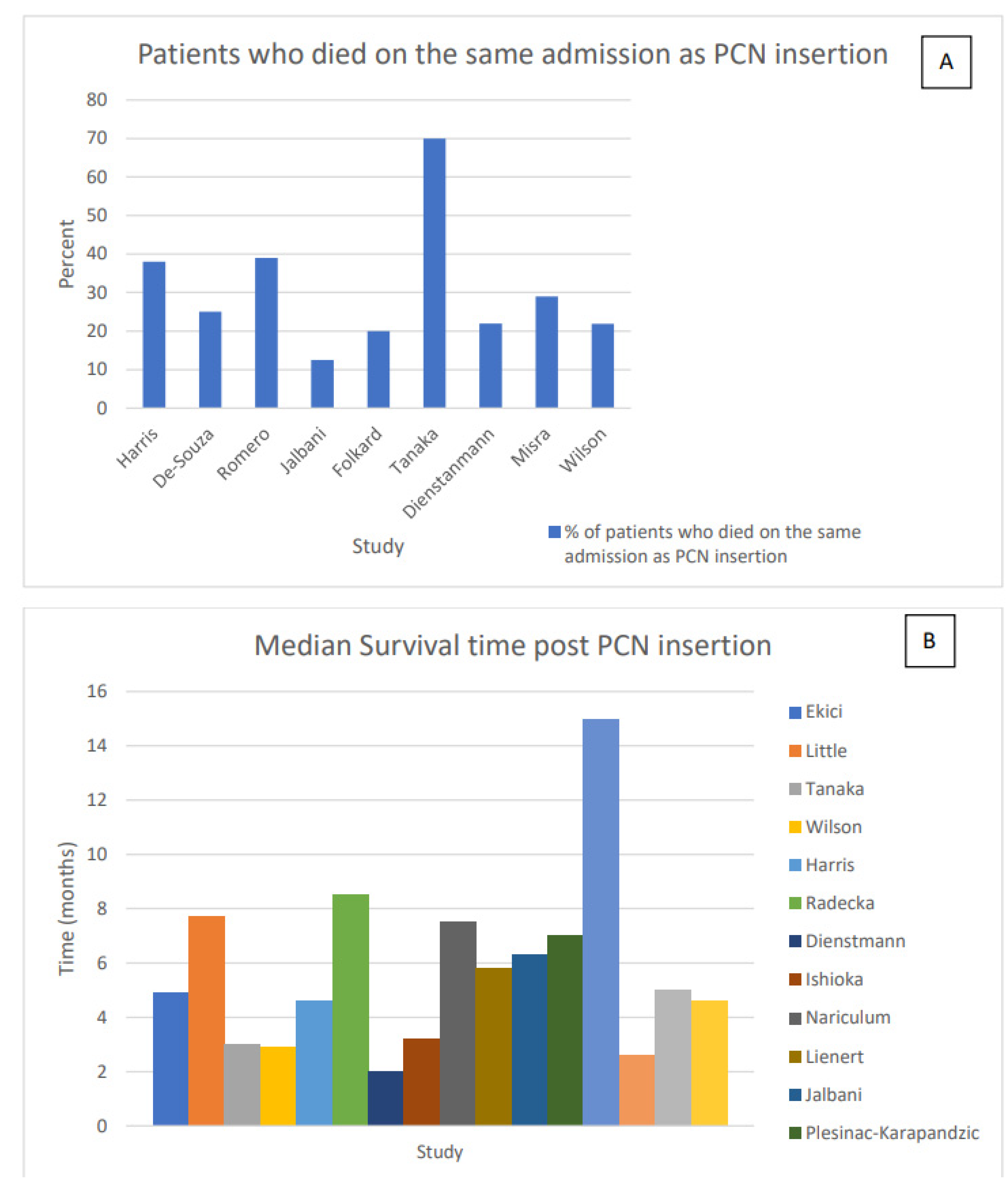
3.3.1. Survival Times after PCN
3.3.2. Prognostic Indicators
3.3.3. Complications of PCN
3.3.4. Bilateral vs. Unilateral PCN
3.3.5. Quality of Life after PCN
3.3.6. In-Hospital Stay after PCN
4. Discussion
4.1. Findings of Our Study
4.2. Patient Counselling
4.3. Quality of Life
4.4. Costs of Replacement of PCN
4.5. Conversion of PCN to Ureteric Stents
4.6. Limitations
4.7. Areas of Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bigum, L.H.; Spielmann, M.E.; Juhl, G.; Rasmussen, A. A qualitative study exploring male cancer patients’ experiences with percutaneous nephrostomy. Scand. J. Urol. 2014, 49, 162–168. [Google Scholar] [CrossRef]
- Kouba, E.; Wallen, E.M.; Pruthi, R.J. Management of ureteral obstruction due to advanced malignancy: Optimising therapeutic and palliative outcomes. J. Urol. 2008, 180, 444–450. [Google Scholar] [CrossRef]
- Radecka, E.; Magnusson, A. Complications associated with percutaneous nephrostomies. A retrospective study. Acta Radiol. 2004, 45, 184–188. [Google Scholar] [CrossRef]
- Wah, T.M.; Weston, M.J.; Irving, H.C. Percutaneous nephrostomy insertion: Outcome data from a prospective multi-operator sudy at a UK training centre. Clin. Radiol. 2004, 59, 255–261. [Google Scholar] [CrossRef]
- Patel, U.; Hussain, F.F. Percutaneous Nephrostomy of Nondilated Renal Collecting Systems with Fluoroscopic Guidance: Technique and Results. Radiology 2004, 233, 226–233. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, J.L.; Acosta-Torres, S.; Zhang, N.; Hu, T.; Odu, A.; Wang, J.; Xi, Y.; Lamus, D.; Miller, D.S.; Pillai, A.K. Long-Term Percutaneous Nephrostomy Management of Malignant Urinary Obstruction: Estimation of Optimal Exchange Frequency and Estimation of the Financial Impact of Patient Compliance. J. Vasc. Interv. Radiol. 2017, 28, 1036–1042.e8. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Jalbani, M.H.; Deenari, R.A.; Dholia, K.R.; Oad, A.K.; Arbani, I.A. Role of percutaneous nephrostomy (PCN) in malignant ureteral obstruction. J. Pak. Med. Assoc. 2010, 60, 280–283. [Google Scholar]
- Alawneh, A.; Tuqan, W.; Innabi, A.; Al-Nimer, Y.; Azzouqah, O.; Rimawi, D.; Taqash, A.; Elkhatib, M.; Klepstad, P. Clinical Factors Associated With a Short Survival Time After Percutaneous Nephrostomy for Ureteric Obstruction in Cancer Patients: An Updated Model. J. Pain Symptom Manag. 2016, 51, 255–261. [Google Scholar] [CrossRef]
- Lienert, A.; Ing, A.; Mark, S. Prognostic factors in malignant ureteric obstruction. BJU Int. 2009, 104, 938–941. [Google Scholar] [CrossRef]
- Plesinac-Karapandzic, V.; Masulovic, D.; Markovic, B.; Djuric-Stefanovic, A.; Plesinac, S.; Vucicevic, D.; Milovanovic, Z.; Milosevic, Z. Percutaneous nephrostomy in the management of advanced and terminal-stage gynecologic malignancies: Outcome and complications. Eur. J. Gynaecol. Oncol. 2010, 31, 645–650. [Google Scholar]
