Trends in Incidence and Outcomes of Hospitalizations for Urinary Tract Infection among Older People in Spain (2001–2018)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Study Variables
2.3. Statistical Analysis
2.4. Ethical Aspects
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gajdács, M. The Importance of Reporting Clinical and Epidemiological Data in Urology: Local Experiences and Insights from the International Literature. Medicina 2020, 56, 581. [Google Scholar] [CrossRef]
- Kline, K.A.; Bowdish, D.M. Infection in an aging population. Curr. Opin. Microbiol. 2016, 29, 63–67. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef]
- Tandogdu, Z.; Wagenlehner, F.M. Global epidemiology of urinary tract infections. Curr. Opin. Infect. Dis. 2016, 29, 73–79. [Google Scholar] [CrossRef]
- Ahmed, H.; Farewell, D.; Jones, H.M.; Francis, N.A.; Paranjothy, S.; Butler, C.C. Incidence and antibiotic prescribing for clinically diagnosed urinary tract infection in older adults in UK primary care, 2004–2014. PLoS ONE 2018, 13, e0190521. [Google Scholar] [CrossRef]
- Gravey, F.; Loggia, G.; de La Blanchardière, A.; Cattoir, V. Bacterial epidemiology and antimicrobial resistance profiles of urinary specimens of the elderly. Med. Mal. Infect. 2017, 47, 271–278. [Google Scholar] [CrossRef]
- López-de-Andrés, A.; Albaladejo-Vicente, R.; Palacios-Ceña, D.; Carabantes-Alarcon, D.; Zamorano-Leon, J.J.; de Miguel-Diez, J.; Lopez-Herranz, M.; Jiménez-García, R. Time Trends in Spain from 2001 to 2018 in the Incidence and Outcomes of Hospitalization for Urinary Tract Infections in Patients with Type 2 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2020, 17, 9427. [Google Scholar] [CrossRef]
- Cortes-Penfield, N.W.; Trautner, B.W.; Jump, R.L.P. Urinary Tract Infection and Asymptomatic Bacteriuria in Older Adults. Infect. Dis. Clin. N. Am. 2017, 31, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.M.; Lowder, J.L. Diagnosis and treatment of urinary tract infections across age groups. Am. J. Obstet. Gynecol. 2018, 219, 40–51. [Google Scholar] [CrossRef]
- Wagenlehner, F.; Wullt, B.; Ballarini, S.; Zingg, D.; Naber, K.G. Social and economic burden of recurrent urinary tract infections and quality of life: A patient web-based study (GESPRIT). Expert Rev. Pharmacoecon. Outcomes Res. 2018, 18, 107–117. [Google Scholar] [CrossRef]
- Clifford, K.M.; Dy-Boarman, E.A.; Haase, K.K.; Maxvill, K.; Pass, S.E.; Alvarez, C.A. Challenges with Diagnosing and Managing Sepsis in Older Adults. Expert Rev. Anti Infect. Ther. 2016, 14, 231–241. [Google Scholar] [CrossRef]
- Leibovici-Weissman, Y.; Tau, N.; Yahav, D. Bloodstream infections in the elderly: What is the real goal? Aging Clin. Exp. Res. 2021, 33, 1101–1112. [Google Scholar] [CrossRef]
- Choe, H.S.; Lee, S.J.; Cho, Y.H.; Çek, M.; Tandoğdu, Z.; Wagenlehner, F.; Bjerklund-Johansen, T.E.; Naber, K.; Nikfallah, A.; Kassem, A.M.; et al. Aspects of urinary tract infections and antimicrobial resistance in hospitalized urology patients in Asia: 10-Year results of the Global Prevalence Study of Infections in Urology (GPIU). J. Infect. Chemother. 2018, 24, 278–283. [Google Scholar] [CrossRef]
- Cek, M.; Tandoğdu, Z.; Wagenlehner, F.; Tenke, P.; Naber, K.; Bjerklund-Johansen, T.E. Healthcare-associated urinary tract infections in hospitalized urological patients--a global perspective: Results from the GPIU studies 2003–2010. World J. Urol. 2014, 32, 1587–1594. [Google Scholar] [CrossRef]
- Öztürk, R.; Murt, A. Epidemiology of urological infections: A global burden. World J. Urol 2020, 38, 2669–2679. [Google Scholar] [CrossRef]
- Smith, D.R.M.; Pouwels, K.B.; Hopkins, S.; Naylor, N.R.; Smieszek, T.; Robotham, J.V. Epidemiology and health-economic burden of urinary-catheter-associated infection in English NHS hospitals: A probabilistic modelling study. J. Hosp. Infect. 2019, 103, 44–54. [Google Scholar] [CrossRef]
- Tang, M.; Quanstrom, K.; Jin, C.; Suskind, A.M. Recurrent Urinary Tract Infections are Associated with Frailty in Older Adults. Urology 2019, 123, 24–27. [Google Scholar] [CrossRef]
- Kranz, J.; Schmidt, S.; Lebert, C.; Schneidewind, L.; Mandraka, F.; Kunze, M.; Helbig, S.; Vahlensieck, W.; Naber, K.; Schmiemann, G.; et al. The 2017 Update of the German Clinical Guideline on Epidemiology, Diagnostics, Therapy, Prevention, and Management of Uncomplicated Urinary Tract Infections in Adult Patients: Part 1. Urol. Int. 2018, 100, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Osakwe, Z.T.; Larson, E.; Shang, J. Urinary tract infection-related hospitalization among older adults receiving home health care. Am. J. Infect. Control 2019, 47, 786–792.e1. [Google Scholar] [CrossRef]
- Ministry of Health. Spanish National Hospital Discharge Database. 2020. Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/cmbdhome.htm (accessed on 1 June 2020).
- Agency for Research and Quality. AHRQ Quality Indicators ICD-9-CM and ICD-10-CM/PCS Specification Enhanced Version 5.0 Prevention Quality Indicators #12 Urinary Tract Infection Admission Rate. Available online: https://www.qualityindicators.ahrq.gov/Downloads/Modules/PQI/V45/TechSpecs/PQI%2012%20Urinary%20Tract%20Infection%20Admission%20Rate.pdf (accessed on 20 May 2021).
