Abstract
Hepatitis B virus (HBV) is a main cause of chronic liver disease worldwide and can lead to severe liver diseases. The World Health Organization has planned to eliminate viral hepatitis, including hepatitis caused by HBV and hepatitis C virus, by 2030. As mother-to-child transmission (MTCT) of HBV is a main cause of chronic HBV infection, MTCT prevention is the main target to reduce the risk of chronic HBV infection and eliminate the disease. Recent clinical trials and meta-analyses found that antiviral therapy could prevent MTCT effectively in mothers with ≥200,000 IU/mL of HBV DNA, in combination with serial vaccination and hepatitis B immune globulin administration in infants. Despite the preventive role of antivirals for MTCT of HBV, there are several concerns regarding antiviral therapy with respect to the safety of the mother and fetus during pregnancy. This review summarizes the benefits and risks of antiviral treatment during pregnancy in women with chronic HBV infection.
1. Introduction
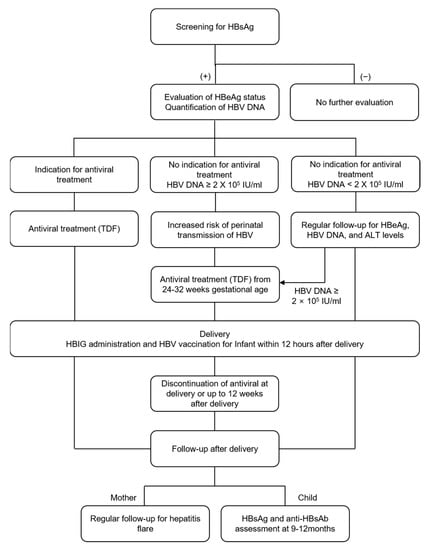
The global prevalence of hepatitis B virus (HBV) was 3.5% in 2015 [1]. Approximately 257 million people, including 65 million women of childbearing age, were infected with HBV, and 884,000 patients died because of HBV-related diseases [1,2,3]. Therefore, the World Health Organization declared the elimination of viral hepatitis by 2030, including hepatitis caused by HBV and hepatitis C virus, as a public health goal. Owing to the hepatitis B vaccine, the number of newly HBV infected patients has decreased. However, there were still approximately 4.7 million new patients with HBV infection in 2015 [4]. The main cause of HBV transmission is mother-to-child transmission (MTCT) during pregnancy and the peripartum period [5]. This is because 90% of the infections in infants become chronic; otherwise only 6% of infections in people over the age of 5 years become chronic [1]. Therefore, preventing MTCT of HBV is the main target to reduce the risk of chronic hepatitis B (CHB) and eliminate the disease. Using an immunoprophylactic strategy of serial vaccination and hepatitis B immune globulin (HBIG) administration to children within 12 h of delivery, the rate of MTCT of HBV has decreased by approximately 90% in infants born to mothers with a high viral load [6,7]. Nevertheless, infants from hepatitis B e antigen (HBeAg)-positive mothers or mothers with a high viral load still have a risk of acquiring the infection by MTCT [8,9]. Therefore, attempts have been made to prevent the MTCT of HBV by administering antiviral drugs to pregnant CHB patients HBeAg-positive and with a high viral load. However, there are several concerns about antiviral therapy in pregnancy. In this review, we discuss the benefits and risks of antiviral treatment during pregnancy to prevent MTCT of HBV.
2. Mechanism of MTCT of HBV
MTCT of HBV can be classified into two categories: intrauterine transmission (IUT) and perinatal transmission. Although the placenta protects against the transmission of the hepatitis B surface antigen (HBsAg) and viral particles, several mechanisms allow HBV transmission from the mother to the fetus before labor. HBV-infected oocytes, transmission through peripheral blood mononuclear cells, and placental infection could be rare causes of IUT [10,11,12]. IUT is mainly caused by placental injury, allowing HBV to be transmitted through the placental barrier to infants [13,14]. Thus, prenatal invasive tests, such as amniocentesis, can result in IUT. Amniocentesis performed in CHB mothers with high viral load (HBV DNA ≥ 7.0 log10 IU/mL) increased the rate of MTCT significantly in a case-control study [15]. Therefore, the risk of MTCT of HBV with amniocentesis should be considered with a risk–benefit assessment in mothers with high HBV DNA [16].