- Misra, S.; Coker, C.; Richenberg, J. Percutaneous nephrostomy for ureteric obstruction due to advanced pelvic malignancy: Have we got the balance right yet? Int. Urol. Nephrol. 2013, 45, 627–632. [Google Scholar] [CrossRef]
- Carrafiello, G.; Laganà, D.; Mangini, M.; Lumia, D.; Recaldini, C.; Bacuzzi, A.; Marconi, A.; Mira, A.; Cuffari, S.; Fugazzola, C. Complications of percutaneous nephrostomy in the treatment of malignant ureteral obstructions: Single–centre review. La Radiol. Med. 2006, 111, 562–571. [Google Scholar] [CrossRef]
- Malik, M.A.; Mahmood, T.; Khan, J.H.; Hanif, A.; Bajwa, I.A. Experience of percutaneous nephrostomy (PCN) in advanced ca prostate. PJMHS 2010, 4, 537–541. [Google Scholar]
- Ishioka, J.; Kzgeyama, Y.; Inoue, M.; Higashi, Y.; Kihara, K. Prognostic Model for predicting survival after palliative urinary diversion for utereral obstruction: Analysis of 140 cases. J. Urol. 2008, 180, 618–621. [Google Scholar] [CrossRef]
- Tanaka, T.; Yanase, M.; Takatsuka, K. Clinical course in patients with percutaneous nephrostomy for hydronephrosis associated with advanced cancer. Hinyokika Kiyo. Acta Urol. Jpn. 2004, 50, 457–462. [Google Scholar]
- Ekici, S.; Şahin, A.; Özen, H. Percutaneous Nephrostomy in the Management of Malignant Ureteral Obstruction Secondary to Bladder Cancer. J. Endourol. 2001, 15, 827–829. [Google Scholar] [CrossRef]
- Romero, F.R.; Broglio, M.; Pires, S.R.; Roca, R.F.; Guibu, I.A.; Perez, M.D. Indications for percutaneous nephrostomy in patients with obstructive uropathy due to malignant Urogenital neoplasias. Int. Braz. J. Urol. 2005, 31, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; Urwin, G.H.; Stower, M.J. The role of percutaneous nephrostomy in malignant ureteric obstruction. Ann. R. Coll. Surg. Engl. 2005, 87, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.R.E.; Speakman, M.J. Nephrostomies in obstructive uropathy; how should hormone resistant prostate cancer patients be managed and can we predict who will benefit? Prostate Cancer Prostatic Dis. 2006, 9, 42–44. [Google Scholar] [CrossRef]
- Aravantinos, E.; Anagnostou, T.; Karatzas, A.D.; Papakonstantinou, W.; Samarinas, M.; Melekos, M.D. Percutaneous nephrostomy in patients with tumors of advanced stage: Treatment dilemmas and impact on clinical course and Qaulity of life. J. Endourol. 2007, 21, 1297–1302. [Google Scholar] [CrossRef]
- Dienstmann, R.; Pinto, C.D.S.; Pereira, M.T.; Small, I.Á.; Gil Ferreira, C. Palliative Percutaneous Nephrostomy in Recurrent Cervical Cancer: A Retrospective Analysis of 50 Consecutive Cases. J. Pain Symptom Manag. 2008, 36, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Nariculam, J.; Murphy, D.G.; Jenner, C.; Sellars, N.; Gwyther, S.; Gordon, S.G.; Swinn, M.J. Nephrostomy insertion for patients with bilateral ureteric obstruction caused by prostate cancer. Br. J. Radiol. 2009, 82, 571–576. [Google Scholar] [CrossRef]