- Agency for Research and Quality. AHRQ Quality Indicators™ (AHRQ QI™) ICD-10-CM/PCS Specification v2020. Prevention Quality Indicator 12 (PQI 12) Urinary Tract Infection Admission Rate. Available online: https://www.qualityindicators.ahrq.gov/Downloads/Modules/PQI/V2020/TechSpecs/PQI_12_Urinary_Tract_Infection_Admission_Rate.pdf (accessed on 20 May 2021).
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estadística (INE). Population Estimates. Available online: http://www.ine.es (accessed on 1 June 2020).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Earlbaum Associates: Hillsdale, NJ, USA, 1988; Available online: http://www.utstat.toronto.edu/~brunner/oldclass/378f16/readings/CohenPower.pdf (accessed on 15 May 2021).
- Artero, E.Á.; Nuñez, A.C.; Bravo, M.G.; Calvo, O.C.; Garcia, M.B.; Lledias, J.P. Urinary infection in the elderly. Rev. Clin. Esp. 2019, 219, 189–193. [Google Scholar]
- de Miguel-Diez, J.; Albaladejo-Vicente, R.; Palacios-Ceña, D.; Carabantes-Alarcon, D.; Zamorano-Leon, J.J.; Lopez-Herranz, M.; Lopez-de-Andres, A. The Impact of COPD in Trends of Urinary Tract Infection Hospitalizations in Spain, 2001–2018, a Population-Based Study Using Administrative Data. J. Clin. Med. 2020, 9, 3979. [Google Scholar] [CrossRef]
- Eliakim-Raz, N.; Babitch, T.; Shaw, E.; Addy, I.; Wiegand, I.; Vank, C.; Torre-Vallejo, L.; Joan-Miquel, V.; Steve, M.; Grier, S.; et al. Risk Factors for Treatment Failure and Mortality Among Hospitalized Patients with Complicated Urinary Tract Infection: A Multicenter Retrospective Cohort Study (RESCUING Study Group). Clin. Infect. Dis. 2019, 68, 29–36. [Google Scholar]
- Godbole, G.P.; Cerruto, N.; Chavada, R. Assessment and management of urinary tract infections in older adults. J. Pharm. Pract. Res. 2020, 50, 276–283. [Google Scholar] [CrossRef]
- Corrao, S.; Santalucia, P.; Argano, C.; Djade, C.D.; Barone, E.; Tettamanti, M.; Pasina, L.; Franchi, C.; Eldin, T.K.; Marengoni, A.; et al. Gender-differences in disease distribution and outcome in hospitalized elderly: Data from the REPOSI study. Eur. J. Intern. Med. 2014, 25, 617–623. [Google Scholar] [CrossRef]
- Mody, L.; Juthani-Mehta, M. Urinary tract infections in older women: A clinical review. JAMA 2014, 311, 844–854. [Google Scholar] [CrossRef]
- Robinson, D.; Giarenis, I.; Cardozo, L. The management of urinary tract infections in octogenarian women. Maturitas 2015, 81, 343–347. [Google Scholar] [CrossRef]
- Tuem, K.B.; Desta, R.; Bitew, H.; Ibrahim, S.; Hishe, H.Z. Antimicrobial resistance patterns of uropathogens isolated between 2012 and 2017 from a tertiary hospital in Northern Ethiopia. J. Glob. Antimicrob. Resist. 2019, 18, 109–114. [Google Scholar] [CrossRef]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Comparative Epidemiology and Resistance Trends of Common Urinary Pathogens in a Tertiary-Care Hospital: A 10-Year Surveillance Study. Medicina 2019, 55, 356. [Google Scholar] [CrossRef]
- Lafon, T.; Padilla, A.C.H.; Baisse, A.; Lavaud, L.; Goudelin, M.; Barraud, O.; Daix, T.; Francois, B.; Vignon, P. Community-acquired Staphylococcus aureus bacteriuria: A warning microbiological marker for infective endocarditis? BMC Infect. Dis. 2019, 19, 504. [Google Scholar] [CrossRef]
