Sex Differences, Genetic and Environmental Influences on Dilated Cardiomyopathy
Abstract
1. Introduction
2. Sex Differences
3. Role of Genes
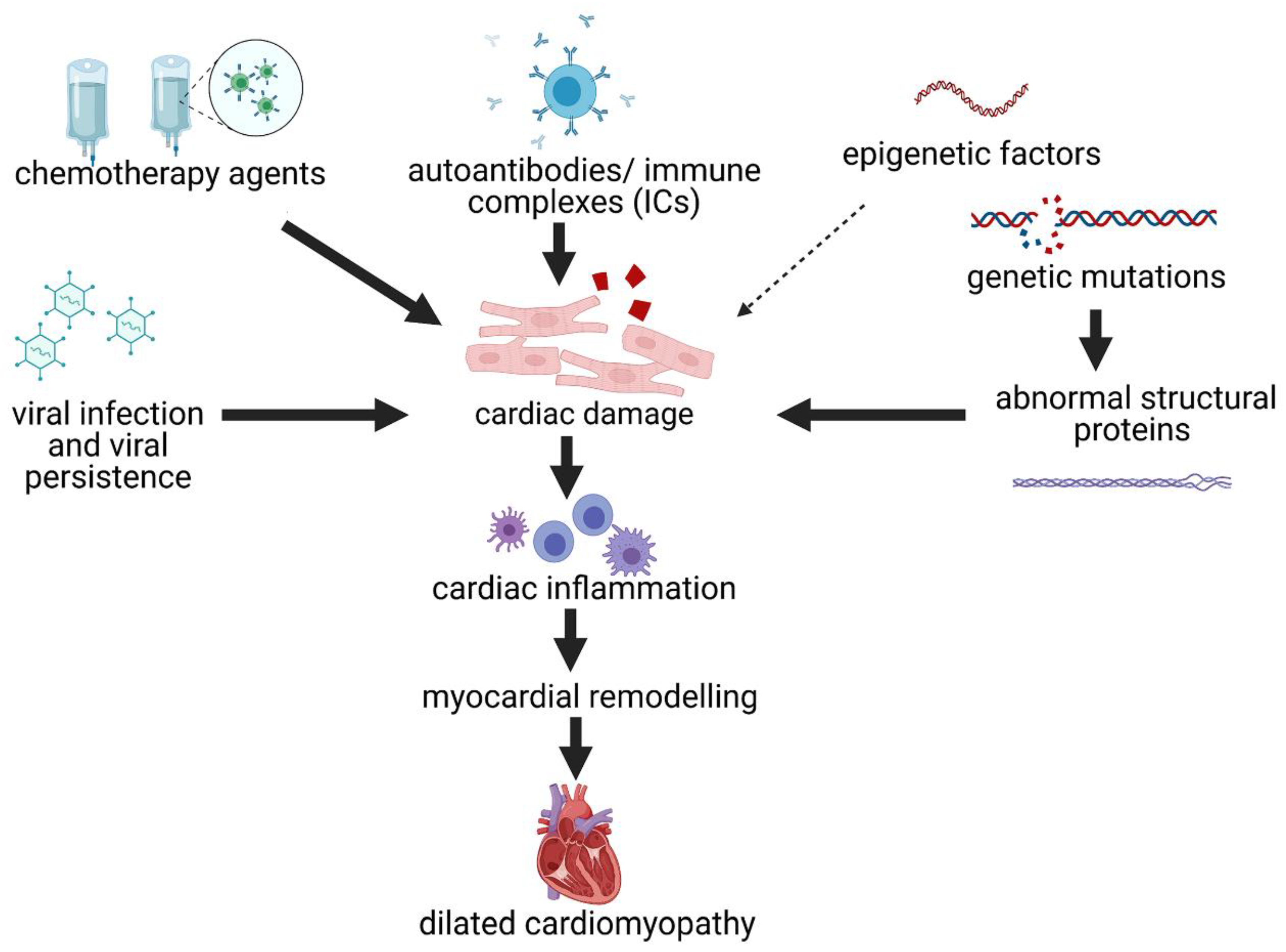
4. Pathogenesis of DCM
5. Sex Differences in Genetic DCM
6. Genetics and Environment
7. Limitations
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schultheiss, H.P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, R.E.; Hedges, D.J.; Morales, A. Dilated cardiomyopathy: The complexity of a diverse genetic architecture. Nat. Rev. Cardiol. 2013, 10, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Dellefave, L.; McNally, E.M. The genetics of dilated cardiomyopathy. Curr. Opin. Cardiol. 2010, 25, 198–204. [Google Scholar] [CrossRef]
- Rosenbaum, A.N.; Agre, K.E.; Pereira, N.L. Genetics of dilated cardiomyopathy: Practical implications for heart failure management. Nat. Rev. Cardiol. 2020, 17, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Norton, N.; Robertson, P.D.; Rieder, M.J.; Zuchner, S.; Rampersaud, E.; Martin, E.; Li, D.; Nickerson, D.A.; Hershberger, R.E.; National Heart, L.; et al. Evaluating pathogenicity of rare variants from dilated cardiomyopathy in the exome era. Circ. Cardiovasc. Genet. 2012, 5, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Isensee, J.; Ruiz Noppinger, P. Sexually dimorphic gene expression in mammalian somatic tissue. Gend. Med. 2007, 4 (Suppl. B), S75–S95. [Google Scholar] [CrossRef]
- Isensee, J.; Witt, H.; Pregla, R.; Hetzer, R.; Regitz-Zagrosek, V.; Noppinger, P.R. Sexually dimorphic gene expression in the heart of mice and men. J. Mol. Med. 2008, 86, 61–74. [Google Scholar] [CrossRef]
- Cleland, J.G.; Swedberg, K.; Follath, F.; Komajda, M.; Cohen-Solal, A.; Aguilar, J.C.; Dietz, R.; Gavazzi, A.; Hobbs, R.; Korewicki, J.; et al. The EuroHeart Failure survey programme—A survey on the quality of care among patients with heart failure in Europe. Part 1: Patient characteristics and diagnosis. Eur. Heart J. 2003, 24, 442–463. [Google Scholar] [CrossRef]
- Luchner, A.; Bröckel, U.; Muscholl, M.; Hense, H.W.; Döring, A.; Riegger, G.A.; Schunkert, H. Gender-specific differences of cardiac remodeling in subjects with left ventricular dysfunction: A population-based study. Cardiovasc. Res. 2002, 53, 720–727. [Google Scholar] [CrossRef][Green Version]
- Sheppard, R.; Bedi, M.; Kubota, T.; Semigran, M.J.; Dec, W.; Holubkov, R.; Feldman, A.M.; Rosenblum, W.D.; McTiernan, C.F.; McNamara, D.M.; et al. Myocardial expression of fas and recovery of left ventricular function in patients with recent-onset cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 1036–1042. [Google Scholar] [CrossRef]
- Cocker, M.; Friedrich, M.G. Cardiovascular magnetic resonance of myocarditis. Curr. Cardiol. Rep. 2010, 12, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Cocker, M.S.; Abdel-Aty, H.; Strohm, O.; Friedrich, M.G. Age and gender effects on the extent of myocardial involvement in acute myocarditis: A cardiovascular magnetic resonance study. Heart 2009, 95, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Gabel, S.A.; Walker, V.R.; London, R.E.; Steenbergen, C.; Korach, K.S.; Murphy, E. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J. Mol. Cell Cardiol. 2005, 38, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Skavdahl, M.; Steenbergen, C.; Clark, J.; Myers, P.; Demianenko, T.; Mao, L.; Rockman, H.A.; Korach, K.S.; Murphy, E. Estrogen receptor-beta mediates male-female differences in the development of pressure overload hypertrophy. Am. J. Physiol Heart Circ. Physiol. 2005, 288, H469–H476. [Google Scholar] [CrossRef]
- Devanathan, S.; Whitehead, T.; Schweitzer, G.G.; Fettig, N.; Kovacs, A.; Korach, K.S.; Finck, B.N.; Shoghi, K.I. An animal model with a cardiomyocyte-specific deletion of estrogen receptor alpha: Functional, metabolic, and differential network analysis. PLoS ONE 2014, 9, e101900. [Google Scholar] [CrossRef]
- Fox, H.S.; Bond, B.L.; Parslow, T.G. Estrogen regulates the IFN-gamma promoter. J. Immunol. 1991, 146, 4362–4367. [Google Scholar]