- De Souza, A.C.P.; Souza, A.N.; Kirsztajn, R.; Kirsztajn, G.M. Cervical cancer: Renal Complications and survival after percutaneous nephrostomy. Rev. Assoc. Med. Bras. 2016, 62, 255–261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Folkard, S.S.; Banerjee, S.; Menzies-Wilson, R.; Reason, J.; Psallidas, E.; Clissold, E.; Al-Mushatat, A.; Chaudhri, S.; Green, J.S.A. Percutaneous nephrostomy in obstructing pelvic malignancy does not facilitate further oncological treatment. Int. Urol. Nephrol. 2020, 52, 1625–1628. [Google Scholar] [CrossRef]
- Little, B.; Ho, K.J.; Gawley, S.; Young, M. Use of nephrostomy tubes in ureteric obstruction from incurable malignancy. Int. J. Clin. Pract. 2003, 57, 180–181. [Google Scholar] [PubMed]
- New, F.; Deverill, S.; Somani, B.K. Role of percutaneous nephrostomy in end of life prostate cancer patients: A systematic review of the literature. Cent. Eur. J. Urol. 2018, 71, 404–409. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Watkinson, A.; A’Hern, R.; Jones, A.; King, D.; Moskovic, E. The role of percutaneous nephrostomy in malignant urinary tract obstruction. Clin. Radiol. 1993, 47, 32–35. [Google Scholar] [CrossRef]
| Author/Year Published | Review Period | Mean Age (Range), Years | Total Number of Patients | Time Spent in Hospital after PCN | Survival Time after Insertion of PCN (Mean) | Malignancy Type | Number of Patients (Subgroup) | Breakdown of Survival per Cancer Type Post PCN Insertion (Months) |
|---|---|---|---|---|---|---|---|---|
| Ekici et al. [17], 2001 | 1987–2000 | 55 (25–76) | 23 | ND | 4.9 months | Bladder | 23 | 4.9 |
| Little et al. [26], 2003 | ND | 69 (50–87) | 31 | 46% of remaining life in hospital | 7.7 months | Bladder | 16 | ND |
| Prostate | 8 | |||||||
| Colorectal | 4 | |||||||
| Gynaecological | 3 | |||||||
| Tanaka et al. [16], 2004 | 1991–2003 | 69.2 | 33 | 70% remained in hospital post PCN | 3 months | Urological | 8 | 3.0 |
| Gynaecological | 8 | 3.0 | ||||||
| Colorectal | 8 | 5.5 | ||||||
| Upper GI | 8 | 1.5 | ||||||
| Lung | 1 | 2.5 | ||||||
| Romero et al. [18], 2005 | 2000–2004 | 52 | 43 | 17% of their remaining life (58% readmission rate) | 40% survival at 6 months, 24.2% survival at 1 year | Urological | 15 | 40% survival at 6 months/10% at 1 year |
| Gynaecological | 28 | 44.6% survival at 6 months/38.45% at 1 year | ||||||
| Wilson et al. [19], 2005 | 1998–2001 | 68.1 (42–84) | 32 | 29 days (81% readmission rate) | 2.9 months | Urological | 17 | 2.4 |
| Gynaecological | 7 | 6.9 | ||||||
| Colorectal | 7 | 4.3 | ||||||
| Breast | 1 | 27.1 | ||||||