- Angioni, D.; Hites, M.; Jacobs, F.; De Breucker, S. Predictive Factors of In-Hospital Mortality in Older Adults with Community-Acquired Bloodstream Infection. J. Frailty Aging 2020, 9, 232–237. [Google Scholar] [PubMed]
- Gharbi, M.; Drysdale, J.H.; Lishman, H.; Goudie, R.; Molokhia, M.; Johnson, A.P.; Holmes, A.H.; Aylin, P. Antibiotic management of urinary tract infection in elderly patients in primary care and its association with bloodstream infections and all-cause mortality: Population-based cohort study. BMJ 2019, 364, l525. [Google Scholar] [CrossRef]
- Hsiao, C.Y.; Chen, T.H.; Lee, Y.C.; Hsiao, M.C.; Hung, P.H.; Wang, M.C. Risk factors for uroseptic shock in hospitalized patients aged over 80 years with urinary tract infection. Ann. Transl. Med. 2020, 8, 477. [Google Scholar] [CrossRef]
- Putot, A.; Astruc, K.; Barben, J.; Mihai, A.M.; Nuss, V.; Bador, J.; Putot, S.; Dipanda, M.; Laborde, C.; Vovelle, J.; et al. Impact of a Diagnosis-Centered Antibiotic Stewardship on Incident Clostridioides difficile Infections in Older Inpatients: An Observational Study. Antibiotics 2020, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Gomila, A.; Carratalà, J.; Eliakim-Raz, N.; Shaw, E.; Tebé, C.; Wolkewitz, M.; Wiegand, I.; Grier, S.; Vank, C.; Cuperus, N.; et al. Clinical outcomes of hospitalised patients with catheter-associated urinary tract infection in countries with a high rate of multidrug-resistance: The COMBACTE-MAGNET RESCUING study. Antimicrob. Resist. Infect. Control 2019, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Gyesi-Appiah, E.; Brown, J.; Clifton, A. Short-term urinary catheters and their risks: An integrated systematic review. Br. J. Nurs. 2020, 29, S16–S22. [Google Scholar] [CrossRef]
- Jones, L.F.; Meyrick, J.; Bath, J.; Dunham, O.; McNulty, C.A.M. Effectiveness of behavioural interventions to reduce urinary tract infections and Escherichia coli bacteraemia for older adults across all care settings: A systematic review. J. Hosp. Infect. 2019, 102, 200–218. [Google Scholar] [CrossRef]
- Grados, M.C.; Thuissard, I.J.; Alós, J.I. Stratification by demographic and clinical data of the antibiotic susceptibility of Escherichia coli from urinary tract infections of the community. Aten. Primaria. 2019, 51, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Aguinaga, A.; Gil-Setas, A.; Mazón Ramos, A.; Alvaro, A.; García-Irure, J.J.; Navascués, A.; Ezpeleta Baquedano, C. Infecciones del tracto urinario. Estudio de sensibilidad antimicrobiana en Navarra [Uncomplicated urinary tract infections. Antimicrobial susceptibility study in Navarre]. An. Sist. Sanit. Navar. 2018, 41, 17–26. [Google Scholar] [CrossRef]