- Buskiewicz, I.A.; Huber, S.A.; Fairweather, D. Sex hormone receptor expression in the immune system. In Sex Differences in Physiology; Academic Press: Amsterdam, The Netherlands, 2016; pp. 45–60. [Google Scholar]
- Benten, W.P.; Lieberherr, M.; Stamm, O.; Wrehlke, C.; Guo, Z.; Wunderlich, F. Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Mol. Biol. Cell 1999, 10, 3113–3123. [Google Scholar] [CrossRef]
- Benten, W.P.; Lieberherr, M.; Giese, G.; Wrehlke, C.; Stamm, O.; Sekeris, C.E.; Mossmann, H.; Wunderlich, F. Functional testosterone receptors in plasma membranes of T cells. FASEB J. 1999, 13, 123–133. [Google Scholar] [CrossRef]
- Johnson, B.D.; Zheng, W.; Korach, K.S.; Scheuer, T.; Catterall, W.A.; Rubanyi, G.M. Increased expression of the cardiac L-type calcium channel in estrogen receptor-deficient mice. J. Gen. Physiol. 1997, 110, 135–140. [Google Scholar] [CrossRef]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: A scientific statement from the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef] [PubMed]
- Batton, K.A.; Austin, C.O.; Bruno, K.A.; Burger, C.D.; Shapiro, B.P.; Fairweather, D. Sex differences in pulmonary arterial hypertension: Role of infection and autoimmunity in the pathogenesis of disease. Biol. Sex. Differ. 2018, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.E.; Priori, R.; Valesini, G.; Fairweather, D. Sex differences in Sjogren’s syndrome: A comprehensive review of immune mechanisms. Biol. Sex. Differ. 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.S.; Harjola, V.P.; Hochadel, M.; Drexler, H.; Komajda, M.; Brutsaert, D.; Dickstein, K.; Ponikowski, P.; Tavazzi, L.; Follath, F.; et al. Gender related differences in patients presenting with acute heart failure. Results from EuroHeart Failure Survey II. Eur J. Heart Fail. 2008, 10, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Codd, M.B.; Sugrue, D.D.; Gersh, B.J.; Melton, L.J. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975–1984. Circulation 1989, 80, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Ludden, T.E.; Edwards, J.E. Carditis in poliomyelitis; an anatomic study of 35 cases and review of the literature. Am. J. Pathol. 1949, 25, 357–381. [Google Scholar]
- Sainani, G.S.; Krompotic, E.; Slodki, S.J. Adult heart disease due to the Coxsackie virus B infection. Medicine 1968, 47, 133–147. [Google Scholar] [CrossRef]
- Woodruff, J.F. Viral myocarditis. A review. Am. J. Pathol. 1980, 101, 425–484. [Google Scholar]
- Bagger, J.P.; Baandrup, U.; Rasmussen, K.; Møller, M.; Vesterlund, T. Cardiomyopathy in western Denmark. Br. Heart J. 1984, 52, 327–331. [Google Scholar] [CrossRef]
- Ikram, H.; Williamson, H.G.; Won, M.; Crozier, I.G.; Wells, E.J. The course of idiopathic dilated cardiomyopathy in New Zealand. Br. Heart J. 1987, 57, 521–527. [Google Scholar] [CrossRef]
- Komajda, M.; Jais, J.P.; Reeves, F.; Goldfarb, B.; Bouhour, J.B.; Juillieres, Y.; Lanfranchi, J.; Peycelon, P.; Geslin, P.; Carrie, D. Factors predicting mortality in idiopathic dilated cardiomyopathy. Eur. Heart J. 1990, 11, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Sachero, A.; Casazza, F.; Recalcati, F.; de Maria, R.; Preti, L.; Mattioli, R.; Ferrari, F.; Capozzi, A.; Camerini, F. Clinical and prognostic significance of echocardiographic parameters in dilated cardiomyopathy: A prospective study on 225 patients. The Italian Multicenter Study of Cardiomyopathies Group. G. Ital. Cardiol. 1992, 22, 1077–1090. [Google Scholar] [PubMed]
- De Maria, R.; Gavazzi, A.; Recalcati, F.; Baroldi, G.; De Vita, C.; Camerini, F. Comparison of clinical findings in idiopathic dilated cardiomyopathy in women versus men. The Italian Multicenter Cardiomyopathy Study Group (SPIC). Am. J. Cardiol. 1993, 72, 580–585. [Google Scholar] [CrossRef]
- Coughlin, S.S.; Gottdiener, J.S.; Baughman, K.L.; Wasserman, A.; Marx, E.S.; Tefft, M.C.; Gersh, B.J. Black-white differences in mortality in idiopathic dilated cardiomyopathy: The Washington, DC, dilated cardiomyopathy study. J. Natl. Med. Assoc. 1994, 86, 583–591. [Google Scholar]
