Cardiopulmonary Exercise Test in Patients with Hypertrophic Cardiomyopathy: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Experimental Section
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Extraction and Synthesis
2.4. Statistical Analyses
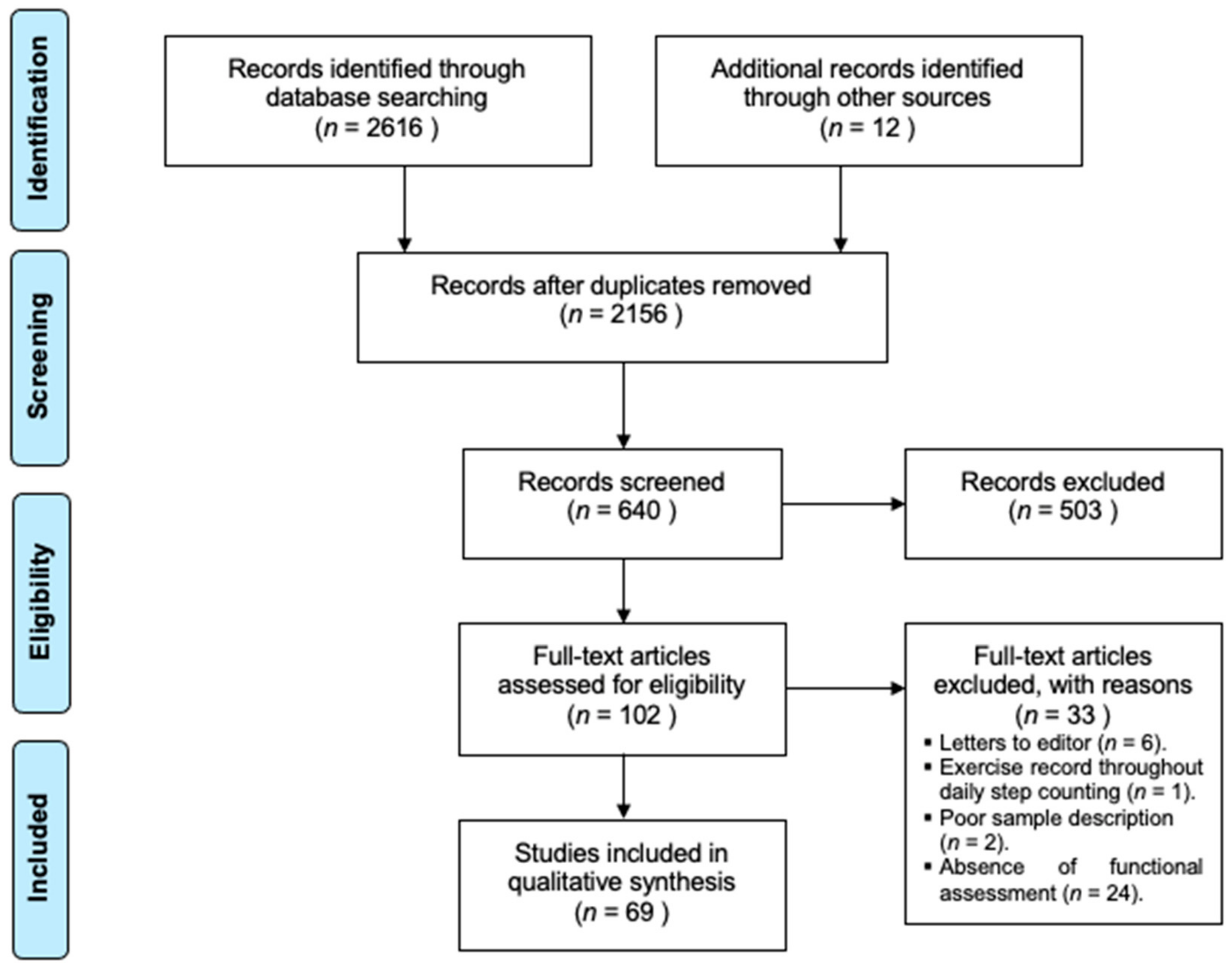
3. Results
3.1. Study Sample
| Author, Year | n | Age (Years) | %W | CPETMaterial | CPET Intensity | Drug Therapy | Extracardiac Variables | Cardiac Variables Measured | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Added | Lapse | LVOT | MR | PCWP | ABPRE | Events | |||||||
| Abozguia, 2010 [14] | 56 | 52 ± 11 | 29 | Treadmill | 4.7 M | 2–3 M | 3′ | Not controlled | - | Out | - | - | - | - |
| Aljaroudi, 2012 [15] | 495 | 50 ± 15 | 32 | Treadmill | 4.7 M | 2–3 M | 3′ | Disc. (12 h) | DM, HL | BL | BL | - | - | Out |
| Arena, 2008 [16] | 83 | 38 ± 10 | 39 | Upright CE | 15 W | 15 W | 1′ | Continued | - | Out | Out | BL, Ex | - | - |
| Austin, 2010 [17] | 50 | 44 ± 13 | 38 | Treadmill | - | - | - | Continued | - | BL, Ex | BL | - | Ex | - |
| Axelsson, 2016 [18] | 130 | 52 ± 13 | 35 | Upright CE | 25 W | 25 W | 2′ | Continued | - | BL | - | - | - | BL |
| Azarbal, 2014 [19] | 265 | 52 ± 15 | 39 | Treadmill | 2 mph | - | - | Continued | DM, HL | BL | BL | - | Ex | - |
| Binder, 2007 [20] | 217 | 56 ± 16 | 41 | - | - | - | - | Not registered | Creatinine | BL | BL | - | - | - |
| Bonow, 1985 [21] | 70 | 47 | 47 | Treadmill | 2 mph | 2.5% | 2′ | Administered | - | BL | - | - | - | Ex |
| Briguori, 1999 [22] | 52 | 41 | 29 | Upright CE | 60 rpm | 1 W | 3″ | Disc. (5H-L) | - | BL | BL | - | Ex | Ex |
| Briguori, 2001 [23] | 44 | 40 ± 15 | 27 | (?) CE | 60 rpm | 1 W | 3″ | Disc. (5H-L) | Blood analysis | BL | BL | - | - | Ex |
| Chan, 1990 [24] | 13 | 42 ± 14 | 38 | Upright CE | 28 W | 22 W | 3′ | Administered | - | BL | - | BL | - | Out |
| Chikamori, 1992 [25] | 81 | 41 | 46 | Treadmill | 4.7 M | 2–3 M | 3′ | Disc. (?) | - | BL | Out | - | Out | Out |
| Choi, 2008 [26] | 32 | 55 ± 11 | 19 | Supine CE | 25 W | 25 W | 3′ | Disc. (?) | Creatinine, HL | BL | Out | - | - | - |
| Ciampi, 2007 [27] | 22 | 36 ± 13 | 23 | Treadmill | 2.7 M | 2–3 M | 3′ | Disc. (5H-L) | - | BL | - | - | Ex | Ex |
| Coats, 2014 [28] | 1898 | 47 ± 15 | 33 | Upright CE | 0 W | 10–30W | 1′ | Continued | - | BL | BL | - | Ex | Ex |
| D’Andrea, 2017 [29] | 45 | 38 ± 15 | 22 | Supine CE | - | 25 W | 2′ | No drugs used | - | BL | - | BL, Ex | - | - |
| de la Morena, 2003 [30] | 98 | 44 ± 15 | 28 | Treadmill | 4.7 M | 2–3 M | 3′ | Disc. (48 h) | - | BL | BL | - | Ex | Ex |
| de la Morena, 2013 [31] | 87 | 54 ± 13 | 33 | Treadmill | 4.7 M | 2–3 M | 3′ | Not controlled | - | BL, Ex | BL, Ex | - | Ex | Ex |
| Desai, 2014 [32] | 426 | 44 ± 14 | 22 | Treadmill | 4.7 M | 2–3 M | 3′ | Continued | DM | BL, Ex | BL, Ex | - | Ex | Ex |
| Dimitrow, 1996 [33] | 10 | 37 ± 7 | - | Treadmill | 2.7 M | 2–3 M | 3′ | Administered | DM, HL | BL | - | - | - | - |
| Dumont, 2007 [34] | 64 | 51 ± 14 | 33 | Treadmill | 4.7 M | 2–3 M | 3′ | Disc. (72 h) | - | BL | - | - | Ex | BL |
| Efthimiadis, 2011 [35] | 68 | 45 ± 15 | 34 | Treadmill | 4.7 M | 2–3 M | 3′ | Not controlled | - | BL | - | - | Ex | BL |
| Ferguson, 2016 [36] | 22 | 14 ± 3 | 44 | Upright CE | 25 W | 25 W | 3′ | Continued | - | BL, Ex | - | - | - | - |
| Finocchiaro, 2015 [37] | 156 | 51 ± 14 | 38 | Treadmill | - | - | - | Continued | - | BL | BL | - | - | - |
| Frenneaux, 1989 [38] | 23 | 30 | 39 | Treadmill | 2.7 M | 2–3 M | 3′ | Continued | - | BL | Out | BL, Ex | - | BL |
| Ghiselli, 2019 [39] | 292 | 46 ± 16 | 28 | Semisup CE | - | 25 W | 2′ | Continued | DM | BL, Ex | BL, Ex | - | Ex | BL, Ex |
| Ha, 2005 [40] | 29 | 57 ± 10 | 16 | Supine CE | 25 W | 25 W | 3′ | Disc. (?) | DM | - | - | - | - | - |
| Hernández, 2015 [41] | 40 | 55 ± 12 | 36 | Treadmill | 2.7 M | 2–3 M | 3′ | Not registered | DM | BL | - | - | - | - |
| Jones, 1998 [42] | 50 | 35 ± 14 | 30 | Upright CE | 0 W | 5–15 W | 1′ | Disc. (48 h) | - | BL | - | - | Out | Ex |
| Kim, 2004 [43] | 21 | 49 ± 15 | 43 | Treadmill | 4.7 M | 2–3 M | 3′ | Administered | - | BL | - | - | - | - |
| Kitaoka, 2009 [44] | 31 | 52 ± 17 | 35 | Upright CE | 0 W | 15 W | 1′ | Continued | - | BL | - | - | Ex | - |
| Kjaergaard, 2005 [45] | 99 | 49 ± 15 | 34 | Treadmill | 2 mph | 2 M | 2′ | Continued | - | BL | BL | Out | - | - |
| Konecny, 2015 [46] | 198 | 53 ± 16 | 38 | Treadmill | 2.5 M | 2,5 M | 2′ | Continued | DM, ∑skinfold | BL | BL | - | - | - |
| Lafitte, 2013 [47] | 107 | 52 ± 15 | 33 | Semisup CE | - | - | - | Continued | - | BL, Ex | BL | - | - | Ex |
| Larsen, 2018 [48] | 510 | 51 ± 15 | 36 | Treadmill | - | 2 M | 2′ | Disc. (?) | - | BL | BL | - | - | BL |
| Le, 2009 [49] | 63 | 48 ± 15 | 28 | Treadmill | - | - | - | Continued | DM, HL | BL, Ex | BL, Ex | - | Ex | Ex |
| Lele, 1995 [50] | 23 | 30 | 39 | SupC + Tread | 25 W | 12.5 W | 3′ | Discont. (120 h) | - | BL | BL | BL, Ex | Ex | BL, Ex |
| Lösse, 1983 [51] | 122 | 42 ± 3 | - | Supine CE | - | - | - | Administered | - | BL, Ex | - | BL, Ex | Ex | - |
| Luo, 2015 [52] | 273 | 50 ± 15 | 30 | Treadmill | 2.7 M | 2–3 M | 3′ | Not registered | - | BL, Ex | - | - | Ex | Ex |
| Ma, 2014 [53] | 27 | 54 ± 12 | 42 | Treadmill | 2.7 M | 2–3 M | 3′ | Not registered | DM | BL, Out | BL, Out | - | - | - |
| Magri, 2014 [54] | 180 | 47 ± 16 | 25 | Upright CE | 0 W | - | - | Continued | - | BL | - | - | - | - |
| Magri, 2016 [55] | 683 | 49 ± 16 | 31 | Supine CE | - | - | - | Continued | DM | BL | - | - | Ex | Ex |
| Magri, 2018 [56] | 767 | 48 ± 16 | 32 | (?) CE | 0 W | 5–15 W | - | Not controlled | - | BL | - | - | Ex | BL, Ex |
| Malek, 2009 [57] | 13 | - | - | Treadmill | - | - | - | Not registered | - | - | - | - | - | - |
| Matsumoto, 2005 [58] | 27 | 35 | 33 | Treadmill | - | 0.5% | 1′ | Continued | - | BL, Out | BL | - | - | - |