The exact proportions of intrauterine and perinatal transmission in MTCT of HBV are unknown, but perinatal transmission is known to be the key form of MTCT of HBV [8]. Fetal trauma during labor, micro-circulation between maternal and fetal blood, and contact with HBV-containing vaginal fluid/epithelium could be possible mechanisms of HBV transmission during the perinatal period [17]. Maternal HBV DNA level is the most significant single risk factor for MTCT of HBV, and the transmission risk increases in mothers with a higher viral load [8,9]. The risk of MTCT with a maternal HBV DNA level of ≥200,000 IU/mL is higher than that with a maternal HBV DNA level of <200,000 IU/mL [18,19,20]. HBeAg-positive status and high serum HBsAg level are also related to an elevated risk of MTCT of HBV, and they can be substituted for HBV DNA to estimate MTCT risk in areas where HBV DNA testing is not available [21,22,23,24]. In general, HBeAg-positive mothers have a higher HBV DNA level than HBeAg-negative mothers. Children from HBeAg-positive mothers have a higher risk of infection compared with those from HBeAg-negative mothers [22]. In a meta-analysis of 66 studies, the pooled analysis showed that the sensitivity of HBeAg status for prediction of HBV DNA levels of ≥200,000 IU/mL was 88.2% (83.9–91.5%), and the specificity was 92.6% (90.0–94.5%) [23]. HBsAg quantification was reported to predict infection in infants in a study on 568 HBsAg-positive mothers in Taiwan [21]. HBeAg can pass the placenta and is transmitted to the fetus during the intrauterine period. In HBeAg-positive mothers, HBeAg that crosses the placenta can weaken the immune response against HBV in children [25]. Likewise, co-inhibitory signaling by macrophages may reduce antiviral effects in children exposed to HBV in the uterus [26].
Cesarean section was shown to reduce the risk of HBV MTCT compared to normal vaginal delivery in a few studies [27,28]. However, no randomized controlled trials (RCTs) have proven that cesarean section reduces the risk of MTCT. Consequently, cesarean section is not indicated to prevent MTCT of HBV.
3. Immunization Strategy to Prevent MTCT of HBV
In addition to serial vaccination of HBV, administration of HBIG is critical for preventing MTCT. The immune barrier formed by HBIG provides protection against MTCT of HBV with safety and efficacy [29]. In a RCT of HBeAg-positive mothers in Hong Kong, the rates of MTCT of HBV without vaccination, with only vaccination, and with vaccination + HBIG administration were 73.2%, 21.0%, and 6.8%, respectively [30]. Furthermore, immunization of infants against HBV prevents the development of HCC in adults [31]. The HBV vaccine and HBIG should be injected within 12–24 h of delivery [1,16]. Delayed administration is associated with immunoprophylaxis failure [32]. However, global coverage of hepatitis B vaccine was 39% in 2015 [1]. Easy access to the hepatitis B vaccine is required to reduce risk of MTCT of HBV and to accomplish the goal of HBV elimination.
7. Postpartum Follow-Up of the Mother and Child
As flare occurrence was not influenced by the discontinuation of antiviral agents (at delivery or at 4–8, 12, or 24 weeks postpartum) [46], antivirals may be discontinued after delivery in mothers who were treated with antivirals to prevent MTCT. Although the timing of discontinuation is not well defined for patients in whom antiviral treatment is initiated only for the prevention of MTCT, most international guidelines recommend that antiviral treatment could be discontinued up to 12 weeks after delivery, as described above [78]. After the discontinuation of antivirals, clinicians should carefully monitor mothers for the occurrence of hepatitis flare.
Children born to mothers with CHB are recommended to be tested for HBsAg and hepatitis B surface antibody (anti-HBsAg) at 9–12 months after birth to identify successful prevention of MTCT of HBV and the production of anti-HBsAg antibody [79]. As HBsAg may appear due to HBV vaccination and anti-HBsAg antibodies can be detected as a result of HBIG administration at birth, testing in children before 9 months of age is not recommended [65]. If children have not acquired immunity against HBV infection and show no HBV infection, they should receive the HBV vaccine again. Children with HBV infection transmitted from the mother are recommended to undergo regular monitoring of HBeAg, ALT, and HBV DNA levels, despite the low risk of developing liver cirrhosis or HCC at this early age [80]. Although data are limited, children also require HCC surveillance, with regular alpha-fetoprotein measurement and sonography, especially children showing HBeAg seroconversion and cirrhosis [81].