- Gavazzi, A.; De Maria, R.; Porcu, M.; Beretta, L.; Casazza, F.; Castelli, G.; Luvini, M.; Parodi, O.; Recalcati, F.; Renosto, G. Dilated cardiomyopathy: A new natural history? The experience of the Italian Multicenter Cardiomyopathy Study (SPIC). G. Ital. Cardiol. 1995, 25, 1109–1125. [Google Scholar]
- Grzybowski, J.; Bilińska, Z.T.; Ruzyłło, W.; Kupść, W.; Michalak, E.; Szcześniewska, D.; Poplawska, W.; Rydlewska-Sadowska, W. Determinants of prognosis in nonischemic dilated cardiomyopathy. J. Card. Fail. 1996, 2, 77–85. [Google Scholar] [CrossRef]
- Fauchier, L.; Babuty, D.; Cosnay, P.; Poret, P.; Rouesnel, P.; Fauchier, J.P. Long-term prognostic value of time domain analysis of signal-averaged electrocardiography in idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2000, 85, 618–623. [Google Scholar] [CrossRef]
- Kadish, A.; Dyer, A.; Daubert, J.P.; Quigg, R.; Estes, N.A.; Anderson, K.P.; Calkins, H.; Hoch, D.; Goldberger, J.; Shalaby, A.; et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N. Engl. J. Med. 2004, 350, 2151–2158. [Google Scholar] [CrossRef]
- Satoh, M.; Nakamura, M.; Akatsu, T.; Shimoda, Y.; Segawa, I.; Hiramori, K. Toll-like receptor 4 is expressed with enteroviral replication in myocardium from patients with dilated cardiomyopathy. Lab. Investig. 2004, 84, 173–181. [Google Scholar] [CrossRef]
- Kubo, T.; Matsumura, Y.; Kitaoka, H.; Okawa, M.; Hirota, T.; Hamada, T.; Hitomi, N.; Hoshikawa, E.; Hayato, K.; Shimizu, Y.; et al. Improvement in prognosis of dilated cardiomyopathy in the elderly over the past 20 years. J. Cardiol. 2008, 52, 111–117. [Google Scholar] [CrossRef]
- Heidecker, B.; Lamirault, G.; Kasper, E.K.; Wittstein, I.S.; Champion, H.C.; Breton, E.; Russell, S.D.; Hall, J.; Kittleson, M.M.; Baughman, K.L.; et al. The gene expression profile of patients with new-onset heart failure reveals important gender-specific differences. Eur. Heart J. 2010, 31, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Kubo, T.; Kitaoka, H.; Hirota, T.; Hoshikawa, E.; Hayato, K.; Shimizu, Y.; Okawa, M.; Yamasaki, N.; Matsumura, Y.; et al. Clinical features of the dilated phase of hypertrophic cardiomyopathy in comparison with those of dilated cardiomyopathy. Clin. Cardiol. 2010, 33, E24–E28. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.M.; Starling, R.C.; Cooper, L.T.; Boehmer, J.P.; Mather, P.J.; Janosko, K.M.; Gorcsan, J.; Kip, K.E.; Dec, G.W.; Investigators, I. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: Results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J. Am. Coll. Cardiol. 2011, 58, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yan, L.; Wang, J.; Zhan, W.; Song, K.; Han, X.; Li, X.; Cao, J.; Liu, H. β1-Adrenoceptor autoantibodies from DCM patients enhance the proliferation of T lymphocytes through the β1-AR/cAMP/PKA and p38 MAPK pathways. PLoS ONE 2012, 7, e52911. [Google Scholar] [CrossRef]
- Castelli, G.; Fornaro, A.; Ciaccheri, M.; Dolara, A.; Troiani, V.; Tomberli, B.; Olivotto, I.; Gensini, G.F. Improving survival rates of patients with idiopathic dilated cardiomyopathy in Tuscany over 3 decades: Impact of evidence-based management. Circ. Heart Fail. 2013, 6, 913–921. [Google Scholar] [CrossRef]
- Hirtle-Lewis, M.; Desbiens, K.; Ruel, I.; Rudzicz, N.; Genest, J.; Engert, J.C.; Giannetti, N. The genetics of dilated cardiomyopathy: A prioritized candidate gene study of LMNA, TNNT2, TCAP, and PLN. Clin. Cardiol. 2013, 36, 628–633. [Google Scholar] [CrossRef]
- Dec, G.W. The natural history of acute dilated cardiomyopathy. Trans. Am. Clin. Clim. Assoc. 2014, 125, 76–86. [Google Scholar]
- Merlo, M.; Pivetta, A.; Pinamonti, B.; Stolfo, D.; Zecchin, M.; Barbati, G.; Di Lenarda, A.; Sinagra, G. Long-term prognostic impact of therapeutic strategies in patients with idiopathic dilated cardiomyopathy: Changing mortality over the last 30 years. Eur. J. Heart Fail. 2014, 16, 317–324. [Google Scholar] [CrossRef]