| Harris et al. [20], 2006 | 2001–2004 | 75.9 (65–89) | 26 | 51 days (Mean) | 4.6 months | Prostate | 26 | 2.9 months |
| Carrafiello et al. [13], 2006 | 2003–2006 | 65.7 (32–102) | 201 | ND | ND | ND | 201 | ND |
| Radecka et al. [3], 2006 | 1998–2002 | 73.1 (51–97) | 151 | ND | 8.5 months | Prostate | 55 | 6.9 |
| Bladder | 43 | 17.6 | ||||||
| Gynaecological | 11 | 31.2 | ||||||
| Colorectal | 16 | 4.3 | ||||||
| other | 26 | 10.1 | ||||||
| Aravantinos et al. [21]. 2007 | 1996–2003 | 63 (40–86) | 270 | ND | 67% of patients died in first 6 months post PCN | Bladder | 54 | 8–270 days |
| Prostate | 54 | 22–723 days | ||||||
| Gynae | 54 | 7–269 days | ||||||
| Colorectal | 54 | 9–272 days | ||||||
| other | 54 | 8–280 days | ||||||
| Dienstmann et al. [22], 2008 | 2002–2006 | 44 (26–67) | 50 | 22% of patients remained in hospital post PCN | 2 months (median) | Cervical | 50 | 2 months |
| Ishioka et al. [15], 2008 | 1995–2007 | 57 (31–85) | 140 | ND | 3.2 months | Urological | 13 | ND |
| Gynaecological | 36 | |||||||
| Colorectal | 34 | |||||||
| other | 57 | |||||||
| Nariculam et al. [23], 2009 | 1998–2006 | 71 (51–85) | 25 | ND | 7.5 months | Prostate | 25 | 7.5 months |
| Lienert et al. [10], 2009 | 2005–2007 | 71 (36–91) median | 49 | ND | 5.8 months (median) | Urological | 33 | ND |
| Gynaecological | 5 | |||||||
| Colorectal | 6 | |||||||
| other | 5 | |||||||
| Jalbani et al. [8], 2010 | 2004–2006 | ND, (range 21–70) | 40 | ND | 6.3 months (median) | Urological | 15 | 14.3 |
| Gynaecological | 17 | 11.3 | ||||||
| Colorectal | 3 | 1.2 | ||||||
| other | 5 | ND | ||||||
| Plesinac-Karapandzic et al. [11], 2010 | 1996–2006 | 51 (28–85) median | 117 | ND | 7 months (median) | Gynaecological | 117 | 7 months |
| Malik et al. [14], 2010 | 2001–2009 | 68.67 (53–85) | 28 | ND | 15 months | Prostate cancer | 28 | 15 months |
| Misra et al. [12], 2013 | 2008 | 75.1 (54–87) | 22 | 29% of life in hospital (100% readmission rate) | 2.6 months | Urological | 18 | 33% survival at 6 months |
| Gynaecological | 2 | 100% survival at 6 months | ||||||
| Colorectal | 2 | 0% survival at 6 months | ||||||
| Alawneh et al. [9], 2016 | 2009–2013 | Not reported | 211 | ND | 5 months (median) | Urological | 122 | 5.5 |
| GI | 61 | 5.2 | ||||||
| Other | 28 | 3.6 | ||||||
| De Souza et al. [24], 2016 | 2010–2012 | 48.2 | 45 | ND | ND | Cervical cancer | 45 | ND |
| McDevitt et al. [6], 2017 | 2011–2013 | 48 (21–79) | 57 | ND | ND | Cervical | 26 | ND |
| Colorectal | 6 | |||||||
| Prostate | 6 | |||||||
| Bladder | 4 | |||||||
| Lymphoma | 3 | |||||||
| Ovarian | 3 | |||||||
| other | 9 | |||||||
| Folkard et al. [25], 2020 | 2015–2018 | 68.8 (30–93) | 105 | Median post procedure 14 days (1–104 days) | 4.6 months | Bladder | 32 | ND |
| Prostate | 18 | |||||||
| Colorectal | 16 | |||||||