9. Future Perspectives
Although TDF is safe in terms of renal function and growth in infants, there is a limitation in that these results did not originate from a randomized controlled study [77]. In addition, while safety has been reported in infants, the long-term safety of TDF exposure during the fetal period should be evaluated in studies with a long-term follow-up period. The role of TAF in preventing MTCT of HBV and the safety of TAF during pregnancy are important issues that need to be studied. Although TAF showed meaningful antiviral effects against HIV and showed safety in both the mother and child [88], the APR reported that TAF use was associated with a higher rate of birth defects due to TAF exposure in the first trimester [59]. Therefore, further studies are required to assess the suitability of the clinical application of TAF for preventing MTCT of HBV. One multicenter prospective observational study reported that TAF is effective for preventing MTCT of HBV (0% of MTCT rate) and safe during pregnancy (no birth defect) [49]. Recently, a prospective study has been conducted in China (NCT04237376) for evaluation of the efficacy and safety of TAF for preventing MTCT of HBV. If the study is completed successfully, the efficacy and safety of TAF in preventing MTCT of HBV can be identified.
10. Conclusions
In addition to sequential vaccination and administration of HBIG, antiviral treatment in pregnant CHB patients with high HBV DNA titers (≥200,000 IU/mL) can effectively prevent MTCT of HBV. Among the currently available antiviral agents, TDF is safe and effective for prevention of MTCT of HBV. If the mother’s serum HBV DNA level is >200,000 IU/mL, clinicians should consider administering TDF to prevent MTCT of HBV from 24–28 weeks of pregnancy to 2–12 weeks after delivery. This strategy is expected to contribute greatly to the elimination of HBV infection as a public health issue.
Author Contributions
Y.S.L. and Y.-S.L. contributed to the conception and design of the study and drafted the manuscript. S.M.B. and Y.-S.L. edited the manuscript. Y.-S.L. approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by Bumsuk Academic Research Fund in 2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Global Hepatitis Report. 2017. Available online: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ (accessed on 14 February 2021).
- Stanaway, J.D.; Flaxman, A.D.; Naghavi, M.; Fitzmaurice, C.; Vos, T.; Abubakar, I.; Abu-Raddad, L.J.; Assadi, R.; Bhala, N.; Cowie, B.; et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the Global Burden of Disease Study 2013. Lancet. 2016, 388, 1081–1088. [Google Scholar] [CrossRef]
- Park, S.H.; Plank, L.D.; Suk, K.T.; Park, Y.E.; Lee, J.; Choi, J.H.; Heo, N.Y.; Park, J.; Kim, T.O.; Moon, Y.S.; et al. Trends in the prevalence of chronic liver disease in the Korean adult population, 1998–2017. Clin. Mol. Hepatol. 2020, 26, 209–215. [Google Scholar] [CrossRef]
- Cooke, G.S.; Andrieux-Meyer, I.; Applegate, T.L.; Atun, R.; Burry, J.R.; Cheinquer, H.; Dusheiko, G.; Feld, J.J.; Gore, C.; Griswold, M.G.; et al. Accelerating the elimination of viral hepatitis: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2019, 4, 135–184. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Q.; Zhang, J.X. Natural History and Clinical Consequences of Hepatitis B Virus Infection. Int. J. Med. Sci. 2005, 2, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; Chen, C.J.; Lai, M.S.; Hsu, H.M.; Wu, T.C.; Kong, M.S.; Liang, D.C.; Shau, W.Y.; Chen, D.S. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med. 1997, 336, 1855–1859. [Google Scholar] [CrossRef]
- Ni, Y.H.; Huang, L.M.; Chang, M.H.; Yen, C.J.; Lu, C.Y.; You, S.L.; Kao, J.H.; Lin, Y.C.; Chen, H.L.; Hsu, H.Y.; et al. Two decades of universal hepatitis B vaccination in taiwan: Impact and implication for future strategies. Gastroenterology 2007, 132, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Chen, Y.; Duan, Z.; Zhang, H.; Pan, C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J. Viral Hepat. 2012, 19, e18–e25. [Google Scholar] [CrossRef]