- Hazebroek, M.R.; Moors, S.; Dennert, R.; van den Wijngaard, A.; Krapels, I.; Hoos, M.; Verdonschot, J.; Merken, J.J.; de Vries, B.; Wolffs, P.F.; et al. Prognostic Relevance of Gene-Environment Interactions in Patients with Dilated Cardiomyopathy: Applying the MOGE(S) Classification. J. Am. Coll. Cardiol. 2015, 66, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Frese, K.S.; Peil, B.; Kloos, W.; Keller, A.; Nietsch, R.; Feng, Z.; Müller, S.; Kayvanpour, E.; Vogel, B.; et al. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur. Heart J. 2015, 36, 1123–1135a. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Laur, O.; Hwa, J.; Depasquale, E.; Bellumkonda, L.; Sugeng, L.; Pomianowski, P.; Testani, J.; Chen, M.; McKenna, W.; et al. Familial dilated cardiomyopathy diagnosis is commonly overlooked at the time of transplant listing. J. Heart Lung Transpl. 2016, 35, 474–480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Halliday, B.P.; Gulati, A.; Ali, A.; Newsome, S.; Lota, A.; Tayal, U.; Vassiliou, V.S.; Arzanauskaite, M.; Izgi, C.; Krishnathasan, K.; et al. Sex—And age-based differences in the natural history and outcome of dilated cardiomyopathy. Eur. J. Heart Fail. 2018, 20, 1392–1400. [Google Scholar] [CrossRef]
- Stojkovic, S.; Kaider, A.; Koller, L.; Brekalo, M.; Wojta, J.; Diedrich, A.; Demyanets, S.; Pezawas, T. GDF-15 is a better complimentary marker for risk stratification of arrhythmic death in non-ischaemic, dilated cardiomyopathy than soluble ST2. J. Cell Mol. Med. 2018, 22, 2422–2429. [Google Scholar] [CrossRef]
- Charron, P.; Elliott, P.M.; Gimeno, J.R.; Caforio, A.L.P.; Kaski, J.P.; Tavazzi, L.; Tendera, M.; Maupain, C.; Laroche, C.; Rubis, P.; et al. The Cardiomyopathy Registry of the EURObservational Research Programme of the European Society of Cardiology: Baseline data and contemporary management of adult patients with cardiomyopathies. Eur. Heart J. 2018, 39, 1784–1793. [Google Scholar] [CrossRef]
- Chen, C.; Liu, J.; Liu, Z.; He, X.; Yuan, X.; Ouyang, X.; Wang, L.; Li, X. Electrocardiogram signs of right ventricular hypertrophy may help identify pulmonary hypertension in patients with dilated cardiomyopathy. Int J. Cardiol. Heart Vasc. 2019, 22, 61–66. [Google Scholar] [CrossRef]
- Kamisago, M.; Sharma, S.D.; DePalma, S.R.; Solomon, S.; Sharma, P.; McDonough, B.; Smoot, L.; Mullen, M.P.; Woolf, P.K.; Wigle, E.D.; et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N. Engl. J. Med. 2000, 343, 1688–1696. [Google Scholar] [CrossRef]
- Lakdawala, N.K.; Dellefave, L.; Redwood, C.S.; Sparks, E.; Cirino, A.L.; Depalma, S.; Colan, S.D.; Funke, B.; Zimmerman, R.S.; Robinson, P.; et al. Familial dilated cardiomyopathy caused by an alpha-tropomyosin mutation: The distinctive natural history of sarcomeric dilated cardiomyopathy. J. Am. Coll. Cardiol. 2010, 55, 320–329. [Google Scholar] [CrossRef]
- De Denus, S.; Mottet, F.; Korol, S.; Feroz Zada, Y.; Provost, S.; Mongrain, I.; Asselin, G.; Oussaid, E.; Busseuil, D.; Lettre, G.; et al. A genetic association study of heart failure: More evidence for the role of BAG3 in idiopathic dilated cardiomyopathy. Esc Heart Fail. 2020. [Google Scholar] [CrossRef]
- Fairweather, D.; Cooper, L.T.; Blauwet, L.A. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr. Probl. Cardiol. 2013, 38, 7–46. [Google Scholar] [CrossRef]
- Herman, D.S.; Lam, L.; Taylor, M.R.; Wang, L.; Teekakirikul, P.; Christodoulou, D.; Conner, L.; DePalma, S.R.; McDonough, B.; Sparks, E.; et al. Truncations of titin causing dilated cardiomyopathy. N. Engl. J. Med. 2012, 366, 619–628. [Google Scholar] [CrossRef]
- Pugh, T.J.; Kelly, M.A.; Gowrisankar, S.; Hynes, E.; Seidman, M.A.; Baxter, S.M.; Bowser, M.; Harrison, B.; Aaron, D.; Mahanta, L.M.; et al. The landscape of genetic variation in dilated cardiomyopathy as surveyed by clinical DNA sequencing. Genet. Med. 2014, 16, 601–608. [Google Scholar] [CrossRef] [PubMed]
- McNally, E.M.; Golbus, J.R.; Puckelwartz, M.J. Genetic mutations and mechanisms in dilated cardiomyopathy. J. Clin. Investig. 2013, 123, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Belkaya, S.; Kontorovich, A.R.; Byun, M.; Mulero-Navarro, S.; Bajolle, F.; Cobat, A.; Josowitz, R.; Itan, Y.; Quint, R.; Lorenzo, L.; et al. Autosomal Recessive Cardiomyopathy Presenting as Acute Myocarditis. J. Am. Coll. Cardiol. 2017, 69, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Badorff, C.; Knowlton, K.U. Dystrophin disruption in enterovirus-induced myocarditis and dilated cardiomyopathy: From bench to bedside. Med. Microbiol. Immunol. 2004, 193, 121–126. [Google Scholar] [CrossRef]