| Gynaecological | 25 | |||||||
| Other | 8 |
| Author | Type of Complication and % | Overall Complications |
|---|---|---|
| Ekici et al. [17] | Occlusion/dislodgement/malposition 30% | 30% |
| Little et al. [26] | Occlusion/dislodgement/malposition 13% | 13% |
| Tanaka et al. [16] | Infection/sepsis 54% | 54% |
| Romero et al. [18] | Nephrectomy 5% | 42% |
| Wilson et al. [19] | Occlusion/dislodgement/malposition 46.2% | 46.2% |
| Carrafiello et al. [13] | Occlusion/dislodgement/malposition 17.3% Haematuria 1% | 18.3% |
| Radecka et al. [3] | Occlusion/dislodgement/malposition 7% | 7% |
| Aravantinos et al. [21] | Infection/sepsis 55% Transfusion 2.9% | 47.9% |
| Dienstmann et al. [22] | Infection/sepsis 32% Occlusion/dislodgement/malposition 18% Death 4% Pain 2% Haematuria 2% | 58% |
| Ishioka et al. [15] | Infection/sepsis 13% Occlusion/dislodgement/malposition 19% Haematuria 8% | 40% |
| Nariculam et al. [23] | Infection/sepsis 4% Occlusion/dislodgement/malposition 12% Haematuria 8% | 24% |
| Lienert et al. [10] | Infection/sepsis 22.4% Occlusion/dislodgement/malposition 63% Haematuria 2% | 87% |
| Jalbani et al. [8] | Infection/sepsis 7.5% Occlusion/dislodgement/malposition 37.5% Haematuria 5% | 50% |
| Plesinac-Karapandzic et al. [11] | Infection/sepsis 39.2% Occlusion/dislodgement/malposition 37.6% | 76.8% |
| Malik et al. [14] | - | 4–25% |
| Misra et al. [12] | - | 27% |
| De Souza et al. [24] | Infection/sepsis 42% Occlusion/dislodgement/malposition 15.5% Perirenal haematoma <5% | 62.5% |
| McDevitt et al. [6] | Infection/sepsis 24% Occlusion/dislodgement/malposition 42.5% | 66.5% |
| Folkard et al. [25] | - | 39% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
New, F.J.; Deverill, S.J.; Somani, B.K. Outcomes Related to Percutaneous Nephrostomies (PCN) in Malignancy-Associated Ureteric Obstruction: A Systematic Review of the Literature. J. Clin. Med. 2021, 10, 2354. https://doi.org/10.3390/jcm10112354
New FJ, Deverill SJ, Somani BK. Outcomes Related to Percutaneous Nephrostomies (PCN) in Malignancy-Associated Ureteric Obstruction: A Systematic Review of the Literature. Journal of Clinical Medicine. 2021; 10(11):2354. https://doi.org/10.3390/jcm10112354
Chicago/Turabian StyleNew, Francesca J., Sally J. Deverill, and Bhaskar K. Somani. 2021. "Outcomes Related to Percutaneous Nephrostomies (PCN) in Malignancy-Associated Ureteric Obstruction: A Systematic Review of the Literature" Journal of Clinical Medicine 10, no. 11: 2354. https://doi.org/10.3390/jcm10112354
APA StyleNew, F. J., Deverill, S. J., & Somani, B. K. (2021). Outcomes Related to Percutaneous Nephrostomies (PCN) in Malignancy-Associated Ureteric Obstruction: A Systematic Review of the Literature. Journal of Clinical Medicine, 10(11), 2354. https://doi.org/10.3390/jcm10112354