- Wiseman, E.; Fraser, M.A.; Holden, S.; Glass, A.; Kidson, B.L.; Heron, L.G.; Maley, M.W.; Ayres, A.; Locarnini, S.A.; Levy, M.T. Perinatal transmission of hepatitis B virus: An Australian experience. Med. J. Aust. 2009, 190, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jiang, Q.; Gu, X.; Ju, L.; Ji, Y.; Wu, K.; Jiang, H. Correlation between vertical transmission of hepatitis B virus and the expression of HBsAg in ovarian follicles and placenta. PLoS ONE 2013, 8, e54246. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Wang, L.; Xu, Y.; Liu, X.; Li, S.; Qian, Q.; Hu, B.; Zhou, A.; Chen, T.; Zhao, Y. Role of maternal viremia and placental infection in hepatitis B virus intrauterine transmission. Microbes. Infect. 2013, 15, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Alla, N.R.; Li, X.; Mynbaev, O.A.; Shi, Z. Mother-to-child transmission of HBV: Review of current clinical management and prevention strategies. Rev. Med. Virol. 2014, 24, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Jonas, M.M. Hepatitis B and pregnancy: An underestimated issue. Liver Int. 2009, 29 (Suppl. S1), 133–139. [Google Scholar] [CrossRef]
- Stevens, C.E.; Beasley, R.P.; Tsui, J.; Lee, W.C. Vertical transmission of hepatitis B antigen in Taiwan. N. Engl. J. Med. 1975, 292, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Pan, C.Q.; Hao, J.; Hu, Y.; Liu, M.; Li, L.; Liang, D. Risk of vertical transmission of hepatitis B after amniocentesis in HBs antigen-positive mothers. J. Hepatol. 2014, 60, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef] [PubMed]
- Mavilia, M.G.; Wu, G.Y. Mechanisms and Prevention of Vertical Transmission in Chronic Viral Hepatitis. J. Clin. Transl. Hepatol. 2017, 5, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, C.Q.; Pang, Q.; Tian, R.; Yan, M.; Liu, X. Telbivudine or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis B virus in real-life practice. Hepatology 2014, 60, 468–476. [Google Scholar] [CrossRef]
- Park, J.S.; Pan, C. Current recommendations of managing HBV infection in preconception or pregnancy. Front. Med. 2014, 8, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Q.; Duan, Z.P.; Bhamidimarri, K.R.; Zou, H.B.; Liang, X.F.; Li, J.; Tong, M.J. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin. Gastroenterol. Hepatol. 2012, 10, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.H.; Huang, C.W.; Chie, W.C.; Yeung, C.Y.; Zhao, L.L.; Lin, W.T.; Wu, J.F.; Ni, Y.H.; Hsu, H.Y.; Chang, M.H.; et al. Quantitative maternal hepatitis B surface antigen predicts maternally transmitted hepatitis B virus infection. Hepatology 2016, 64, 1451–1461. [Google Scholar] [CrossRef]
- Wen, W.H.; Chang, M.H.; Zhao, L.L.; Ni, Y.H.; Hsu, H.Y.; Wu, J.F.; Chen, P.J.; Chen, D.S.; Chen, H.L. Mother-to-infant transmission of hepatitis B virus infection: Significance of maternal viral load and strategies for intervention. J. Hepatol. 2013, 59, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Boucheron, P.; Lu, Y.; Yoshida, K.; Zhao, T.; Funk, A.L.; Lunel-Fabiani, F.; Guingané, A.; Tuaillon, E.; van Holten, J.; Chou, R.; et al. Accuracy of HBeAg to identify pregnant women at risk of transmitting hepatitis B virus to their neonates: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 85–96. [Google Scholar] [CrossRef]
- Okada, K.; Kamiyama, I.; Inomata, M.; Imai, M.; Miyakawa, Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N. Engl. J. Med. 1976, 294, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Kuo, C.F.; Akbari, O.; Ou, J.H. Maternal-Derived Hepatitis B Virus e Antigen Alters Macrophage Function in Offspring to Drive Viral Persistence after Vertical Transmission. Immunity 2016, 44, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Kinder, J.M.; Jiang, T.T.; Way, S.S. Offspring’s Tolerance of Mother Goes Viral. Immunity 2016, 44, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qin, Q.; Fang, Q.; Jiang, L.; Nie, S. Cesarean section to prevent mother-to-child transmission of hepatitis B virus in China: A meta-analysis. BMC Pregnancy Childbirth 2017, 17, 303. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Shi, X.H.; Feng, Y.L.; Wang, B.; Feng, L.P.; Wang, S.P.; Zhang, Y.W. Risk factors of HBV intrauterine transmission among HBsAg-positive pregnant women. J. Viral Hepat. 2013, 20, 317–321. [Google Scholar] [CrossRef]