- Losada, I.; Barbeito, G.; García-Garrote, F.; Fernández-Pérez, B.; Malvar, A.; Hervada, X. Estudio de Sensibilidad de Escherichia coli Productores de Infecciones del Tracto Urinario Comunitarias en Galicia. Período: 2016–2017 [Antimicrobial susceptibility of Escherichia coli producers of community urinary tract infections in Galicia (Spain). Period: 2016–2017]. Aten. Primaria 2020, 52, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.R.; Motter, J.D.; Bae, S.; Kernodle, A.; Long, J.J.; Werbel, W.; Avery, R.; Durand, C.; Massie, A.B.; Desai, N.; et al. Characterizing the landscape and impact of infections following kidney transplantation. Am. J. Transplant. 2021, 21, 198–207. [Google Scholar] [CrossRef] [PubMed]
| 2001–2003 | 2004–2006 | 2007–2009 | 2010–2012 | 2013–2015 | 2016–2018 | Total | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Age Groups | n (Inc/105) | n (Inc/105) | n (Inc/105) | n (Inc/105) | n (Inc/105) | n (Inc/105) | n (Inc/105) | |
| MEN | 65–74 years | 8706 (161.54) | 9572 (181.81) | 10,854 (206.91) | 11,789 (216.18) | 15,102 (255.37) | 18,328 (290.83) | 74,351 (221.49) | <0.001 |
| 75–84 years | 10,541 (372.04) | 13,318 (415.05) | 17,018 (480.5) | 19,883 (523.75) | 22,354 (588.51) | 25,255 (668.11) | 108,369 (517.06) | <0.001 | |
| >84 years | 5312 (826.15) | 6544 (929.91) | 9395 (1084.28) | 12,288 (1164.5) | 15,414 (1234.06) | 20,745 (1434.24) | 69,698 (1168.67) | <0.001 | |
| Total | 24,559 (277.01) | 29,434 (320.73) | 37,267 (386.03) | 43,960 (426.59) | 52,870 (482.34) | 64,328 (557.99) | 252,418 (417.28) | <0.001 | |
| WOMEN | 65–74 years | 8769 (137.12) | 9382 (151.39) | 9405 (154.76) | 10,364 (166.75) | 11,859 (177.56) | 13,438 (189.04) | 63,217 (163.47) | <0.001 |
| 75–84 years | 13,278 (309.54) | 16,813 (354.97) | 20,626 (404.24) | 24,852 (464.53) | 27,408 (519.33) | 29,841 (579.07) | 132,818 (444.07) | <0.001 | |
| >84 years | 9235 (618.04) | 11,859 (724.92) | 18,002 (936.43) | 24,457 (1084.81) | 30,760 (1197.25) | 40,927 (1432.6) | 135,240 (1062.12) | <0.001 | |
| Total | 31,282 (256.86) | 38,054 (302.75) | 48,033 (366.61) | 59,673 (431.79) | 70,027 (482.09) | 84,206 (556.96) | 331,275 (407.4) | <0.001 | |
| BOTH | 65–74 years | 17,475 (148.29) | 18,954 (165.36) | 20,259 (178.92) | 22,153 (189.85) | 26,961 (214.1) | 31,766 (236.87) | 137,568 (190.43) | <0.001 |
| 75–84 years | 23,819 (334.4) | 30,131 (379.23) | 37,644 (435.48) | 44,735 (489.11) | 49,762 (548.28) | 55,096 (616.75) | 241,187 (474.14) | <0.001 | |
| >84 years | 14,547 (680.65) | 18,403 (786.58) | 27,397 (982.37) | 36,745 (1110.22) | 46,174 (1209.29) | 61,672 (1433.15) | 204,938 (1096.1) | <0.001 | |
| Total | 55,841 (265.35) | 67,488 (310.33) | 85,300 (374.85) | 103,633 (429.57) | 12,2897 (482.2) | 148,534 (557.41) | 583,693 (411.61) | <0.001 |
| 2001–2003 | 2004–2006 | 2007–2009 | 2010–2012 | 2013–2015 | 2016–2018 | Total | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Age; mean (SD) | 78.01 (7.61) | 78.54 (7.48) | 79.15 (7.52) | 79.56 (7.61) | 79.63 (7.88) | 79.99 (8.03) | 79.35 (7.77) | <0.001 |
| CCI; mean (SD) | 1.08 (1) | 1.2 (1.05) | 1.28 (1.06) | 1.37 (1.07) | 1.4 (1.08) | 1.49 (1.17) | 1.35 (1.1) | <0.001 |
| AMI; n (%) | 984 (4.01) | 1385 (4.71) | 1768 (4.74) | 1745 (3.97) | 1949 (3.69) | 3704 (5.76) | 11,535 (4.57) | <0.001 |
| CHF; n (%) | 1199 (4.88) | 1760 (5.98) | 2530 (6.79) | 3417 (7.77) | 4215 (7.97) | 6325 (9.83) | 19,446 (7.7) | <0.001 |