| Matsumura, 2002 [59] | 85 | 38 ± 14 | 34 | Upright CE | 0 W | 5–15 W | 1′ | Disc. (5H-L) | - | BL | Out | - | - | - |
| Menon, 2008 [60] | 88 | 14 | 25 | Treadmill | 2.7 M | 2–3 M | 3′ | Not registered | - | BL | BL | - | - | - |
| Miki, 1990 [61] | 22 | 47 ± 15 | 18 | Treadmill | 2.7 M | 2–3 M | 3′ | Disc. (120 h) | - | - | - | BL, Ex | - | - |
| Mizukoshi, 2013 [62] | 33 | 59 ± 16 | 27 | Semisup CE | 20 W | 1–2 W | 6″ | Continued | - | Out | - | - | - | Ex |
| Moneghetti, 2017 [63] | 131 | 52 ± 13 | 37 | Treadmill | - | - | - | Not controlled | - | BL | BL | - | Ex | BL |
| Nihoyannopoulos, 1992 [64] | 40 | 41 | 45 | Treadmill | 2.7 M | 2–3 M | 3′ | Continued | - | BL | BL | - | - | - |
| Nistri, 2010 [65] | 74 | 45 ± 16 | 28 | Upright CE | - | 25 W | 2′ | Disc. (5H-L) | - | BL, Ex | BL | - | - | Ex |
| Payá, 2008 [66] | 120 | 47 ± 16 | 30 | Treadmill | 4.7 M | 2–3 M | 3′ | Not registered | - | BL | - | - | Ex | BL |
| Peteiro, 2012 [67] | 239 | 52 ± 15 | 39 | Treadmill | 2.7 M | 2–3 M | 3′ | Continued | DM, HL | BL, Ex | BL, Ex | - | Ex | BL, Ex |
| Pozios, 2018 [68] | 95 | 49 ± 16 | 31 | Treadmill | 2.7 M | 2–3 M | 3′ | Continued | DM, HL | BL, Ex | - | - | - | Ex |
| Re, 2017 [69] | 197 | 45 ± 15 | 35 | Upright CE | - | 10W | 1′ | Disc. (72 h) | - | BL, Ex | BL | - | Ex | Out |
| Romero, 2008 [70] | 98 | 46 ± 15 | 29 | Treadmill | 2.7 M | 2–3 M | 3′ | Not registered | - | BL | - | - | Ex | BL |
| Rosing, 1979 [71] | 27 | 44 ± 3 | 41 | Treadmill | 2 mph | 2.5% | 2′ | Administered | - | BL | - | BL | - | - |
| Sachdev, 2005 [72] | 43 | 36 ± 10 | 40 | Upright CE | - | 15 W | - | Not registered | - | Out | BL | BL, Ex | - | - |
| Saura, 2009 [73] | 75 | 46 ± 14 | 25 | Treadmill | 4.7 M | 2–3 M | 3′ | Disc. (48 h) | DM | BL | - | - | Ex | BL |
| Shah, 2019 [74] | 9 | 29 ± 8 | 0 | Upright CE | 0 W | 20 W | 1′ | Continued | Blood analysis | Out | - | - | - | Ex |
| Shizukuda, 2005 [75] | 49 | 36 ± 10 | 41 | Upright CE | - | 5 W | - | Not registered | - | Out | - | BL, Ex | - | - |
| Smith, 2018 [76] | 589 | 51 ± 14 | 39 | Treadmill | - | - | - | Continued | - | BL | - | - | - | - |
| Smith, 2018 [77] | 1177 | 53 ± 14 | 43 | Treadmill | - | 2 M | 2′ | Continued | DM, HL, Hb | BL | - | - | Ex | - |
| Thaman, 2006 [78] | 171 | 46 ± 18 | 63 | Upright CE | - | 10–25W | 1′ | Continued | - | BL | - | - | - | BL |
| Wu, 2019 [79] | 67 | 53 ± 11 | 12 | Semisup CE | 25 W | 25 W | 2′ | Disc. (24 h) | - | Out | - | - | - | - |
| Wu, 2019 [80] | 88 | 52 ± 12 | 14 | Semisup CE | 25 W | 25 W | 2′ | Disc. (24 h) | - | BL | - | - | - | - |
| Wu, 2019 [81] | 76 | 48 ± 12 | 20 | Semisup CE | 25 W | 25 W | 2′ | Disc. (24 h) | - | BL, Ex | - | Ex | Ex | - |
| Yetman, 2001 [82] | 17 | 12 ± 3 | 30 | Treadmill | 4.7 M | 2–3 M | 3′ | Not controlled | - | BL, Ex | BL | - | - | - |
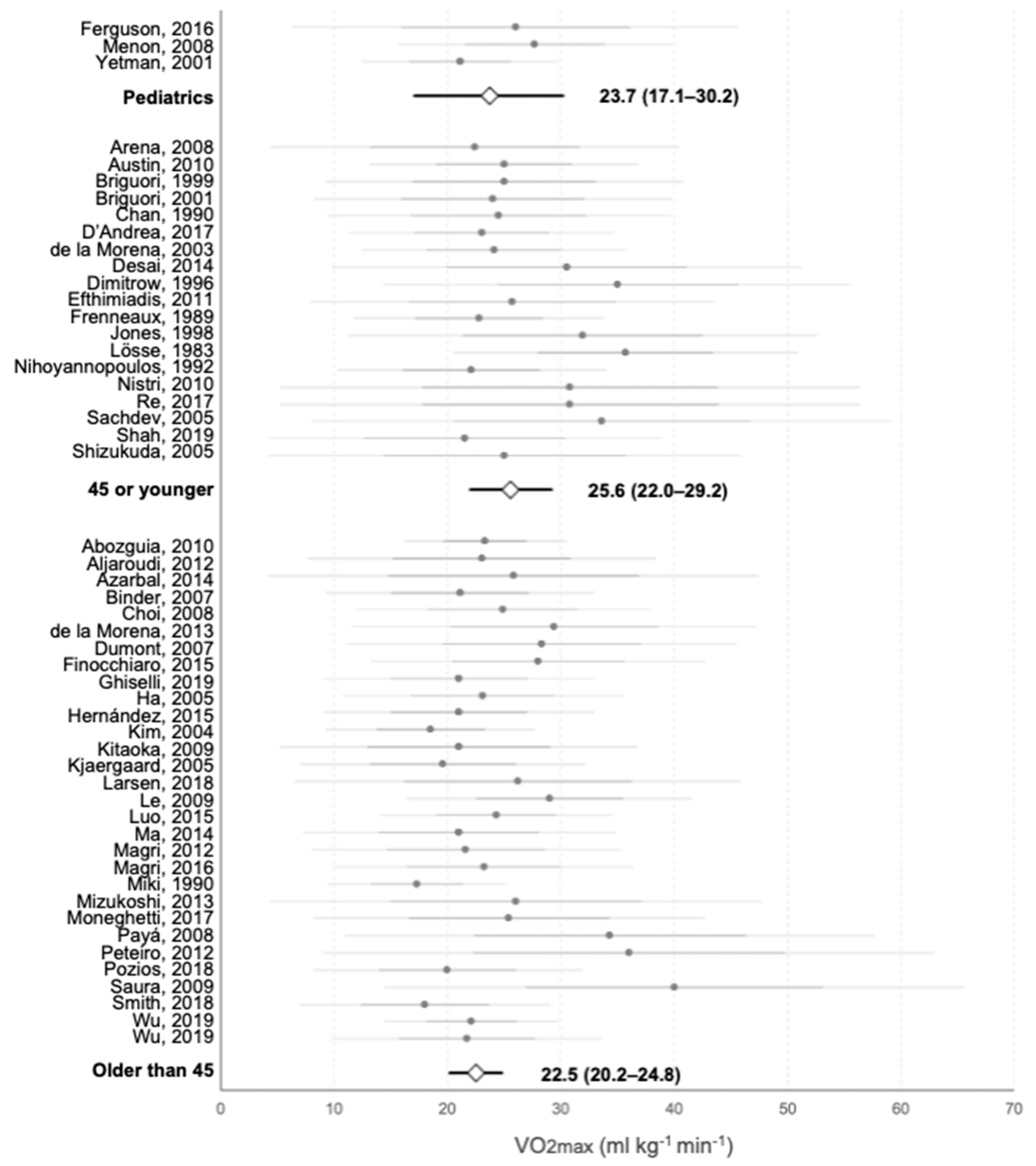
3.2. Maximal Oxygen Consumption
3.3. Cardiac Variables
3.4. Analysis of Subgroups
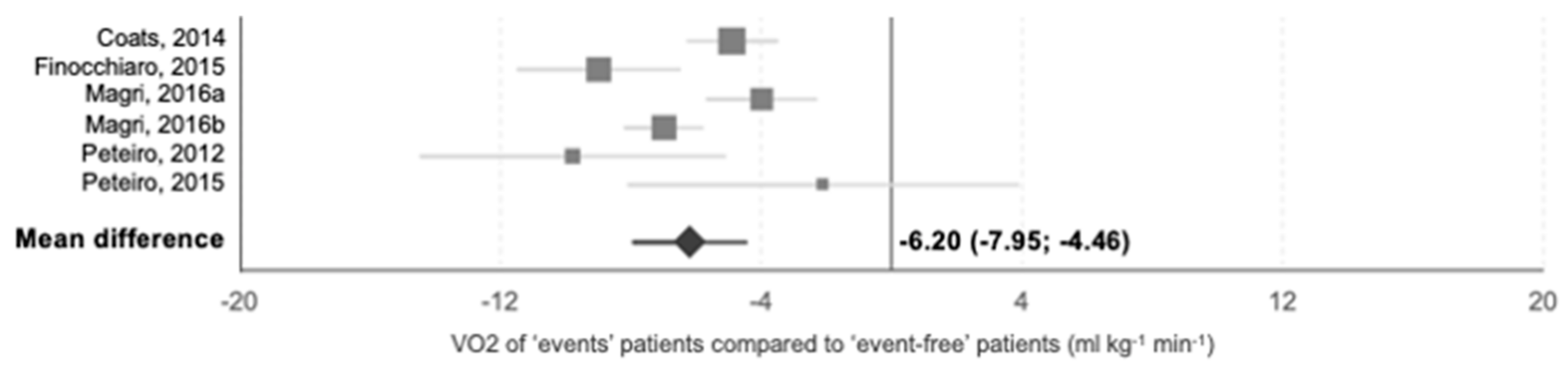
3.5. Prognostic Studies
| Author, Year | n | Age | %W | F-up | Drug Therapy | Prospective Outcomes (Cases) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCD | ICD | HFm | Res | Str | HT | CVm | All | ||||||
| Caselli, 2014 [85] | 25 | 26 | 0 | 4.0 | Not controlled | 0 | - | 0 | - | - | - | 0 | 0 |
| Coats, 2014 [28] | 1898 | 47 | 33 | 5.6 | Continued | 40 | - | 31 | - | - | 22 | 117 | 178 |
| Desai, 2014 [32] | 426 | 44 | 22 | 8.7 | Continued | 30 | 13 | 7 | 0 | 8 | - | 37 | 40 |
| Feneon, 2016 [86] | 126 | 47 | 22 | 2.4 | Continued | - | 1 | - | 3 | - | - | 7 | - |
| Finocchiaro, 2015 [37] | 156 | 51 | 38 | 2.3 | Continued | - | - | - | - | - | 5 | 4 | - |
| Ghiselli, 2019 [39] | 292 | 46 | 28 | 5.9 | Continued | 1 | - | - | 1 | 13 | - | 6 | - |
| Gimeno, 2009 [87] | 1380 | 42 | 38 | 4.5 | Not controlled | - | - | - | - | - | - | 158 | - |
| Limongelli, 2019 [88] | 51 | 39 | 24 | 1.8 | Not controlled | 0 | - | 0 | - | - | - | 0 | 0 |
| Magri, 2016 [61] | 683 | 49 | 31 | 3.7 | Continued | 25 | 18 | - | 4 | - | - | - | - |
| Magri, 2016 [89] | 620 | 49 | 31 | 3.8 | Not controlled | 25 | 18 | 4 | 3 | 3 | 5 | 36 | 50 |
| Magri, 2018 [56] | 767 | 48 | 32 | 4.2 | Continued | 23 | 14 | 4 | 5 | 3 | 5 | 30 | 30 |
| Masri, 2015 [90] | 1005 | 50 | 36 | 5.5 | Not controlled | - | 17 | - | 2 | 11 | - | - | 126 |
| Michaelides, 2009 [91] | 81 | 42 | 30 | 5.3 | Continued | 8 | 6 | 0 | 6 | - | - | 8 | - |
| Moneghetti, 2017 [63] | 131 | 52 | 37 | 4.7 | Not controlled | - | - | - | - | - | - | - | 6 |
| Nagata, 2003 [92] | 65 | 50 | 23 | 6.3 | Disc. (24 h) | 0 | 0 | - | 0 | - | 0 | 0 | 0 |
| Olivotto, 1999 [93] | 126 | 42 | 29 | 4.7 | Disc. (5H-L) | 3 | - | 6 | - | - | - | 9 | 9 |
| Peteiro, 2012 [67] | 239 | 52 | 39 | 4.1 | Continued | - | 2 | - | - | 1 | 1 | 5 | - |
| Peteiro, 2015 [94] | 148 | 51 | 34 | 7.1 | Continued | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 |
| Pozios, 2018 [68] | 95 | 49 | 31 | 3.4 | Continued | 0 | 18 | 0 | - | - | - | 0 | 0 |
| Reant, 2015 [95] | 115 | 52 | 34 | 1.6 | Continued | 0 | - | 1 | - | - | - | 1 | - |
| Sadoul, 1997 [96] | 161 | 27 | 35 | 3.7 | Disc. (5H-L) | 12 | 2 | 1 | 2 | - | 2 | 15 | 17 |
| Smith, 2018 [76] | 589 | 51 | 39 | 4.3 | Continued | - | - | - | - | - | - | - | - |
| Sorajja, 2012 [97] | 182 | 53 | 35 | 4.0 | Continued | 2 | - | 1 | - | - | - | 10 | 18 |
4. Discussion
4.1. Clinical and Prognostic Value of CPET
4.2. Exercise Protocols
4.3. Extracardiac Factors and Functional Capacity
4.4. Exercise Prescription
4.5. Future Perspectives
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gersh, B.J.; Maron, B.J.; Bonow, R.O.; Dearani, J.A.; Fifer, M.A.; Link, M.S.; Naidu, S.S.; Nishimura, R.A.; Ommen, S.R.; Rakowski, H.; et al. ACCF/AHA guideline 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 2011, 124, 2761–2796. [Google Scholar] [CrossRef] [PubMed]
- Wigle, E.; Sasson, Z.; Henderson, M.; Ruddy, T.; Fulop, J.; Rakowski, H.; Williams, W.G. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog. Cardiovasc. Dis. 1985, 28, 1–83. [Google Scholar] [CrossRef]