- Diegoli, M.; Grasso, M.; Favalli, V.; Serio, A.; Gambarin, F.I.; Klersy, C.; Pasotti, M.; Agozzino, E.; Scelsi, L.; Ferlini, A.; et al. Diagnostic work-up and risk stratification in X-linked dilated cardiomyopathies caused by dystrophin defects. J. Am. Coll. Cardiol. 2011, 58, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M.; Fairweather, D.; Huber, S.A.; Cunningham, M.W. Autoimmune myocarditis, valvulitis, and cardiomyopathy. Curr. Protoc. Immunol. 2013, 14, 11–51. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M.; Cooper, L.T.; Kem, D.C.; Stavrakis, S.; Kosanke, S.D.; Shevach, E.M.; Fairweather, D.; Stoner, J.A.; Cox, C.J.; Cunningham, M.W. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.J.; Bruno, K.A.; Blauwet, L.A.; Tschope, C.; Cunningham, M.W.; Pankuweit, S.; van Linthout, S.; Jeon, E.S.; McNamara, D.M.; Krejci, J.; et al. Elevated Sera sST2 Is Associated with Heart Failure in Men ≤50 Years Old with Myocarditis. J. Am. Heart Assoc. 2019, 8, e008968. [Google Scholar] [CrossRef]
- Kindermann, I.; Kindermann, M.; Kandolf, R.; Klingel, K.; Bultmann, B.; Muller, T.; Lindinger, A.; Bohm, M. Predictors of outcome in patients with suspected myocarditis. Circulation 2008, 118, 639–648. [Google Scholar] [CrossRef]
- Fatkin, D.; MacRae, C.; Sasaki, T.; Wolff, M.R.; Porcu, M.; Frenneaux, M.; Atherton, J.; Vidaillet, H.J., Jr.; Spudich, S.; De Girolami, U.; et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med. 1999, 341, 1715–1724. [Google Scholar] [CrossRef]
- Norton, N.; Li, D.; Rieder, M.J.; Siegfried, J.D.; Rampersaud, E.; Zuchner, S.; Mangos, S.; Gonzalez-Quintana, J.; Wang, L.; McGee, S.; et al. Genome-wide studies of copy number variation and exome sequencing identify rare variants in BAG3 as a cause of dilated cardiomyopathy. Am. J. Hum. Genet. 2011, 88, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Arimura, T.; Ishikawa, T.; Nunoda, S.; Kawai, S.; Kimura, A. Dilated cardiomyopathy-associated BAG3 mutations impair Z-disc assembly and enhance sensitivity to apoptosis in cardiomyocytes. Hum. Mutat. 2011, 32, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Duboscq-Bidot, L.; Xu, P.; Charron, P.; Neyroud, N.; Dilanian, G.; Millaire, A.; Bors, V.; Komajda, M.; Villard, E. Mutations in the Z-band protein myopalladin gene and idiopathic dilated cardiomyopathy. Cardiovasc. Res. 2008, 77, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Syrris, P.; Salas, C.; Evans, A.; Mirelis, J.G.; Cobo-Marcos, M.; Vilches, C.; Bornstein, B.; Segovia, J.; Alonso-Pulpon, L.; et al. Desmosomal protein gene mutations in patients with idiopathic dilated cardiomyopathy undergoing cardiac transplantation: A clinicopathological study. Heart 2011, 97, 1744–1752. [Google Scholar] [CrossRef]
- Begay, R.L.; Tharp, C.A.; Martin, A.; Graw, S.L.; Sinagra, G.; Miani, D.; Sweet, M.E.; Slavov, D.B.; Stafford, N.; Zeller, M.J.; et al. FLNC gene splice mutations cause dilated cardiomyopathy. JACC Basic Transl. Sci. 2016, 1, 344–359. [Google Scholar] [CrossRef]
- Brauch, K.M.; Karst, M.L.; Herron, K.J.; de Andrade, M.; Pellikka, P.A.; Rodeheffer, R.J.; Michels, V.V.; Olson, T.M. Mutations in ribonucleic acid binding protein gene cause familial dilated cardiomyopathy. J. Am. Coll. Cardiol. 2009, 54, 930–941. [Google Scholar] [CrossRef]
- McNair, W.P.; Ku, L.; Taylor, M.R.; Fain, P.R.; Dao, D.; Wolfel, E.; Mestroni, L.; Familial Cardiomyopathy Registry Research, G. SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation 2004, 110, 2163–2167. [Google Scholar] [CrossRef]
- Olson, T.M.; Michels, V.V.; Ballew, J.D.; Reyna, S.P.; Karst, M.L.; Herron, K.J.; Horton, S.C.; Rodeheffer, R.J.; Anderson, J.L. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA 2005, 293, 447–454. [Google Scholar] [CrossRef]