- Liu, J.; Feng, Y.; Wang, J.; Li, X.; Lei, C.; Jin, D.; Feng, W.; Yang, Y.; He, Y.; Li, Y.; et al. An “immune barrier” is formed in the placenta by hepatitis B immunoglobulin to protect the fetus from hepatitis B virus infection from the mother. Hum. Vaccin. Immunother. 2015, 11, 2068–2076. [Google Scholar] [CrossRef]
- Wong, V.C.; Ip, H.M.; Reesink, H.W.; Lelie, P.N.; Reerink-Brongers, E.E.; Yeung, C.Y.; Ma, H.K. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin. Double-blind randomised placebo-controlled study. Lancet 1984, 1, 921–926. [Google Scholar] [CrossRef]
- Chang, M.H.; You, S.L.; Chen, C.J.; Liu, C.J.; Lai, M.W.; Wu, T.C.; Wu, S.F.; Lee, C.M.; Yang, S.S.; Chu, H.C.; et al. Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology 2016, 151, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C.; Jia, Z.F.; Wu, X.; Wen, S.M.; Kong, F.; Hu, K.Q.; Li, J.; Jiang, J.; Niu, J.Q. Protective effect of an improved immunization practice of mother-to-infant transmission of hepatitis B virus and risk factors associated with immunoprophylaxis failure. Medicine 2016, 95, e4390. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.M.; Sung, J.; Yang, S.; Choe, Y.H.; Chang, Y.S.; Park, W.S. Factors associated with immunoprophylaxis failure against vertical transmission of hepatitis B virus. Eur. J. Pediatr. 2007, 166, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Bzowej, N.H. Optimal Management of the Hepatitis B Patient Who Desires Pregnancy or Is Pregnant. Curr. Hepat. Rep. 2012, 11, 82–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ayoub, W.S.; Cohen, E. Hepatitis B Management in the Pregnant Patient: An Update. J. Clin. Transl. Hepatol. 2016, 4, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Q.; Lee, H.M. Antiviral therapy for chronic hepatitis B in pregnancy. Semin. Liver Dis. 2013, 33, 138–146. [Google Scholar] [CrossRef]
- Brown, R.S., Jr.; McMahon, B.J.; Lok, A.S.; Wong, J.B.; Ahmed, A.T.; Mouchli, M.A.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Mohammed, K. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: A systematic review and meta-analysis. Hepatology 2016, 63, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Owusu-Edusei, K., Jr.; Schillie, S.F.; Murphy, T.V. Cost-effectiveness of active-passive prophylaxis and antiviral prophylaxis during pregnancy to prevent perinatal hepatitis B virus infection. Hepatology 2016, 63, 1471–1480. [Google Scholar] [CrossRef]
- Li, X.M.; Yang, Y.B.; Hou, H.Y.; Shi, Z.J.; Shen, H.M.; Teng, B.Q.; Li, A.M.; Shi, M.F.; Zou, L. Interruption of HBV intrauterine transmission: A clinical study. World J. Gastroenterol. 2003, 9, 1501–1503. [Google Scholar] [CrossRef]
- Han, G.R.; Cao, M.K.; Zhao, W.; Jiang, H.X.; Wang, C.M.; Bai, S.F.; Yue, X.; Wang, G.J.; Tang, X.; Fang, Z.X. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J. Hepatol. 2011, 55, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for management of chronic hepatitis B. Clin. Mol. Hepatol. 2019, 25, 93–159. [Google Scholar] [CrossRef]
- Pan, C.Q.; Duan, Z.; Dai, E.; Zhang, S.; Han, G.; Wang, Y.; Zhang, H.; Zou, H.; Zhu, B.; Zhao, W.; et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. N. Engl. J. Med. 2016, 374, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, G.; Ngo-Giang-Huong, N.; Harrison, L.; Decker, L.; Khamduang, W.; Tierney, C.; Salvadori, N.; Cressey, T.R.; Sirirungsi, W.; Achalapong, J.; et al. Tenofovir versus Placebo to Prevent Perinatal Transmission of Hepatitis B. N. Engl. J. Med. 2018, 378, 911–923. [Google Scholar] [CrossRef]