- Maron, B.J. Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Circulation 2010, 121, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Argulian, E.; Chaudhry, F.A. Stress testing in patients with hypertrophic cardiomyopathy. Prog. Cardiovasc. Dis. 2012, 54, 477–482. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Zenovich, A.G.; Link, M.S.; Pandian, N.G.; Kuvin, J.T.; Nistri, S.; Cecchi, F.; Udelson, J.E.; Maron, B.J. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 2006, 114, 2232–2239. [Google Scholar] [CrossRef]
- Shah, J.S.; Esteban, M.T.T.; Thaman, R.; Sharma, R.; Mist, B.; Pantazis, A.; Ward, D.; Kohli, S.K.; Page, S.P.; Demetrescu, C. Prevalence of exercise-induced left ventricular outflow tract obstruction in symptomatic patients with non-obstructive hypertrophic cardiomyopathy. Heart 2008, 94, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Patel, U.K.; Yao, S.; Castenada, V.; Isambert, A.; Winson, G.; Chaudhry, F.A.; Sherrid, M.V. Standing and exercise doppler echocardiography in obstructive hypertrophic cardiomyopathy: The range of gradients with upright activity. J. Am. Soc. Echocardiogr. 2011, 24, 75–82. [Google Scholar] [CrossRef]
- Peteiro, J.; Bouzas-Mosquera, A. Exercise echocardiography. World J. Cardiol. 2010, 2, 223–232. [Google Scholar] [CrossRef]
- Sharma, S.; Whyte, G.; Elliott, P.; Padula, M.; Kaushal, R.; Mahon, N.; McKenna, W.J. Electrocardiographic changes in 1000 highly trained junior elite athletes. Br. J. Sports Med. 1999, 33, 319–324. [Google Scholar] [CrossRef]
- Grazioli, G.; Usín, D.; Trucco, E.; Sanz, M.; Montserrat, S.; Vidal, B.; Gutiérrez, J.; Canal, R.; Brugada, J.; Mont, L.; et al. Differentiating hypertrophic cardiomyopathy from athlete’s heart: An electrocardiographic and echocardiographic approach. J. Electrocardiol. 2016, 49, 539–544. [Google Scholar] [CrossRef]
- Wijnhoven, T.M.A.; van Raaij, J.M.A.; Spinelli, A.; Starc, G.; Hassapidou, M.; Spiroski, I.; Rutter, H.; Martos, E.; Rito, A.I.; Hovengen, R.; et al. WHO European Childhood Obesity Surveillance Initiative: Body mass index and level of overweight among 6–9-year-old children from school year 2007/2008 to school year 2009/2010. BMC Public Health 2014, 14, 1–16. [Google Scholar] [CrossRef]
- Jetté, M.; Sidney, K.; Blümchen, G. Metabolic Equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Abozguia, K.; Nallur-Shivu, G.; Phan, T.T.; Ahmed, I.; Kalra, R.; Weaver, R.; McKenna, W.J.; Sanderson, J.E.; Elliott, P.; Frenneaux, M.P. Left ventricular strain and untwist in hypertrophic cardiomyopathy: Relation to exercise capacity. Am. Heart J. 2010, 159, 825–832. [Google Scholar] [CrossRef]
- Aljaroudi, W.A.; Desai, M.Y.; Alraies, M.C.; Thamilarasan, M.; Menon, V.; Rodriguez, L.L.; Smedira, N.; Grimm, R.A.; Lever, H.M.; Jaber, W.A. Relationship between baseline resting diastolic function and exercise capacity in patients with hypertrophic cardiomyopathy undergoing treadmill stress echocardiography: A cohort study. Br. Med. J. 2012, 2, e0022104. [Google Scholar] [CrossRef]
- Arena, R.; Owens, D.S.; Arevalo, J.; Smith, K.; Mohiddin, S.A.; McAreavey, D.; Ulisney, K.L.; Tripodi, D.; Fananapazir, L.; Plehn, J.F. Ventilatory efficiency and resting hemodynamics in hypertrophic cardiomyopathy. Med. Sci. Sport Exerc. 2008, 40, 799–805. [Google Scholar] [CrossRef]
- Austin, B.A.; Popovic, Z.B.; Kwon, D.H.; Thamilarasan, M.; Boonyasirinant, T.; Flamm, S.D.; Lever, H.M.; Desai, M.Y. Aortic stiffness independently predicts exercise capacity in hypertrophic cardiomyopathy: A multimodality imaging study. Heart 2010, 96, 1303–1310. [Google Scholar] [CrossRef]
- Axelsson, A.; Iversen, K.; Vejlstrup, N.; Langhoff, L.; Thomsen, A.; Ho, C.Y.; Havndrup, O.; Kofoed, K.F.; Jensen, M.; Bundgaard, H. Left ventricular volume predicts exercise capacity in hypertrophic cardiomyopathy. Int. J. Cardiol. 2016, 203, 676–678. [Google Scholar] [CrossRef]
- Azarbal, F.; Singh, M.; Finocchiaro, G.; Le, V.V.; Schnittger, I.; Wang, P.; Myers, J.; Ashley, E.; Perez, M. Exercise capacity and paroxysmal atrial fibrillation in patients with hypertrophic cardiomyopathy. Heart 2014, 100, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.; Ommen, S.R.; Chen, H.H.; Ackerman, M.J.; Tajik, A.J.; Jaffe, A.S. Usefulness of brain natriuretic peptide levels in the clinical evaluation of patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2007, 100, 712–714. [Google Scholar] [CrossRef]
- Bonow, R.; Dilsizian, V.; Rosing, D.R.; Maron, B.J.; Bacharach, S.; Green, M. Verapamil-induced improvement in left ventricular diastolic filling and increased exercise tolerance in patients with hypertrophic cardiomyopathy: Short- and long-term effects. Circulation 1985, 72, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Briguori, C.; Betocchi, S.; Romano, M.; Manganelli, F.; Losi, M.A.; Ciampi, Q.; Gottilla, R.; Lombardi, R.; Condorelli, M.; Chiariello, M. Exercise capacity in hypertrophic cardiomyopathy depends on left ventricular diastolic function. Am. J. Cardiol. 1999, 84, 309–315. [Google Scholar] [CrossRef]
- Briguori, C.; Betocchi, S.; Manganelli, F.; Gigante, B.; Losi, M.A.; Ciampi, Q.; Gottilla, R.; Violante, A.; Toccheti, C.G.; Volpe, M.; et al. Determinants and clinical significance of natriuretic peptides in hypertrophic cardiomyopathy. Eur. Heart J. 2001, 22, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.L.; Gilligan, D.M.; Ang, E.L.; Oakley, C.M. Effect of preload change on resting and exercise cardiac performance in hypertrophic cardiomyopathy. Am. J. Cardiol. 1990, 66, 746–751. [Google Scholar] [CrossRef]
- Chikamori, T.; Counihan, P.J.; Doi, Y.L.; Takata, J.; Stewart, J.T.; Frenneaux, M.P.; McKenna, W.J. Mechanisms of exercise limitation in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1992, 19, 507–512. [Google Scholar] [CrossRef]