| PVD; n (%) | 1059 (4.31) | 1539 (5.23) | 2119 (5.69) | 2699 (6.14) | 3453 (6.53) | 4488 (6.98) | 15,357 (6.08) | <0.001 |
| CEVD; n (%) * | 2297 (9.35) | 2955 (10.04) | 3899 (10.46) | 4733 (10.77) | 5416 (10.24) | 6113 (9.5) | 25,413 (10.07) | <0.001 |
| Dementia; n (%) | 2855 (11.63) | 3312 (11.25) | 4305 (11.55) | 5214 (11.86) | 5726 (10.83) | 9162 (14.24) | 30,574 (12.11) | <0.001 |
| COPD; n (%) * | 4471 (18.21) | 5710 (19.4) | 7007 (18.8) | 8722 (19.84) | 10,236 (19.36) | 11,274 (17.53) | 47,420 (18.79) | <0.001 |
| Rheumatoid disease; n (%) * | 186 (0.76) | 286 (0.97) | 405 (1.09) | 644 (1.46) | 777 (1.47) | 885 (1.38) | 3183 (1.26) | <0.001 |
| Peptic ulcer; n (%) * | 318 (1.29) | 312 (1.06) | 316 (0.85) | 264 (0.6) | 307 (0.58) | 378 (0.59) | 1895 (0.75) | <0.001 |
| Diabetes; n (%) | 5890 (22.53) | 7695 (26.14) | 10,733 (28.80) | 13,783 (31.36) | 19,959 (32.08) | 21,905 (34.05) | 76,608 (30.35) | <0.001 |
| HP/PAPL; n (%) | 336 (1.37) | 395 (1.34) | 511 (1.37) | 644 (1.46) | 815 (1.54) | 901 (1.4) | 3602 (1.43) | 0.124 |
| Chronic renal disease; n (%) | 3071 (12.5) | 4291 (14.58) | 6495 (17.43) | 9227 (20.99) | 12,345 (23.35) | 16,489 (25.63) | 51,918 (20.57) | <0.001 |
| Chronic liver disease; n (%) * | 1055 (4.30) | 1342 (4.54) | 1697 (4.56) | 2110 (4.80) | 2554 (4.83) | 3201 (4.98) | 11,961 (4.74) | <0.001 |
| Cancer; n (%) | 2372 (9.66) | 3264 (11.09) | 4235 (11.36) | 5186 (11.8) | 6414 (12.13) | 7463 (11.6) | 28,934 (11.46) | <0.001 |
| Metastatic cancer; n (%) | 758 (3.09) | 1138 (3.87) | 1481 (3.97) | 2011 (4.57) | 2686 (5.08) | 3484 (5.42) | 11,558 (4.58) | <0.001 |
| AIDS; n (%) | 6 (0.02) | 15 (0.05) | 20 (0.05) | 32 (0.07) | 44 (0.08) | 59 (0.09) | 176 (0.07) | 0.275 |
| Urinary catheter; n (%) | 1367 (5.57) | 1887 (6.41) | 3117 (8.36) | 3953 (8.99) | 5356 (10.13) | 5477 (8.51) | 21,157 (8.38) | <0.001 |
| Urinary incontinence; n (%) | 364 (1.48) | 634 (2.15) | 940 (2.52) | 1516 (3.45) | 1919 (3.63) | 2112 (3.28) | 7485 (2.97) | <0.001 |
| LOHS; median (IQR) | 6 (7) | 6 (6) | 6 (6) | 6 (6) | 6 (6) | 6 (6) | 6 (6) | 0.089 |
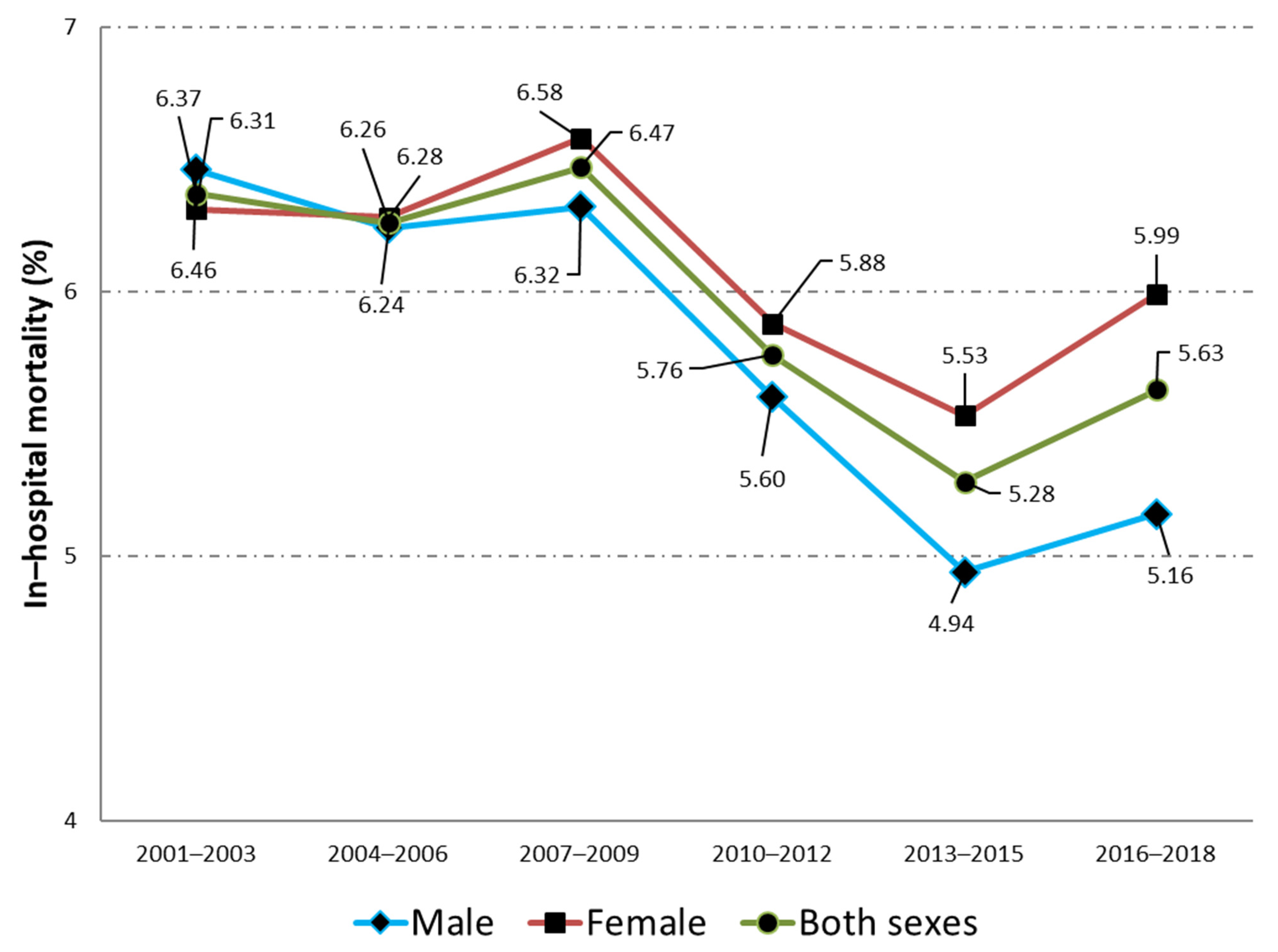
| IHM; n (%) | 1586 (6.46) | 1836 (6.24) | 2356 (6.32) | 2462 (5.6) | 2614 (4.94) | 3316 (5.15) | 14,170 (5.61) | <0.001 |
| 2001–2003 | 2004–2006 | 2007–2009 | 2010–2012 | 2013–2015 | 2016–2018 | Total | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Age; mean (SD) | 79.7 (7.78) | 80.34 (7.65) | 81.51 (7.58) | 82.11 (7.64) | 82.52 (7.72) | 83.25 (7.8) | 81.97 (7.79) | <0.001 |
| CCI; mean (SD) | 0.99 (0.92) | 1.08 (0.96) | 1.15 (0.98) | 1.23 (1) | 1.27 (1.02) | 1.37 (1.08) | 1.22 (1.02) | <0.001 |
| AMI; n (%) * | 605 (1.93) | 913 (2.4) | 1114 (2.32) | 1138 (1.91) | 1077 (1.54) | 2190 (2.6) | 7037 (2.12) | <0.001 |
| CHF; n (%) | 2293 (7.33) | 3141 (8.25) | 4606 (9.59) | 6588 (11.04) | 8224 (11.74) | 11,858 (14.08) | 36,710 (11.08) | <0.001 |
| PVD; n (%) * | 674 (2.15) | 1053 (2.77) | 1260 (2.62) | 1545 (2.59) | 1843 (2.63) | 2109 (2.5) | 8484 (2.56) | <0.001 |
| CEVD; n (%) | 2777 (8.88) | 3726 (9.79) | 5140 (10.7) | 6708 (11.24) | 7812 (11.16) | 8219 (9.76) | 34,382 (10.38) | <0.001 |