- Hyun, M.H.; Lee, Y.S.; Kim, J.H.; Je, J.H.; Yoo, Y.J.; Yeon, J.E.; Byun, K.S. Systematic review with meta-analysis: The efficacy and safety of tenofovir to prevent mother-to-child transmission of hepatitis B virus. Aliment. Pharmacol. Ther. 2017, 45, 1493–1505. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, H.S.; Kim, J.H.; Chang, S.W.; Hyun, M.H.; Bak, H.; Kim, S.; Lee, M.J.; Lee, C.U.; Jung, Y.K.; et al. Role of tenofovir disoproxil fumarate in prevention of perinatal transmission of hepatitis B virus from mother to child: A systematic review and meta-analysis. Korean J. Intern. Med. 2021, 36, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Funk, A.L.; Lu, Y.; Yoshida, K.; Zhao, T.; Boucheron, P.; van Holten, J.; Chou, R.; Bulterys, M.; Shimakawa, Y. Efficacy and safety of antiviral prophylaxis during pregnancy to prevent mother-to-child transmission of hepatitis B virus: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 70–84. [Google Scholar] [CrossRef]
- Bartholomeusz, A.; Locarnini, S.A. Antiviral drug resistance: Clinical consequences and molecular aspects. Semin. Liver Dis. 2006, 26, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Ayres, A.; Yuen, L.; Jackson, K.M.; Manoharan, S.; Glass, A.; Maley, M.; Yoo, W.; Hong, S.P.; Kim, S.O.; Luciani, F.; et al. Short duration of lamivudine for the prevention of hepatitis B virus transmission in pregnancy: Lack of potency and selection of resistance mutations. J. Viral Hepat. 2014, 21, 809–817. [Google Scholar] [CrossRef]
- Zeng, Q.-L.; Yu, Z.; Wang, F.-S. Tenofovir Alafenamide to prevent perinatal hepatitis B transmission in mothers with high viral load: A multicenter, prospective, observational study. Hepatology 2020, 72 (Suppl. S1), 115A–116A. [Google Scholar]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S. Management of Antiviral Resistance in Chronic Hepatitis B. Gut Liver 2017, 11, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, A.L.; Chan, H.L.; Bhardwaj, N.; Liu, Y.; Marcellin, P.; Pan, C.Q.; Shalimar; Buti, M.; Cox, S.; Parhy, B.; et al. No Resistance to Tenofovir Alafenamide Detected through 96 Weeks of Treatment in Patients with Chronic Hepatitis B Infection. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, Y.; Ma, L.; Li, X.; Schreiber, A. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: A systematic review and meta-analysis. Obstet. Gynecol. 2010, 116, 147–159. [Google Scholar] [CrossRef]
- Njei, B.; Gupta, N.; Ewelukwa, O.; Ditah, I.; Foma, M.; Lim, J.K. Comparative efficacy of antiviral therapy in preventing vertical transmission of hepatitis B: A network meta-analysis. Liver Int. 2016, 36, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, H.W.; Xie, J.X.; Zhang, Q.; Wang, H.Y.; Cao, G.W. A meta-analysis of lamivudine for interruption of mother-to-child transmission of hepatitis B virus. World J. Gastroenterol. 2011, 17, 4321–4333. [Google Scholar] [CrossRef]
- Lu, Y.P.; Liang, X.J.; Xiao, X.M.; Huang, S.M.; Liu, Z.W.; Li, J.; Hocher, B.; Chen, Y.P. Telbivudine during the second and third trimester of pregnancy interrupts HBV intrauterine transmission: A systematic review and meta-analysis. Clin. Lab. 2014, 60, 571–586. [Google Scholar] [CrossRef]
- Liu, M.H.; Sheng, Y.J.; Liu, J.Y.; Hu, H.D.; Zhang, Q.F.; Ren, H. Efficacy of telbivudine on interruption of hepatitis B virus vertical transmission: A meta-analysis. Ann. Saudi Med. 2013, 33, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Zhou, X.; Gao, S.; Yang, S.G.; Wang, B.; Chen, H.Z.; Ruan, B. The effects of telbivudine in late pregnancy to prevent intrauterine transmission of the hepatitis B virus: A systematic review and meta-analysis. Virol. J. 2012, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- The Antiretroviral Pregnancy Registry. Interim Report. 1 January 1989 through 31 July 2020. Available online: http://www.apregistry.com/forms/interim_report.pdf (accessed on 5 May 2021).