- Choi, E.Y.; Ha, J.W.; Rim, S.J.; Kim, S.A.; Yoon, S.J.; Shim, C.Y.; Kim, J.M.; Jang, Y.; Chung, N.; Cho, S.Y. Incremental value of left ventricular diastolic function reserve index for predicting exercise capacity in patients with hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2008, 21, 487–492. [Google Scholar] [CrossRef]
- Ciampi, Q.; Betocchi, S.; Losi, M.A.; Ferro, A.; Cuocolo, A.; Lombardi, R.; Villari, B.; Chiariello, M. Abnormal blood-pressure response to exercise and oxygen consumption in patients with hypertrophic cardiomyopathy. J. Nucl. Cardiol. 2007, 14, 869–875. [Google Scholar] [CrossRef]
- Coats, C.; Rantell, K.; Bartnik, A.; Patel, A.; Mist, B.; McKenna, W.J.; Elliott, P.M. Cardiopulmonary Exercise Testing and Prognosis in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2014, 8, 1022–1031. [Google Scholar] [CrossRef]
- D’Andrea, A.; Limongelli, G.; Baldini, L.; Verrengia, M.; Carbone, A.; Di Palma, E.; Vastarella, R.; Masarone, D.; Tagliamonte, G.; Riegler, L.; et al. Exercise speckle-tracking strain imaging demonstrates impaired right ventricular contractile reserve in hypertrophic cardiomyopathy. Int. J. Cardiol. 2017, 227, 209–216. [Google Scholar] [CrossRef]
- De la Morena, G.; Sánchez, R.F.; García, F.J.; González, E.; Figal, D.P.; Soria, F.; Villegas, M.; Ruipérez, J.A.; Valdés, M. Functional assessment of patients with hypertrophic cardiomyopathy by maximal oxygen consumption. Rev. Esp. Cardiol. 2003, 56, 865–872. [Google Scholar]
- De la Morena, G.; Caro, C.; Saura, D.; Marín, F.; Gimeno, J.R.; González, J.; Oliva, M.J.; García-Navarro, M.; López-Cuenca, A.; Espinosa, M.D.; et al. Exercise eco-doppler in hypertrophic cardiomyopathy patients. determinant factors of exercise intolerance. Rev. Esp. Cardiol. 2013, 66, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.Y.; Bhonsale, A.; Patel, P.; Naji, P.; Smedira, N.G.; Thamilarasan, M.; Lytle, B.W.; Lever, H.M. Exercise echocardiography in asymptomatic HCM. JACC Cardiovasc. Imaging 2014, 7, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Dimitrow, P.; Krzanowski, M.; Bodzon, W.; Szczeklik, A.; Dubiel, J.S. Coronary flow reserve and exercise capacity in hypertrophic cardiomyopathy. Heart Vessel. 1996, 11, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Dumont, C.A.; Monserrat, L.; Peteiro, J.; Soler, R.; Rodriguez, E.; Bouzas, A.; Fernández, X.; Pérez, R.; Bouzas, B.; Castro-Beiras, A. Relation of left ventricular chamber stiffness at rest to exercise capacity in hypertrophic cardiomyopathy. Am. J. Cardiol. 2007, 99, 1454–1457. [Google Scholar] [CrossRef]
- Efthimiadis, G.K.; Giannakoulas, G.; Parcharidou, D.G.; Pagourelias, E.D.; Kouidi, E.J.; Spanos, G.; Kamperidis, V.; Gavrielides, S.; Karvounis, H.; Styliadis, I.; et al. Chronotropic incompetence and its relation to exercise intolerance in hypertrophic cardiomyopathy. Int. J. Cardiol. 2011, 153, 179–184. [Google Scholar] [CrossRef]
- Ferguson, M.E.; Sachdeva, R.; Gillespie, S.; Morrow, G.; Border, W. Tissue doppler imaging during exercise stress echocardiography demonstrates a mechanism for impaired exercise performance in children with hypertrophic cardiomyopathy. Electrocardiography 2016, 33, 1718–1725. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Haddad, F.; Knowles, J.; Caleshu, C.; Pavlovic, A.; Homburger, J.; Shmargad, Y.; Sinagra, G.; Magavern, E.; Wong, M.; et al. Cardiopulmonary responses and prognosis in hypertrophic cardiomyopathy: A potential role for comprehensive noninvasive hemodynamic assessment. JACC Heart Fail. 2015, 3, 408–418. [Google Scholar] [CrossRef]
- Frenneaux, M.P.; Porter, A.; Caforio, A.L.; Odawara, H.; Counihan, P.J.; McKenna, W.J. Determinants of exercise capacity in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1989, 13, 1521–1526. [Google Scholar] [CrossRef]
- Ghiselli, L.; Marchi, A.; Fumagalli, C.; Maurizi, N.; Oddo, A.; Pieri, F.; Girolami, F.; Rowin, E.; Mazzarotto, F.; Cicoira, M.; et al. Sex-related differences in exercise performance and outcome of patients with hypertrophic cardiomyopathy. Eur. J. Prev. Cardiol. 2019, 27, 1821–1831. [Google Scholar] [CrossRef]
- Ha, J.W.; Cho, J.R.; Kim, J.M.; Ahn, J.A.; Choi, E.Y.; Kang, S.M.; Rim, S.J.; Chung, N. Tissue doppler-derived indices predict exercise capacity in patients with apical hypertrophic cardiomyopathy. Chest 2005, 128, 3428–3433. [Google Scholar] [CrossRef]
- Hernández-Romero, D.; Jover, E.; Martínez, C.M.; Andreu-Cayuelas, J.M.; Orenes-Piñero, E.; Romero-Aniorte, A.I.; Casas, T.; Cánovas, S.; Montero-Argudo, J.A.; Valdés, M.; et al. TWEAK and NT-proBNP levels predict exercise capacity in hypertrophic cardiomyopathy. Eur. J. Clin. Investig. 2015, 45, 179–186. [Google Scholar] [CrossRef]
- Jones, S.; Elliott, P.M.; Sharma, S.; McKenna, W.J.; Whipp, B.J. Cardiopulmonary responses to exercise in patients with hypertrophic cardiomyopathy. Heart 1998, 80, 60–67. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, Y.J.; Sohn, D.W.; Park, Y.B.; Choi, Y.S. Transthoracic echocardiographic evaluation of coronary flow reserve in patients with hypertrophic cardiomyopathy. Int. J. Cardiol. 2004, 94, 167–171. [Google Scholar] [CrossRef]
- Kitaoka, H.; Kubo, T.; Okawa, M.; Hirota, T.; Hayato, K.; Yamakasi, N.; Matsumura, Y.; Doi, Y.L. Utility of tissue doppler imaging to predict exercise capacity in hypertrophic cardiomyopathy: Comparison with B-type natriuretic peptide. J. Cardiol. 2009, 53, 361–367. [Google Scholar] [CrossRef]
- Kjaergaard, J.; Johnson, B.D.; Pellikka, P.A.; Cha, S.S.; Oh, J.K.; Ommen, S.R. Left atrial index is a predictor of exercise capacity in patients with hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2005, 18, 1373–1380. [Google Scholar] [CrossRef]
- Konecny, T.; Geske, J.B.; Ludka, O.; Orban, M.; Brady, P.A.; Abudiab, M.M.; Albuquerque, F.N.; Placek, A.; Kara, T.; Sahakyan, K.R.; et al. Decreased exercise capacity and sleep-disordered breathing in patients with hypertrophic cardiomyopathy. Chest 2015, 147, 1574–1581. [Google Scholar] [CrossRef]