| Dementia; n (%) | 5645 (18.05) | 6563 (17.25) | 8809 (18.34) | 11,122 (18.64) | 12,724 (18.17) | 21,264 (25.25) | 66,127 (19.96) | <0.001 |
| COPD; n (%) | 1911 (6.11) | 2508 (6.59) | 3625 (7.55) | 5201 (8.72) | 6605 (9.43) | 6890 (8.18) | 26,740 (8.07) | <0.001 |
| Rheumatoid disease; n (%) | 661 (2.11) | 955 (2.51) | 1364 (2.84) | 1988 (3.33) | 2685 (3.83) | 2851 (3.39) | 10,504 (3.17) | <0.001 |
| Peptic ulcer; n (%) * | 247 (0.79) | 246 (0.65) | 237 (0.49) | 257 (0.43) | 253 (0.36) | 319 (0.38) | 1559 (0.47) | <0.001 |
| Diabetes; n (%) | 10,037 (32.08) | 13,162 (34.59) | 17,100 (35.60) | 21,814 (36.55) | 24,778 (35.38) | 29,705 (35.27) | 116,596 (25.19) | <0.001 |
| HP/PAPL; n (%) | 236 (0.75) | 273 (0.72) | 330 (0.69) | 504 (0.84) | 596 (0.85) | 658 (0.78) | 2597 (0.78) | 0.071 |
| Chronic renal disease; n (%) | 3010 (9.62) | 4437 (11.66) | 6563 (13.66) | 10,280 (17.23) | 14,370 (20.52) | 19,913 (23.65) | 58,573 (17.68) | <0.001 |
| Chronic liver disease; n (%) * | 1306 (4.18) | 1849 (4.86) | 2241 (4.67) | 2848 (4.76) | 3351 (4.78) | 3794 (4.50) | 15,389 (4.64) | <0.001 |
| Cancer; n (%) | 1065 (3.4) | 1454 (3.82) | 1946 (4.05) | 2506 (4.2) | 3141 (4.49) | 3781 (4.49) | 13,893 (4.19) | <0.001 |
| Metastatic cancer; n (%) * | 464 (1.48) | 627 (1.65) | 870 (1.81) | 1170 (1.96) | 1488 (2.12) | 1879 (2.23) | 6498 (1.96) | <0.001 |
| AIDS; n (%) | 3 (0.01) | 6 (0.02) | 7 (0.01) | 7 (0.01) | 14 (0.02) | 21 (0.02) | 58 (0.02) | 0.362 |
| Urinary catheter; n (%) | 619 (1.98) | 1025 (2.69) | 1670 (3.48) | 2467 (4.13) | 3386 (4.84) | 3224 (3.83) | 12,391 (3.74) | <0.001 |
| Urinary incontinence; n (%) | 805 (2.57) | 1345 (3.53) | 2266 (4.72) | 3305 (5.54) | 4596 (6.56) | 5065 (6.02) | 17,382 (5.25) | <0.001 |
| LOHS; median (IQR) | 7 (7) | 7 (7) | 6 (7) | 6 (6) | 6 (6) | 6 (6) | 6 (6) | <0.001 |
| IHM; n (%) | 1973 (6.31) | 2391 (6.28) | 3161 (6.58) | 3511 (5.88) | 3874 (5.53) | 5043 (5.99) | 19,953 (6.02) | <0.001 |
| 2001–2003 | 2004–2006 | 2007–2009 | 2010–2012 | 2013–2015 | 2016–2018 | Total | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Age Groups | IHM n (%) | IHM n (%) | IHM n (%) | IHM n (%) | IHM n (%) | IHM n (%) | IHM n (%) | |
| MEN | 65–74 years | 308 (3.54) | 304 (3.18) | 357 (3.29) | 328 (2.78) | 337 (2.23) | 445 (2.43) | 2079 (2.8) | <0.001 |
| 75–84 years | 682 (6.47) | 878 (6.59) | 1014 (5.96) | 1042 (5.24) | 995 (4.45) | 1099 (4.35) | 5710 (5.27) | <0.001 | |
| >84 years | 596 (11.22) | 654 (9.99) | 985 (10.48) | 1092 (8.89) | 1282 (8.32) | 1772 (8.54) | 6381 (9.16) | <0.001 | |
| WOMEN | 65–74 years | 258 (2.94) | 272 (2.9) | 278 (2.96) | 244 (2.35) | 265 (2.23) | 317 (2.36) | 1634 (2.58) | <0.001 |
| 75–84 years | 800 (6.03) | 981 (5.83) | 1163 (5.64) | 1212 (4.88) | 1205 (4.4) | 1330 (4.46) | 6691 (5.04) | <0.001 | |
| >84 years | 915 (9.91) | 1138 (9.6) | 1720 (9.55) | 2055 (8.4) | 2404 (7.82) | 3396 (8.3) | 11,628 (8.6) | <0.001 | |
| BOTH | 65–74 years | 566 (3.24) | 576 (3.04) | 635 (3.13) | 572 (2.58) | 602 (2.23) | 762 (2.4) | 3713 (2.7) | <0.001 |