- Lorenzi, P.; Spicher, V.M.; Laubereau, B.; Hirschel, B.; Kind, C.; Rudin, C.; Irion, O.; Kaiser, L. Antiretroviral therapies in pregnancy: Maternal, fetal and neonatal effects. Swiss HIV Cohort Study, the Swiss Collaborative HIV and Pregnancy Study, and the Swiss Neonatal HIV Study. AIDS 1998, 12, F241–F247. [Google Scholar] [CrossRef] [PubMed]
- Blanche, S.; Tardieu, M.; Rustin, P.; Slama, A.; Barret, B.; Firtion, G.; Ciraru-Vigneron, N.; Lacroix, C.; Rouzioux, C.; Mandelbrot, L.; et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet 1999, 354, 1084–1089. [Google Scholar] [CrossRef]
- Liang, L.Y.; Wong, G.L. Unmet need in chronic hepatitis B management. Clin. Mol. Hepatol. 2019, 25, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Siberry, G.K.; Jacobson, D.L.; Kalkwarf, H.J.; Wu, J.W.; DiMeglio, L.A.; Yogev, R.; Knapp, K.M.; Wheeler, J.J.; Butler, L.; Hazra, R.; et al. Lower Newborn Bone Mineral Content Associated With Maternal Use of Tenofovir Disoproxil Fumarate During Pregnancy. Clin. Infect. Dis. 2015, 61, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Kinai, E.; Hosokawa, S.; Gomibuchi, H.; Gatanaga, H.; Kikuchi, Y.; Oka, S. Blunted fetal growth by tenofovir in late pregnancy. AIDS 2012, 26, 2119–2120. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Levy, M.T.; Cheung, K.W.; Jourdain, G. Viral hepatitis and pregnancy. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Bzowej, N.H.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Murad, M.H. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016, 63, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, A.A.; Myers, R.P. The outcomes of pregnancy in patients with cirrhosis: A population-based study. Liver Int. 2010, 30, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Mave, V.; Kadam, D.; Kinikar, A.; Gupte, N.; Bhattacharya, D.; Bharadwaj, R.; McIntire, K.; Kulkarni, V.; Balasubramanian, U.; Suryavanshi, N.; et al. Impact of maternal hepatitis B virus coinfection on mother-to-child transmission of HIV. HIV Med. 2014, 15, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Guleria, I.; Sayegh, M.H. Maternal acceptance of the fetus: True human tolerance. J. Immunol. 2007, 178, 3345–3351. [Google Scholar] [CrossRef]
- ter Borg, M.J.; Leemans, W.F.; de Man, R.A.; Janssen, H.L. Exacerbation of chronic hepatitis B infection after delivery. J. Viral Hepat. 2008, 15, 37–41. [Google Scholar] [CrossRef]
- Nguyen, V.; Tan, P.K.; Greenup, A.J.; Glass, A.; Davison, S.; Samarasinghe, D.; Holdaway, S.; Strasser, S.I.; Chatterjee, U.; Jackson, K.; et al. Anti-viral therapy for prevention of perinatal HBV transmission: Extending therapy beyond birth does not protect against post-partum flare. Aliment. Pharmacol. Ther. 2014, 39, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.; Chen, C.J.; Chen, D.S.; Chen, H.L.; Chen, P.J.; Chien, R.N.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [CrossRef]
- Fontana, R.J. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology 2009, 49, S185–S195. [Google Scholar] [CrossRef]
- Pernia, S.; DeMaagd, G. The New Pregnancy and Lactation Labeling Rule. Phys. Ther. 2016, 41, 713–715. [Google Scholar]
- Hu, X.; Wang, L.; Xu, F. Guides concerning tenofovir exposure via breastfeeding: A comparison of drug dosages by developmental stage. Int. J. Infect. Dis. 2019, 87, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kourtis, A.P.; Ellington, S.; Legardy-Williams, J.; Bulterys, M. Safety of tenofovir during pregnancy for the mother and fetus: A systematic review. Clin. Infect. Dis. 2013, 57, 1773–1781. [Google Scholar] [CrossRef]