- Lafitte, S.; Reant, P.; Touche, C.; Pillois, X.; Dijos, M.; Arsac, F.; Peyrou, J.; Montaudon, M.; Ritter, P.; Roudaut, R.; et al. Paradoxical response to exercise in asymptomatic hypertrophic cardiomyopathy: A new description of outflow tract obstruction dynamics. J. Am. Coll. Cardiol. 2013, 62, 842–850. [Google Scholar] [CrossRef]
- Larsen, C.M.; Ball, C.A.; Hebl, V.B.; Ong, K.C.; Siontis, K.C.; Olson, T.P.; Ackerman, M.J.; Ommen, S.R.; Allison, T.G.; Geske, J.B. Effect of body mass index on exercise capacity in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2018, 121, 100–106. [Google Scholar] [CrossRef]
- Le, V.V.; Perez, M.V.; Wheeler, M.T.; Myers, J.; Schnittger, I.; Ashley, E.A. mechanisms of exercise intolerance in patients with hypertrophic cardiomyopathy. Am. Heart J. 2009, 158, e27–e34. [Google Scholar] [CrossRef] [PubMed]
- Lele, S.; Thomson, H.; Seo, H.; Belenkie, I.; McKenna, W.J.; Frenneaux, M.P. Exercise capacity in hypertrophic cardiomyopathy role of stroke volume limitation, heart rate, and diastolic filling characteristics. Circulation 1995, 92, 2886–2894. [Google Scholar] [CrossRef]
- Lösse, B.; Kuhn, H.; Loogen, F.; Schulte, H.D. Exercise performance in hypertrophic cardiomyopathies. Eur. Heart J. 1983, 4, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.C.; Dimaano, V.L.; Kembro, J.M.; Hilser, A.; Hurtado-de-Mendoza, D.; Pozios, I.; Tomas, M.S.; Yalcin, H.; Dolores-Cerna, K.; Mormontoy, W.; et al. Exercise heart rates in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2015, 115, 1144–1150. [Google Scholar] [CrossRef]
- Ma, G.; Xu, M.; Gao, W.; Li, Z.; Li, W.; Chen, B.; Feng, J.; Wang, H.; Ma, W.; Chen, H.; et al. Left ventricular filling pressure assessed by exercise TDI was correlated with early HFNEF in patients with non-obstructive hypertrophic cardiomyopathy. BMC Cardiovasc. Disord. 2014, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Magri, D.; Agostoni, P.; Cauti, F.M.; Musumeci, B.; Assenza, G.E.; De Cecco, C.N.; Muscogiuri, G.; Maruotti, A.; Ricotta, A.; Pagannone, E.; et al. Determinants of peak oxygen uptake in patients with hypertrophic cardiomyopathy: A single-center study. Intern. Emerg. Med. 2014, 9, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Magrì, D.; Limongelli, G.; Re, F.; Agostoni, P.; Zachara, E.; Correale, M.; Mastromarino, V.; Santolamazza, C.; Casenghi, M.; Pacileo, G.; et al. Cardiopulmonary exercise test and sudden cardiac death risk in hypertrophic cardiomyopathy. Heart 2016, 102, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Magri, D.; Agostoni, P.; Sinagra, G.; Re, F.; Correale, M.; Limongelli, G.; Zachara, E.; Mastromarino, V.; Santolamazza, C.; Casenghi, M.; et al. Clinical and prognostic impact of chronotropic incompetence in patients with hypertrophic cardiomyopathy. Int. J. Cardiol. 2018, 15, 125–131. [Google Scholar] [CrossRef]
- Malek, L.A.; Chojnowska, L.; Klopotowski, M.; Misko, J.; Dabrowski, M.; Kusmierczyk-Droszcz, B.; Maczynska, R.; Piotrowicz, E.; Ruzyllo, W. Left ventricular diastolic function assessed with cardiovascular magnetic resonance imaging and exercise capacity in patients with non-obstructive hypertrophic cardiomyopathy. Kardiol. Pol. 2009, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.Y.; Arteaga, E.; Ianni, B.M.; Braga, A.M.F.W.; Buck, P.C.; Mady, C. Relationships among exercise capacity, hypertrophy, and left ventricular diastolic function in nonobstructive hypertrophic cardiomyopathy. Am. Heart J. 2005, 150, 144–149. [Google Scholar] [CrossRef]
- Matsumura, Y.; Elliott, P.M.; Virdee, M.S.; Sorajja, P.; Doi, Y.; McKenna, W.J. Left ventricular diastolic function assessed using doppler tissue imaging in patients with hypertrophic cardiomyopathy: Relation to symptoms and exercise capacity. Heart 2002, 87, 247–251. [Google Scholar] [CrossRef]
- Menon, S.C.; Ackerman, M.J.; Cetta, F.; O’Leary, P.W.; Eiden, B.W. Significance of left atrial volume in patients <20 years of age with hypertrophic cardiomyopathy. Am. J. Cardiol. 2008, 102, 1390–1393. [Google Scholar] [CrossRef]
- Miki, T.; Yokota, Y.; Fukuzaki, H. Afterload mismatch in patients with hypertrophic cardiomyopathy. Jpn. Circ. J. 1990, 54, 603–615. [Google Scholar] [CrossRef]
- Mizukoshi, K.; Suzuki, K.; Yoneyama, K.; Kamijima, R.; Kou, S.; Takai, M.; Izumo, M.; Hayashi, A.; Ohtaki, E.; Akashi, Y.J.; et al. Early diastolic function during exertion influences exercise intolerance in patients with hypertrophic cardiomyopathy. J. Echocardiogr. 2013, 11, 9–17. [Google Scholar] [CrossRef]
- Moneghetti, K.; Stolfo, D.; Christle, J.W.; Kobayashi, Y.; Finocchiaro, G.; Sinagra, G.; Myers, J.; Ashley, E.A.; Haddad, F.; Wheeler, M. Value of strain imaging and maximal oxygen consumption in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2017, 120, 1203–1208. [Google Scholar] [CrossRef]
- Nihoyannopoulos, P.; Karatasakis, G.; Frenneaux, M.P.; McKenna, W.J.; Oakley, C.M. Diastolic function in hypertrophic cardiomyopathy: Relation to exercise capacity. J. Am. Coll. Cardiol. 1992, 19, 536–540. [Google Scholar] [CrossRef]
- Nistri, S.; Olivotto, I.; Maron, M.S.; Grifoni, C.; Baldini, K.; Baldi, M.; Sgalambro, A.; Cecchi, F.; Maron, B.J. Timing and significance of exercise-induced left ventricular outflow tract pressure gradients in hypertrophic cardiomyopathy. Am. J. Cardiol. 2010, 106, 1301–1306. [Google Scholar] [CrossRef]
- Payá, E.; Marín, F.; González, J.; Gimeno, J.R.; Feliu, E.; Romero, A.; Ruiz-Espejo, F.; Roldán, V.; Climent, V.; de la Morena, G.; et al. Variables associated with contrast-enhanced cardiovascular magnetic resonance in hypertrophic cardiomyopathy: Clinical implications. J. Card. Fail. 2008, 14, 414–419. [Google Scholar] [CrossRef]