| 75–84 years | 1482 (6.22) | 1859 (6.17) | 2177 (5.78) | 2254 (5.04) | 2200 (4.42) | 2429 (4.41) | 12,401 (5.14) | <0.001 | |
| >84 years | 1511 (10.39) | 1792 (9.74) | 2705 (9.87) | 3147 (8.56) | 3686 (7.98) | 5168 (8.38) | 18,009 (8.79) | <0.001 | |
| Total | 3559 (6.37) | 4227 (6.26) | 5517 (6.47) | 5973 (5.76) | 6488 (5.28) | 8359 (5.63) | 34,123 (5.85) | <0.001 |
| Isolated Pathogen | 2001–2003 | 2004–2006 | 2007–2009 | 2010–2012 | 2013–2015 | 2016–2018 | Total | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
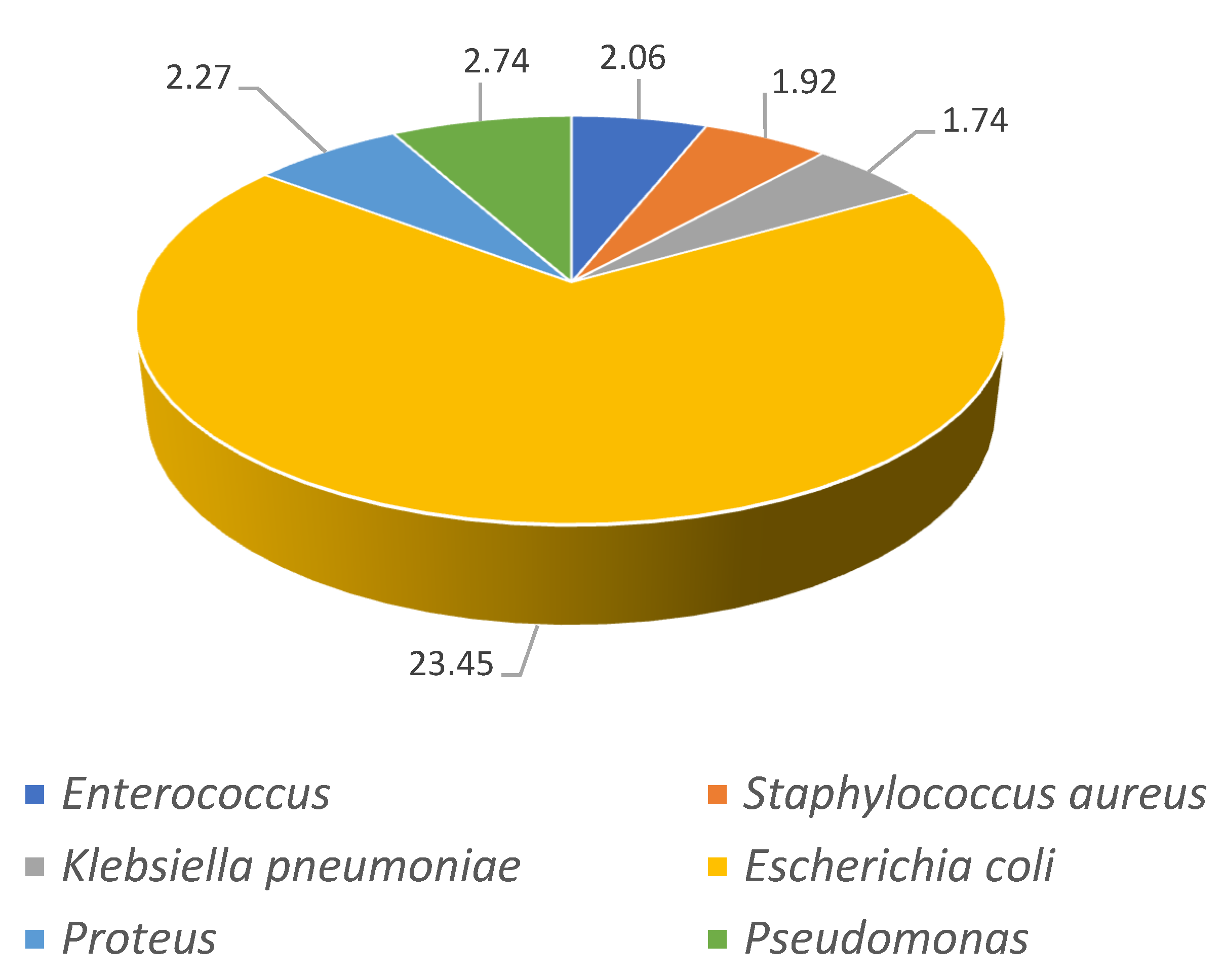
| MEN | Escherichia coli n (%) | 4548 (18.52) | 6217 (21.12) | 8524 (22.87) | 11,571 (26.32) | 14,492 (27.41) | 17,752 (27.6) | 63,104 (25) | <0.001 |
| Pseudomonasn (%) | 1005 (4.09) | 1409 (4.79) | 2043 (5.48) | 2833 (6.44) | 3654 (6.91) | 4044 (6.29) | 14,988 (5.94) | <0.001 | |
| Klebsiella pneumoniae n (%) | 429 (1.75) | 667 (2.27) | 1163 (3.12) | 1937 (4.41) | 3582 (6.78) | 5441 (8.46) | 13,219 (5.24) | <0.001 | |
| Enterococcus n (%) | 672 (2.74) | 944 (3.21) | 1438 (3.86) | 2074 (4.72) | 3232 (6.11) | 4154 (6.46) | 12,514 (4.96) | <0.001 | |
| Proteus n (%) | 544 (2.22) | 715 (2.43) | 1040 (2.79) | 1305 (2.97) | 1810 (3.42) | 2419 (3.76) | 7833 (3.1) | <0.001 | |
| Staphylococcus aureus n (%) | 588 (2.39) | 832 (2.83) | 1116 (2.99) | 1245 (2.83) | 1438 (2.72) | 1698 (2.64) | 6917 (2.74) | <0.001 | |
| Bacteriemia n (%) | 944 (3.84) | 1307 (4.44) | 1890 (5.07) | 2497 (5.68) | 3060 (5.79) | 3362 (5.23) | 13,060 (5.17) | <0.001 | |
| Sepsis n (%) | 753 (3.07) | 839 (2.85) | 1067 (2.86) | 1156 (2.63) | 1091 (2.06) | 1286 (2) | 6192 (2.45) | <0.001 | |
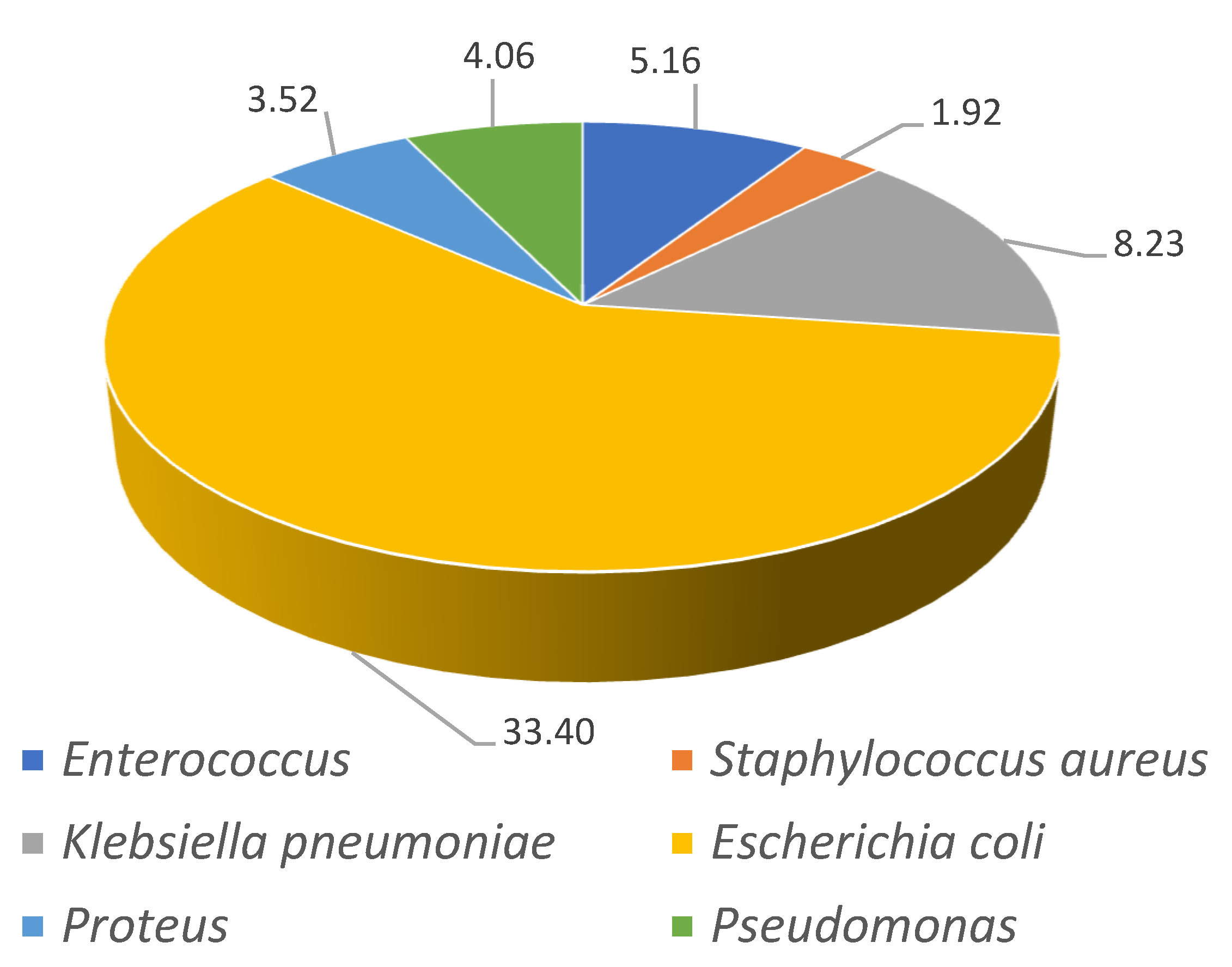
| WOMEN | Escherichia coli n (%) | 8547 (27.32) | 11,438 (30.06) | 15,215 (31.68) | 21,026 (35.24) | 26,256 (37.49) | 31,969 (37.97) | 114,451 (34.55) | <0.001 |
| Pseudomonas n (%) | 524 (1.68) | 749 (1.97) | 1101 (2.29) | 1479 (2.48) | 1844 (2.63) | 1991 (2.36) | 7688 (2.32) | <0.001 | |
| Klebsiella pneumoniae n (%) | 541 (1.73) | 957 (2.51) | 1570 (3.27) | 2729 (4.57) | 4495 (6.42) | 6778 (8.05) | 17,070 (5.15) | <0.001 | |
| Enterococcus n (%) | 478 (1.53) | 758 (1.99) | 1232 (2.56) | 1848 (3.1) | 2587 (3.69) | 3511 (4.17) | 10,414 (3.14) | <0.001 | |
| Proteus n (%) | 725 (2.32) | 931 (2.45) | 1326 (2.76) | 1749 (2.93) | 2267 (3.24) | 2808 (3.33) | 9806 (2.96) | <0.001 | |
| Staphylococcus aureus n (%) | 485 (1.55) | 654 (1.72) | 934 (1.94) | 1007 (1.69) | 1177 (1.68) | 1148 (1.36) | 5405 (1.63) | <0.001 | |
| Bacteriemia n (%) | 1443 (4.61) | 2052 (5.39) | 2538 (5.28) | 3297 (5.53) | 3978 (5.68) | 4195 (4.98) | 17,503 (5.28) | <0.001 | |
| Sepsis n (%) | 989 (3.16) | 1100 (2.89) | 1266 (2.64) | 1438 (2.41) | 1269 (1.81) | 1423 (1.69) | 7485 (2.26) | <0.001 |
| MEN | WOMEN | BOTH | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| 75–84 years | 1.89 (1.79–1.99) | 2.04 (1.92–2.16) | 1.96 (1.89–2.04) |
| >84 years | 3.6 (3.41–3.8) | 3.71 (3.51–3.92) | 3.63 (3.5–3.78) |
| AMI | 1.17 (1.08–1.26) | 1.35 (1.24–1.48) | 1.24 (1.17–1.32) |
| CHF | 2.03 (1.93–2.14) | 1.79 (1.72–1.86) | 1.88 (1.82–1.94) |