- Wen, W.H.; Chen, H.L.; Shih, T.T.; Wu, J.F.; Ni, Y.H.; Lee, C.N.; Zhao, L.L.; Lai, M.W.; Mu, S.C.; Tung, Y.C.; et al. Long-term growth and bone development in children of HBV-infected mothers with and without fetal exposure to tenofovir disoproxil fumarate. J. Hepatol. 2020, 72, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.J.; Kim, J.H.; Park, J.Y.; Yoon, E.L.; Park, H.; Kwon, J.H.; Sinn, D.H.; Lee, S.H.; Lee, J.H.; Lee, H.W. Comparison of clinical practice guidelines for the management of chronic hepatitis B: When to start, when to change, and when to stop. Clin. Mol. Hepatol. 2020, 26, 411–429. [Google Scholar] [CrossRef]
- Schillie, S.; Murphy, T.V.; Fenlon, N.; Ko, S.; Ward, J.W. Update: Shortened Interval for Postvaccination Serologic Testing of Infants Born to Hepatitis B-Infected Mothers. Morb. Mortal. Wkly. Rep. 2015, 64, 1118–1120. [Google Scholar] [CrossRef]
- Indolfi, G.; Easterbrook, P.; Dusheiko, G.; Siberry, G.; Chang, M.H.; Thorne, C.; Bulterys, M.; Chan, P.L.; El-Sayed, M.H.; Giaquinto, C.; et al. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol. Hepatol. 2019, 4, 466–476. [Google Scholar] [CrossRef]
- Tajiri, H.; Takano, T.; Tanaka, H.; Ushijima, K.; Inui, A.; Miyoshi, Y.; Ozono, K.; Abukawa, D.; Endo, T.; Brooks, S.; et al. Hepatocellular carcinoma in children and young patients with chronic HBV infection and the usefulness of alpha-fetoprotein assessment. Cancer Med. 2016, 5, 3102–3110. [Google Scholar] [CrossRef]
- Michie, C.A.; Gilmour, J. Breast feeding and the risks of viral transmission. Arch. Dis. Child. 2001, 84, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Gonzalez, L.S., 3rd; Barnhart, D.J. Medications in the breast-feeding mother. Am. Fam. Physician 2001, 64, 119–126. [Google Scholar] [PubMed]
- Benaboud, S.; Pruvost, A.; Coffie, P.A.; Ekouévi, D.K.; Urien, S.; Arrivé, E.; Blanche, S.; Théodoro, F.; Avit, D.; Dabis, F.; et al. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d’Ivoire, in the ANRS 12109 TEmAA Study, Step 2. Antimicrob. Agents Chemother. 2011, 55, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Mirochnick, M.; Taha, T.; Kreitchmann, R.; Nielsen-Saines, K.; Kumwenda, N.; Joao, E.; Pinto, J.; Santos, B.; Parsons, T.; Kearney, B.; et al. Pharmacokinetics and safety of tenofovir in HIV-infected women during labor and their infants during the first week of life. J. Acquir. Immune Defic. Syndr. 2014, 65, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Mugwanya, K.K.; Hendrix, C.W.; Mugo, N.R.; Marzinke, M.; Katabira, E.T.; Ngure, K.; Semiyaga, N.B.; John-Stewart, G.; Muwonge, T.R.; Muthuri, G.; et al. Pre-exposure Prophylaxis Use by Breastfeeding HIV-Uninfected Women: A Prospective Short-Term Study of Antiretroviral Excretion in Breast Milk and Infant Absorption. PLoS Med. 2016, 13, e1002132. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yang, Y.; Wang, H.; Ma, L.; Schreiber, A.; Li, X.; Sun, W.; Zhao, X.; Yang, X.; Zhang, L.; et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: A meta-analysis and systematic review. Arch. Pediatr. Adolesc. Med. 2011, 165, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Eke, A.C.; Brooks, K.M.; Gebreyohannes, R.D.; Sheffield, J.S.; Dooley, K.E.; Mirochnick, M. Tenofovir alafenamide use in pregnant and lactating women living with HIV. Expert Opin. Drug Metab. Toxicol. 2020, 16, 333–342. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).