- Peteiro, J.; Bouzas-Mosquera, A.; Fernández, X.; Monserrat, L.; Pazos, P.; Estevez-Loureiro, R.; Castro-Beiras, A. Prognostic value of exercise echocardiography in patients with hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2012, 25, 182–189. [Google Scholar] [CrossRef]
- Pozios, I.; Pinheiro, A.; Corona-Villalobos, C.; Sorensen, L.L.; Dardari, Z.; Liu, H.Y.; Cresswell, K.; Phillip, S.; Bluemke, D.A.; Zimmerman, S.L.; et al. Rest and stress longitudinal systolic left ventricular mechanics in hypertrophic cardiomyopathy: Implications for prognostication. J. Am. Soc. Echocardiogr. 2018, 31, 578–586. [Google Scholar] [CrossRef]
- Re, F.; Zachara, E.; Avella, A.; Baratta, P.; di Mauro, M.; Uguccioni, M.; Olivotto, I. Dissecting functional impairment in hypertrophic cardiomyopathy by dynamic assessment of diastolic reserve and outflow obstruction: A combined cardiopulmonary-echocardiographic study. Int. J. Cardiol. 2017, 15, 743–750. [Google Scholar] [CrossRef]
- Romero-Puche, A.; Marín, F.; González-Carrillo, J.; García-Honrubia, A.; Climent, V.; Feliu, E.; Ruiz-Espejo, F.; Payá, E.; Gimeno-Blanes, J.R.; de la Morena, G.; et al. Gadolinium-enhanced cardiovascular magnetic resonance and exercise capacity in hypertrophic cardiomyopathy. Rev. Esp. Cardiol. 2008, 61, 853–860. [Google Scholar] [CrossRef]
- Rosing, D.R.; Kent, K.M.; Borer, J.S.; Seides, S.F.; Maron, B.J.; Epstein, S.E. Verapamil therapy: A new approach to the pharmacologic treatment of hypertrophic cardiomyopathy. I. Hemodynamic effects. Circulation 1979, 60, 1201–1207. [Google Scholar] [CrossRef]
- Sachdev, V.; Shizukuda, Y.; Brenneman, C.L.; Birdsall, C.W.; Waclawiw, M.A.; Arai, A.E.; Mohiddin, S.A.; Tripodi, D.; Fananapazir, L.; Plehn, J.F. Left atrial volumetric remodeling is predictive of functional capacity in nonobstructive hypertrophic cardiomyopathy. Am. Heart J. 2005, 149, 730–736. [Google Scholar] [CrossRef]
- Saura, D.; Marín, F.; Climent, V.; González, J.; Roldán, V.; Hernández-Romero, D.; Oliva, M.J.; Sabater, M.; de la Morena, G.; Lip, G.Y.H.; et al. Left atrial remodelling in hypertrophic cardiomyopathy: Relation with exercise capacity and biochemical markers of tissue strain and remodelling. Int. J. Clin. Pract. 2009, 63, 1465–1471. [Google Scholar] [CrossRef]
- Shah, A.B.; Bechis, M.Z.; Brown, M.; Finch, J.M.; Loomer, G.; Groezinger, E.; Weiner, R.B.; Wasfy, M.M.; Picard, M.H.; Fifer, M.A.; et al. Catecholamine response to exercise in patients with non-obstructive hypertrophic cardiomyopathy. J. Physiol. 2019, 597, 1337–1346. [Google Scholar] [CrossRef]
- Shizukuda, Y.; Sachdev, V.; Fananapazir, L.; Tripodi, D.; Mohiddin, S.A.; Arai, A.E.; Waclawiw, M.A.; Plehn, J.F. Is functional capacity related to left atrial contractile function in nonobstructive hypertrophic cardiomyopathy? Congest. Heart Fail. 2005, 11, 234–240. [Google Scholar] [CrossRef]
- Smith, E.D.; Tome, J.; Mcgrath, R.; Kumar, S.; Concannon, M.; Day, S.M.; Saberi, S.; Helms, A.S. Exercise hemodynamics in hypertrophic cardiomyopathy identify risk of incident heart failure but not ventricular arrhythmias or sudden cardiac death. Int. J. Cardiol. 2018, 1, 226–231. [Google Scholar] [CrossRef]
- Smith, J.R.; Medina-Inojosa, J.R.; Layrisse, V.; Ommen, S.R.; Olson, T.P. Predictors of exercise capacity in patients with hypertrophic obstructive cardiomyopathy. J. Clin. Med. 2018, 7, 447. [Google Scholar] [CrossRef]
- Thaman, R.; Tomé-Esteban, M.; Barnes, S.; Gimeno-Blanes, J.R.; Mist, B.; Murphy, R.; Collinson, P.O.; McKenna, W.J.; Elliott, P.E. Usefulness of N-terminal Pro-B-Type natriuretic peptide levels to predict exercise capacity in hypertrophic cardiomyopathy. Am. J. Cardiol. 2006, 98, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.D.; Wang, Y.D.; Zhang, M.; Zhu, W.Z.; Cai, Q.Z.; Jiang, W.; Sun, L.L.; Ding, X.Y.; Ye, X.G.; et al. Decreased biventricular mechanics and functional reserve in nonobstructive hypertrophic cardiomyopathy patients: Implications for exercise capacity. Int. J. Cardiovasc. Imaging 2019, 35, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Zhang, M.; Zhu, W.; Cai, Q.; Jiang, W.; Sun, L.L.; Ding, X.Y.; Ye, X.G.; Qin, Y.Y.; et al. Impaired left ventricular mechanics and functional reserve are associated with reduced exercise capacity in patients with hypertrophic cardiomyopathy. Echocardiography 2019, 36, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Wang, Y.; Zhang, M.; Zhu, W.; Cai, Q.; Jiang, W.; Sun, L.L.; Ding, X.Y.; Ye, X.G.; et al. Impaired right ventricular mechanics at rest and during exercise are associated with exercise capacity in patients with hypertrophic cardiomyopathy. J. Am. Heart Assoc. 2019, 8, e011269. [Google Scholar] [CrossRef]
- Yetman, A.T.; Gow, R.M.; Seib, P.; Morrow, W.R.; McCrindle, B.W. Exercise capacity in children with hypertrophic cardiomyopathy and its relation to diastolic left ventricular function. Am. J. Cardiol. 2001, 87, 491–493. [Google Scholar] [CrossRef]
- Bruce, R.A.; McDonough, J.R. Stress testing in screening for cardiovascular disease. Bull. N. Y. Acad. Med. 1969, 45, 1288–1305. [Google Scholar]
- Bruce, R.A.; Blackmon, J.R.; Jones, J.W.; Strait, G. Exercising testing in adult normal subjects and cardiac patients. Pediatrics 1963, 32, 742–756. [Google Scholar] [CrossRef]
- Caselli, S.; Maron, M.S.; Urbano-Moral, J.A.; Pandian, N.G.; Maron, B.J.; Pelliccia, A. Differentiating left ventricular hypertrophy in athletes from that in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2014, 114, 1383–1389. [Google Scholar] [CrossRef]
- Feneon, D.; Schnell, F.; Galli, E.; Bernard, A.; Mabo, P.; Daubert, J.C.; Leclercq, C.; Carre, F.; Donal, E. Impact of exercise-induced mitral regurgitation on hypertrophic cardiomyopathy outcomes. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1110–1117. [Google Scholar] [CrossRef][Green Version]