- Olson, T.M.; Kishimoto, N.Y.; Whitby, F.G.; Michels, V.V. Mutations that alter the surface charge of alpha-tropomyosin are associated with dilated cardiomyopathy. J. Mol. Cell. Cardiol. 2001, 33, 723–732. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Morales, A.; Siegfried, J.D. Clinical and genetic issues in dilated cardiomyopathy: A review for genetics professionals. Genet. Med. 2010, 12, 655–667. [Google Scholar] [CrossRef]
- Liu, L.Y.; Schaub, M.A.; Sirota, M.; Butte, A.J. Sex differences in disease risk from reported genome-wide association study findings. Hum. Genet. 2012, 131, 353–364. [Google Scholar] [CrossRef]
- Haddad, G.E.; Saunders, L.J.; Crosby, S.D.; Carles, M.; del Monte, F.; King, K.; Bristow, M.R.; Spinale, F.G.; Macgillivray, T.E.; Semigran, M.J.; et al. Human cardiac-specific cDNA array for idiopathic dilated cardiomyopathy: Sex-related differences. Physiol. Genom. 2008, 33, 267–277. [Google Scholar] [CrossRef][Green Version]
- Stefanelli, C.B.; Rosenthal, A.; Borisov, A.B.; Ensing, G.J.; Russell, M.W. Novel troponin T mutation in familial dilated cardiomyopathy with gender-dependant severity. Mol. Genet. Metab. 2004, 83, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Crispell, K.A.; Wray, A.; Ni, H.; Nauman, D.J.; Hershberger, R.E. Clinical profiles of four large pedigrees with familial dilated cardiomyopathy: Preliminary recommendations for clinical practice. J. Am. Coll. Cardiol. 1999, 34, 837–847. [Google Scholar] [CrossRef][Green Version]
- Kushner, J.D.; Nauman, D.; Burgess, D.; Ludwigsen, S.; Parks, S.B.; Pantely, G.; Burkett, E.; Hershberger, R.E. Clinical characteristics of 304 kindreds evaluated for familial dilated cardiomyopathy. J. Card Fail. 2006, 12, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Van Spaendonck-Zwarts, K.Y.; van Rijsingen, I.A.; van den Berg, M.P.; Lekanne Deprez, R.H.; Post, J.G.; van Mil, A.M.; Asselbergs, F.W.; Christiaans, I.; van Langen, I.M.; Wilde, A.A.; et al. Genetic analysis in 418 index patients with idiopathic dilated cardiomyopathy: Overview of 10 years’ experience. Eur. J. Heart Fail. 2013, 15, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Franaszczyk, M.; Chmielewski, P.; Truszkowska, G.; Stawinski, P.; Michalak, E.; Rydzanicz, M.; Sobieszczanska-Malek, M.; Pollak, A.; Szczygieł, J.; Kosinska, J.; et al. Titin truncating variants in dilated cardiomyopathy—Prevalence and genotype-phenotype correlations. PLoS ONE 2017, 12, e0169007. [Google Scholar] [CrossRef]
- Meyer, S.; van der Meer, P.; van Tintelen, J.P.; van den Berg, M.P. Sex differences in cardiomyopathies. Eur. J. Heart Fail. 2014, 16, 238–247. [Google Scholar] [CrossRef]
- Pelliccia, F.; Limongelli, G.; Autore, C.; Gimeno-Blanes, J.R.; Basso, C.; Elliott, P. Sex-related differences in cardiomyopathies. Int. J. Cardiol. 2019, 286, 239–243. [Google Scholar] [CrossRef]
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O′Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [CrossRef]
- Noutsias, M.; Rohde, M.; Goldner, K.; Block, A.; Blunert, K.; Hemaidan, L.; Hummel, M.; Blohm, J.H.; Lassner, D.; Kuhl, U.; et al. Expression of functional T-cell markers and T-cell receptor Vbeta repertoire in endomyocardial biopsies from patients presenting with acute myocarditis and dilated cardiomyopathy. Eur. J. Heart Fail. 2011, 13, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: An expert consensus document. Circ. Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef]
- Kang, M.; An, J. Viral myocarditis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Nguyen, T.; Waseem, M. Chagas disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kaya, Z.; Afanasyeva, M.; Wang, Y.; Dohmen, K.M.; Schlichting, J.; Tretter, T.; Fairweather, D.; Holers, V.M.; Rose, N.R. Contribution of the innate immune system to autoimmune myocarditis: A role for complement. Nat. Immunol. 2001, 2, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Frisancho-Kiss, S.; Njoku, D.B.; Nyland, J.F.; Kaya, Z.; Yusung, S.A.; Davis, S.E.; Frisancho, J.A.; Barrett, M.A.; Rose, N.R. Complement receptor 1 and 2 deficiency increases coxsackievirus B3-induced myocarditis, dilated cardiomyopathy, and heart failure by increasing macrophages, IL-1beta, and immune complex deposition in the heart. J. Immunol. 2006, 176, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T., Jr.; Onuma, O.K.; Sagar, S.; Oberg, A.L.; Mahoney, D.W.; Asmann, Y.W.; Liu, P. Genomic and proteomic analysis of myocarditis and dilated cardiomyopathy. Heart Fail. Clin. 2010, 6, 75–85. [Google Scholar] [CrossRef]