| PVD | 1.15 (1.08–1.24) | 1.52 (1.41–1.64) | 1.3 (1.23–1.37) |
| CEVD | 1.37 (1.3–1.44) | 1.32 (1.27–1.38) | 1.35 (1.3–1.39) |
| Dementia | 1.67 (1.59–1.75) | 1.42 (1.37–1.47) | 1.5 (1.46–1.55) |
| Diabetes | 0.95 (0.91–0.99) | 0.97 (0.93–1) | 0.95 (0.93–0.98) |
| HP/PAPL | 1.65 (1.43–1.9) | 1.97 (1.71–2.27) | 1.8 (1.62–1.99) |
| Chronic renal disease | 1.27 (1.21–1.32) | 1.26 (1.21–1.31) | 1.27 (1.23–1.3) |
| Chronic liver disease | 2.09 (1.78–2.45) | 1.8 (1.55–2.09) | 1.93 (1.73–2.16) |
| Cancer | 1.49 (1.42–1.57) | 1.73 (1.62–1.84) | 1.57 (1.51–1.64) |
| Metastatic cancer | 4.04 (3.8–4.3) | 3.85 (3.56–4.17) | 3.94 (3.76–4.14) |
| Urinary catheter | 0.71 (0.66–0.76) | 1.1 (1.02–1.18) | 0.87 (0.83–0.91) |
| Escherichia coli | 0.51 (0.49–0.54) | 0.45 (0.44–0.47) | 0.47 (0.46–0.49) |
| Pseudomonas | 0.72 (0.67–0.78) | NS | 0.82 (0.77–0.87) |
| Klebsiella pneumoniae | 0.71 (0.65–0.78) | 0.75 (0.7–0.8) | 0.73 (0.69–0.77) |
| Enterococcus | NS | 0.92 (0.84–0.99) | 0.92 (0.87–0.98) |
| Proteus | 0.73 (0.66–0.81) | 0.83 (0.76–0.9) | 0.79 (0.74–0.84) |
| Staphylococcus aureus | 1.4 (1.28–1.53) | 1.6 (1.46–1.76) | 1.49 (1.4–1.59) |
| Sepsis | 5.41 (4.91–5.97) | 6.5 (5.97–7.09) | 5.98 (5.61–6.38) |
| Time periods (Continous) | 0.97 (0.96–0.98) | 0.98 (0.97–0.99) | 0.98 (0.97–0.99) |
| Women | NA | NA | 1.06 (1.03–1.08) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios-Ceña, D.; Florencio, L.L.; Hernández-Barrera, V.; Fernandez-de-las-Peñas, C.; de Miguel-Diez, J.; Martínez-Hernández, D.; Carabantes-Alarcón, D.; Jimenez-García, R.; Lopez-de-Andres, A.; Lopez-Herranz, M. Trends in Incidence and Outcomes of Hospitalizations for Urinary Tract Infection among Older People in Spain (2001–2018). J. Clin. Med. 2021, 10, 2332. https://doi.org/10.3390/jcm10112332
Palacios-Ceña D, Florencio LL, Hernández-Barrera V, Fernandez-de-las-Peñas C, de Miguel-Diez J, Martínez-Hernández D, Carabantes-Alarcón D, Jimenez-García R, Lopez-de-Andres A, Lopez-Herranz M. Trends in Incidence and Outcomes of Hospitalizations for Urinary Tract Infection among Older People in Spain (2001–2018). Journal of Clinical Medicine. 2021; 10(11):2332. https://doi.org/10.3390/jcm10112332
Chicago/Turabian StylePalacios-Ceña, Domingo, Lidiane Lima Florencio, Valentín Hernández-Barrera, Cesar Fernandez-de-las-Peñas, Javier de Miguel-Diez, David Martínez-Hernández, David Carabantes-Alarcón, Rodrigo Jimenez-García, Ana Lopez-de-Andres, and Marta Lopez-Herranz. 2021. "Trends in Incidence and Outcomes of Hospitalizations for Urinary Tract Infection among Older People in Spain (2001–2018)" Journal of Clinical Medicine 10, no. 11: 2332. https://doi.org/10.3390/jcm10112332
APA StylePalacios-Ceña, D., Florencio, L. L., Hernández-Barrera, V., Fernandez-de-las-Peñas, C., de Miguel-Diez, J., Martínez-Hernández, D., Carabantes-Alarcón, D., Jimenez-García, R., Lopez-de-Andres, A., & Lopez-Herranz, M. (2021). Trends in Incidence and Outcomes of Hospitalizations for Urinary Tract Infection among Older People in Spain (2001–2018). Journal of Clinical Medicine, 10(11), 2332. https://doi.org/10.3390/jcm10112332