- Gimeno, J.R.; Tomé-Esteban, M.T.; Lofiego, C.; Hurtado, J.; Pantazis, A.; Mist, B.; Lambiase, P.; McKenna, W.J.; Elliott, P.E. Exercise-induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Eur. Heart J. 2009, 30, 2599–2605. [Google Scholar] [CrossRef]
- Limongelli, G.; Fioretti, V.; Di Maio, M.; Verrengia, M.; Rubino, M.; Gravino, R.; Masarone, D.; D’Andrea, A.; Ciampi, Q.; Picano, E.; et al. Left atrial volume during stress is associated with increased risk of arrhythmias in patients with hypertrophic cardiomyopathy. J. Cardiovasc. Echogr. 2019, 29, 1–6. [Google Scholar] [CrossRef]
- Magri, D.; Re, F.; Agostoni, P.; Zachara, E.; Correale, M.; Mastromarino, V.; Santolamazza, C.; Casenghi, M.; Pacileo, G.; Valente, F.; et al. Heart failure progression in hypertrophic cardiomyopathy-possible insights from cardiopulmonary exercise testing. Circ. J. 2016, 80, 2204–2211. [Google Scholar] [CrossRef]
- Masri, A.; Pierson, L.M.; Smedira, N.G.; Agarwal, S.; Lytle, B.W.; Naji, P.; Thamilarasan, M.; Lever, H.M.; Cho, L.S.; Desai, M.Y. Predictors of long-term outcomes in patients with hypertrophic cardiomyopathy undergoing cardiopulmonary stress testing and echocardiography. Am. Heart J. 2015, 169, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, A.P.; Stamatopoulos, I.; Antoniades, C.; Anastasakis, A.; Kotsiopoulou, C.; Theopistou, A.; Misailidou, M.; Fourlas, C.; Elliott, P.M.; Stefanadis, C. ST Segment “hump” during exercise testing and the risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Ann. Noninvasive Electrocardiol. 2009, 14, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Shimizu, M.; Ino, H.; Yamaguchi, M.; Hayashi, K.; Taki, J.; Mabuchi, H. Hemodynamic changes and prognosis in patients with hypertrophic cardiomyopathy and abnormal blood pressure responses during exercise. Clin. Cardiol. 2003, 26, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Maron, B.J.; Montereggi, A.; Mazzuoli, F.; Dolara, A.; Cecchi, F. Prognostic value of systemic blood pressure response during exercise in a community-based patient population with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1999, 33, 2044–2051. [Google Scholar] [CrossRef]
- Peteiro, J.; Fernández, X.; Bouzas-Mosquera, A.; Montserrat, L.; Méndez, C.; Rodriguez-Garcia, E.; Soler, R.; Couto, D.; Castro-Beiras, A. Exercise echocardiography and cardiac magnetic resonance imaging to predict outcome in patients with hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 423–432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reant, P.; Reynaud, A.; Pillois, X.; Dijos, M.; Arsac, F.; Touche, C.; Landelle, M.; Rooryck, C.; Roudaut, R.; Lafitte, S. comparison of resting and exercise echocardiographic parameters as indicators of outcomes in hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2015, 28, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, N.; Prasad, K.; Elliott, P.M.; Bannerjee, S.; Frenneaux, M.P.; McKenna, W.J. Prospective prognostic assessment of blood pressure response during exercise in patients with hypertrophic cardiomyopathy. Circulation 1997, 96, 2987–2991. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Allison, T.; Hayes, C.; Nishimura, R.A.; Lam, C.S.P.; Ommen, S.R. Prognostic utility of metabolic exercise testing in minimally symptomatic patients with obstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 2012, 109, 1494–1498. [Google Scholar] [CrossRef]
- Patel, V.; Critoph, C.; Elliot, P.M. Mechanisms and medical management of exercise intolerance in hypertrophic cardiomyopathy. Curr. Pharm. Des. 2015, 21, 466–472. [Google Scholar] [CrossRef]
- Frenneaux, M.P.; Counihan, P.J.; Caforio, A.L.; Chikamori, T.; McKenna, W.J. Abnormal blood pressure response during exercise in hypertrophic cardiomyopathy. Circulation 1990, 82, 1995–2002. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar]
- Myers, J.; Bellin, D. Ramp exercise protocols for clinical and cardiopulmonary exercise testing. Sport Med. 2000, 30, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Buchfuhrer, M.; Hansen, J.; Robinson, T.; Sue, D.; Wasserman, K.; Whipp, B.J. Optimizing the exercise protocol for cardiopulmonary assessment. J. Appl. Physiol. 1983, 55, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, J.G.; Cerezuela-Espejo, V.; Morán-Navarro, R.; Martínez-Cava, A.; Conesa, E.; Courel-Ibáñez, J. A new short track test to estimate the VO2max and maximal aerobic speed in well-trained runners. J. Strength Cond. Res. 2019, 33, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, D.; Nihoyannopoulos, P.; Fletcher, A.; Sbarouni, E.; Dritsas, A.; Oakley, C.M. Symptoms of hypertrophic cardiomyopathy, with special emphasis on syncope and postprandial exacerbation of symptoms. Clin. Cardiol. 1996, 19, 371–378. [Google Scholar] [CrossRef]
- Feiner, E.; Arabadjian, M.; Winson, G.; Kim, B.; Chaudhry, F.; Sherrid, M.V. Post-prandial upright exercise echocardiography in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2013, 61, 2487–2488. [Google Scholar] [CrossRef]
- Gilligan, D.; Marsonis, A.; Joshi, J.; Nihoyannopoulos, P.; Ghatei, M.A.; Bloom, S.; Oakley, C.M. Cardiovascular and hormonal responses to a meal in hypertrophic cardiomyopathy: A comparison of patients with and without postprandial exacerbation of symptoms. Clin. Cardiol. 1996, 19, 129–135. [Google Scholar] [CrossRef]
- Gilligann, D.M.; Chan, W.L.; Ang, E.L.; Oakley, C.M. Effects of a meal on hemodynamic function at rest and during exercise in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1991, 18, 429–436. [Google Scholar] [CrossRef][Green Version]
- Braun, L.A.; Rosenfeldt, F. Pharmaco-nutrient interactions—A systematic review of zinc and antihypertensive therapy. Int. J. Clin. Pract. 2013, 67, 717–725. [Google Scholar] [CrossRef]
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Pupek-Musialik, D. The influence of antihypertensive drugs on mineral status in hypertensive patients. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 58–65. [Google Scholar]