- Fairweather, D.; Yusung, S.; Frisancho, S.; Barrett, M.; Gatewood, S.; Steele, R.; Rose, N.R. IL-12 receptor beta 1 and Toll-like receptor 4 increase IL-1 beta- and IL-18-associated myocarditis and coxsackievirus replication. J. Immunol. 2003, 170, 4731–4737. [Google Scholar] [CrossRef]
- Frisancho-Kiss, S.; Davis, S.E.; Nyland, J.F.; Frisancho, J.A.; Cihakova, D.; Barrett, M.A.; Rose, N.R.; Fairweather, D. Cutting edge: Cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J. Immunol. 2007, 178, 6710–6714. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.J.; Brandt, J.E.; Kim, E.; Bucek, A.; Bedja, D.; Abston, E.D.; Shin, J.; Gabrielson, K.L.; Mitzner, W.; Fairweather, D. Testosterone and interleukin-1β increase cardiac remodeling during coxsackievirus B3 myocarditis via serpin A 3n. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1726–H1736. [Google Scholar] [CrossRef]
- Fairweather, D.; Frisancho-Kiss, S.; Yusung, S.A.; Barrett, M.A.; Davis, S.E.; Gatewood, S.J.; Njoku, D.B.; Rose, N.R. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am. J. Pathol. 2004, 165, 1883–1894. [Google Scholar] [CrossRef]
- Fairweather, D.; Frisancho-Kiss, S.; Yusung, S.A.; Barrett, M.A.; Davis, S.E.; Steele, R.A.; Gatewood, S.J.; Rose, N.R. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. J. Immunol. 2005, 174, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Abston, E.D.; Coronado, M.J.; Bucek, A.; Bedja, D.; Shin, J.; Kim, J.B.; Kim, E.; Gabrielson, K.L.; Georgakopoulos, D.; Mitzner, W.; et al. Th2 regulation of viral myocarditis in mice: Different roles for TLR3 versus TRIF in progression to chronic disease. Clin. Dev. Immunol. 2012, 2012, 129486. [Google Scholar] [CrossRef] [PubMed]
- Abston, E.D.; Coronado, M.J.; Bucek, A.; Onyimba, J.A.; Brandt, J.E.; Frisancho, J.A.; Kim, E.; Bedja, D.; Sung, Y.K.; Radtke, A.J.; et al. TLR3 deficiency induces chronic inflammatory cardiomyopathy in resistant mice following coxsackievirus B3 infection: Role for IL-4. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R267–R277. [Google Scholar] [CrossRef] [PubMed]
- Afanasyeva, M.; Wang, Y.; Kaya, Z.; Park, S.; Zilliox, M.J.; Schofield, B.H.; Hill, S.L.; Rose, N.R. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. Am. J. Pathol. 2001, 159, 193–203. [Google Scholar] [CrossRef]
- Diny, N.L.; Baldeviano, G.C.; Talor, M.V.; Barin, J.G.; Ong, S.; Bedja, D.; Hays, A.G.; Gilotra, N.A.; Coppens, I.; Rose, N.R.; et al. Eosinophil-derived IL-4 drives progression of myocarditis to inflammatory dilated cardiomyopathy. J. Exp. Med. 2017, 214, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ong, S.; Talor, M.V.; Barin, J.G.; Baldeviano, G.C.; Kass, D.A.; Bedja, D.; Zhang, H.; Sheikh, A.; Margolick, J.B.; et al. Cardiac fibroblasts mediate IL-17A-driven inflammatory dilated cardiomyopathy. J. Exp. Med. 2014, 211, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Abston, E.D.; Barin, J.G.; Cihakova, D.; Bucek, A.; Coronado, M.J.; Brandt, J.E.; Bedja, D.; Kim, J.B.; Georgakopoulos, D.; Gabrielson, K.L.; et al. IL-33 independently induces eosinophilic pericarditis and cardiac dilation: ST2 improves cardiac function. Circ. Heart Fail. 2012, 5, 366–375. [Google Scholar] [CrossRef]
- Fairweather, D.; Petri, M.A.; Coronado, M.J.; Cooper, L.T. Autoimmune heart disease: Role of sex hormones and autoantibodies in disease pathogenesis. Expert Rev. Clin. Immunol. 2012, 8, 269–284. [Google Scholar] [CrossRef]
- Fairweather, D.; Frisancho-Kiss, S.; Gatewood, S.; Njoku, D.; Steele, R.; Barrett, M.; Rose, N.R. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity 2004, 37, 131–145. [Google Scholar] [CrossRef]