- Madden, A.M.; Smith, S. Body composition and morphological assessment of nutritional status in adults: A review of anthropometric variables. J. Hum. Nutr. Diet. 2016, 29, 7–25. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colaguiri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Goff, D.C.; Bertoni, A.G.; Kramer, H.; Bonds, D.; Blumenthal, R.S.; Tsai, M.Y.; Psaty, B.M. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): Gender, ethnicity, and coronary artery calcium. Circulation 2006, 113, 647–656. [Google Scholar] [CrossRef]
- Olivotto, I.; Maron, B.J.; Tomberli, B.; Appelbaum, E.; Salton, C.; Haas, T.S.; Gimbson, M.; Nistri, S.; Servettini, E.; Chan, R.H.; et al. Obesity and its association to phenotype and clinical course in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2013, 62, 449–457. [Google Scholar] [CrossRef]
- Wasserstrum, Y.; Barbarova, I.; Lotan, D.; Kuperstein, R.; Shechter, M.; Freimark, D.; Segal, G.; Klempfner, R.; Arad, M. Efficacy and safety of exercise rehabilitation in patients with hypertrophic cardiomyopathy. J. Cardiol. 2019, 74, 466–472. [Google Scholar] [CrossRef]
- Saberi, S.; Wheeler, M.; Bragg-Gresham, J.; Hornsby, W.; Agarwal, P.P.; Attili, A.; Concannon, W.; Dries, A.M.; Shmargad, Y.; Salisbury, H. Effect of moderate-intensity exercise training on peak oxygen consumption in patients with hypertrophic cardiomyopathy a randomized clinical trial. J. Am. Med. Assoc. 2017, 317, 1349–1357. [Google Scholar] [CrossRef]
- Klempfner, R.; Kamerman, T.; Schwammenthal, E.; Nahshon, A.; Hay, I.; Goldenberg, I.; Dov, F.; Arad, M. Efficacy of exercise training in symptomatic patients with hypertrophic cardiomyopathy: Results of a structured exercise training program in a cardiac rehabilitation center. Eur. J. Prev. Cardiol. 2015, 22, 13–19. [Google Scholar] [CrossRef]
- Olivotto, I.; Oreziak, A.; Barriales-Villa, R.; Abraham, T.P.; Masri, A.; Garcia-Pavia, P.; Saberi, S.; Lakdawala, N.K.; Wheeler, M.T.; Owens, A.; et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 396, 759–769. [Google Scholar] [CrossRef]
- Ho, C.Y.; Mealife, M.E.; Bach, R.G.; Bhattacharya, M.; Choudhury, L.; Edelberg, J.M.; Hedge, S.M.; Jacoby, D.; Lakdawala, N.K.; Lester, S.J.; et al. Evaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2020, 75, 2649–2660. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Dias, K.; Link, M.S.; Levine, B.D. Exercise training for patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2018, 72, 1157–1165. [Google Scholar] [CrossRef]
- Hart, P.D.; Buck, D.J. The effect of resistance training on health-related quality of life in older adults: Systematic review and meta-analysis. Health Promot. Perspect. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Gomes-Neto, M.; Rodrigues, A.; Conceição, L.S.; Roever, L.; Magalhães, C.; Nogueira, I.G.; Ellingsen, Ø.; Oliveira, V. Effect of combined aerobic and resistance training on peak oxygen consumption, muscle strength and health-related quality of life in patients with heart failure with reduced left ventricular ejection fraction: A systematic review and meta-analysis. Int. J. Cardiol. 2019, 293, 165–175. [Google Scholar] [CrossRef]




| Author, Year | VO2max | AT VO2 | METs | % VO2 | ABPRE | Arrhythmia |
|---|---|---|---|---|---|---|
| Coats, 2014 [28] | 0.79 (0.74–0.83) | 0.71 (0.62–0.82) | - | - | 2.15 (1.58–2.94) | 1.66 (1.23–2.23) |
| Desai, 2014 [32] | - | - | 0.73 (0.63–0.85) | - | 1.96 (0.20–3.27) | 1.25 (0.51–3.10) |
| Feneon, 2016 [86] | - | - | 0.99 (0.96–1.02) | - | - | - |
| Finocchiaro, 2015 [37] | 0.52 (0.45–0.60) | - | - | 5.36 (1.96–14.6) † | - | - |
| Ghiselli, 2019 [39] | - | - | 0.68 (0.53–0.87) | - | - | - |
| Gimeno, 2009 [87] | - | - | - | - | - | 2.18 (1.07–4.45) |
| Magri, 2016 [55] | 0.91 (0.85–0.98) | 0.82 (0.72–0.94) | - | 0.96 (0.93–0.99) | 2.33 (1.003–5.4) | 3.13 (1.38–7.01) |
| Magri, 2016 [89] | 0.80 (0.76–0.84) | 0.81 (0.74–0.88) | - | 0.94 (0.92–0.95) | 4.49 (2.91–6.93) | - |
| Magri, 2018 [56] | 0.85 (0.81–0.89) | - | - | 0.95 (0.94–0.97) | 3.50 (2.22–5.31) | 3.00 (1.22–7.39) |
| Masri, 2015 [90] | - | - | - | 0.96 (0.93–0.98) | - | - |
| Michaelides, 2009 [91] | - | - | - | - | 3.6 (0.8–15.6) | - |
| Moneghetti, 2017 [63] | 0.57 (0.42–0.76) | - | 0.55 (0.41–0.75) | 0.59 (0.46–0.76) | - | - |
| Olivotto, 1999 [93] | - | - | - | - | 4.50 (1.1–20.1) | - |
| Peteiro, 2012 [67] | - | - | 0.74 (0.63–0.86) | - | - | 3.13 (1.128.71) |
| Sorajja, 2012 [97] | 0.92 (0.89–0.96) | 0.99 (0.99–0.99) | 0.81 (0.71–0.93) | 0.98 (0.96–0.99) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayonas-Ruiz, A.; Muñoz-Franco, F.M.; Ferrer, V.; Pérez-Caballero, C.; Sabater-Molina, M.; Tomé-Esteban, M.T.; Bonacasa, B. Cardiopulmonary Exercise Test in Patients with Hypertrophic Cardiomyopathy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2312. https://doi.org/10.3390/jcm10112312
Bayonas-Ruiz A, Muñoz-Franco FM, Ferrer V, Pérez-Caballero C, Sabater-Molina M, Tomé-Esteban MT, Bonacasa B. Cardiopulmonary Exercise Test in Patients with Hypertrophic Cardiomyopathy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(11):2312. https://doi.org/10.3390/jcm10112312
Chicago/Turabian StyleBayonas-Ruiz, Adrián, Francisca M. Muñoz-Franco, Vicente Ferrer, Carlos Pérez-Caballero, María Sabater-Molina, María Teresa Tomé-Esteban, and Bárbara Bonacasa. 2021. "Cardiopulmonary Exercise Test in Patients with Hypertrophic Cardiomyopathy: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 11: 2312. https://doi.org/10.3390/jcm10112312
APA StyleBayonas-Ruiz, A., Muñoz-Franco, F. M., Ferrer, V., Pérez-Caballero, C., Sabater-Molina, M., Tomé-Esteban, M. T., & Bonacasa, B. (2021). Cardiopulmonary Exercise Test in Patients with Hypertrophic Cardiomyopathy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(11), 2312. https://doi.org/10.3390/jcm10112312