- Tschope, C.; Muller, I.; Xia, Y.; Savvatis, K.; Pappritz, K.; Pinkert, S.; Lassner, D.; Heimesaat, M.M.; Spillmann, F.; Miteva, K.; et al. NOD2 (Nucleotide-Binding Oligomerization Domain 2) is a major pathogenic mediator of coxsackievirus B3-Induced myocarditis. Circ. Heart Fail. 2017, 10. [Google Scholar] [CrossRef]
- Fuse, K.; Chan, G.; Liu, Y.; Gudgeon, P.; Husain, M.; Chen, M.; Yeh, W.C.; Akira, S.; Liu, P.P. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of Coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation 2005, 112, 2276–2285. [Google Scholar] [CrossRef]
- Epelman, S.; Liu, P.P.; Mann, D.L. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 2015, 15, 117–129. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. Ca Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Perez, I.E.; Taveras Alam, S.; Hernandez, G.A.; Sancassani, R. Cancer therapy-related cardiac dysfunction: An overview for the clinician. Clin. Med. Insights Cardiol. 2019, 13, 1179546819866445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Di Florio, D.N.; Sin, J.; Coronado, M.J.; Atwal, P.S.; Fairweather, D. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. 2020, 31, 101482. [Google Scholar] [CrossRef] [PubMed]
- Cowan, J.R.; Salyer, L.; Wright, N.T.; Kinnamon, D.D.; Amaya, P.; Jordan, E.; Bamshad, M.J.; Nickerson, D.A.; Hershberger, R.E. SOS1 Gain-of-Function Variants in Dilated Cardiomyopathy. Circ. Genom. Precis. Med. 2020, 13, e002892. [Google Scholar] [CrossRef]
- Melchert, R.B.; Welder, A.A. Cardiovascular effects of androgenic-anabolic steroids. Med. Sci Sports Exerc 1995, 27, 1252–1262. [Google Scholar] [CrossRef]
- Scheuer, J.; Malhotra, A.; Schaible, T.F.; Capasso, J. Effects of gonadectomy and hormonal replacement on rat hearts. Circ. Res. 1987, 61, 12–19. [Google Scholar] [CrossRef]
- Vitale, C.; Mendelsohn, M.E.; Rosano, G.M. Gender differences in the cardiovascular effect of sex hormones. Nat. Rev. Cardiol. 2009, 6, 532–542. [Google Scholar] [CrossRef]
- Calippe, B.; Douin-Echinard, V.; Delpy, L.; Laffargue, M.; Lelu, K.; Krust, A.; Pipy, B.; Bayard, F.; Arnal, J.F.; Guery, J.C.; et al. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J. Immunol. 2010, 185, 1169–1176. [Google Scholar] [CrossRef]
- Kraft, L.; Erdenesukh, T.; Sauter, M.; Tschope, C.; Klingel, K. Blocking the IL-1beta signalling pathway prevents chronic viral myocarditis and cardiac remodeling. Basic Res. Cardiol. 2019, 114, 11. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Arenas, I.A.; Armstrong, S.J.; Plahta, W.C.; Xu, H.; Davidge, S.T. Estrogen improves cardiac recovery after ischemia/reperfusion by decreasing tumor necrosis factor-alpha. Cardiovasc. Res. 2006, 69, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Fliegner, D.; Gustafsson, J.A.; Regitz-Zagrosek, V. Role of the estrogen/estrogen-receptor-beta axis in the genomic response to pressure overload-induced hypertrophy. Physiol. Genom. 2011, 43, 438–446. [Google Scholar] [CrossRef]
- Planavila, A.; Laguna, J.C.; Vazquez-Carrera, M. Nuclear factor-kappaB activation leads to down-regulation of fatty acid oxidation during cardiac hypertrophy. J. Biol. Chem. 2005, 280, 17464–17471. [Google Scholar] [CrossRef]
- Blanco, J.G.; Sun, C.L.; Landier, W.; Chen, L.; Esparza-Duran, D.; Leisenring, W.; Mays, A.; Friedman, D.L.; Ginsberg, J.P.; Hudson, M.M.; et al. Anthracycline-related cardiomyopathy after childhood cancer: Role of polymorphisms in carbonyl reductase genes—A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
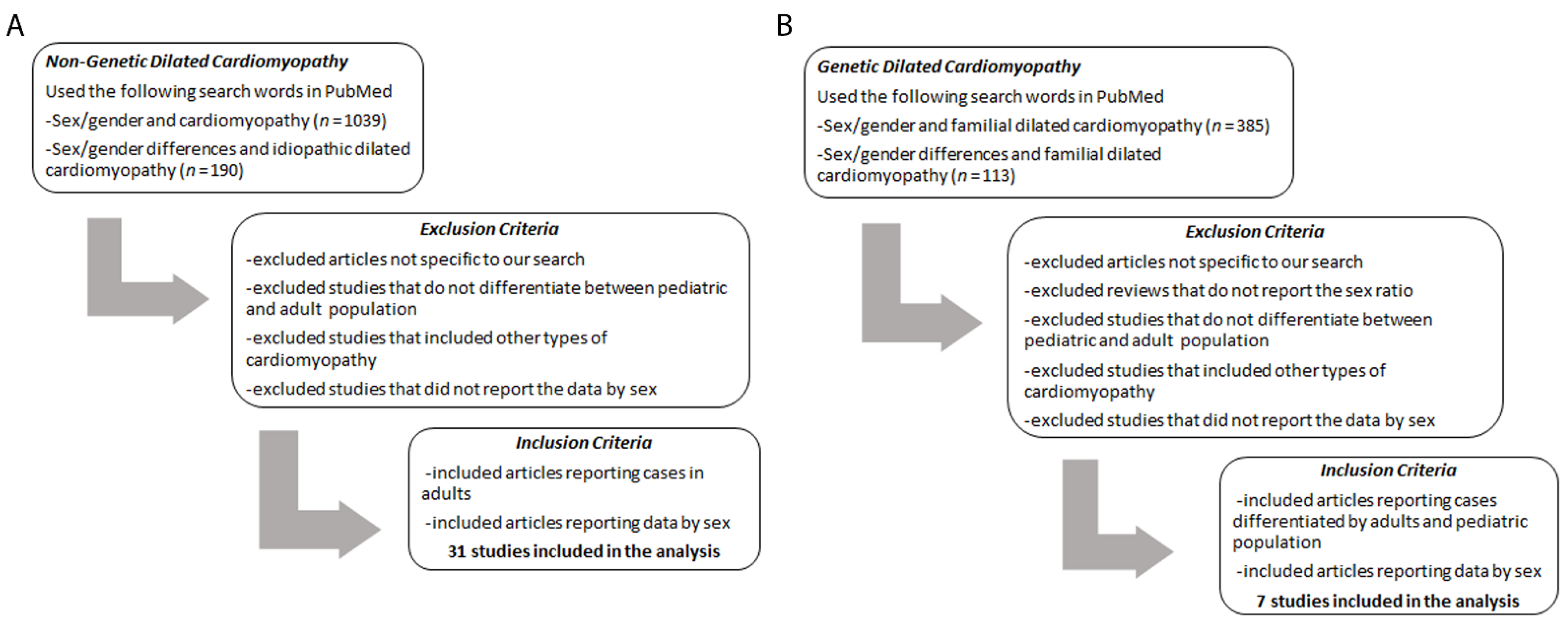
| Year of Publication | Patients (n) | Male:Female (n) | Sex Ratio (M:F) | Mean Age of Patients | Additional Information | References |
|---|---|---|---|---|---|---|
| 1949 | 35 | 25:10 | 2.5:1 | – | [27] | |
| 1968 | 22 | 13:9 | 1.4:1 | – | Coxsackievirus heart disease | [28] |
| 1980 | 164 | 109:55 | 1.9:1 | – | Coxsackievirus myocarditis progressing to DCM | [29] |
| 1984 | 41 | 27:14 | 1.9:1 | – | DCM | [30] |
| 1987 | 72 | 59:13 | 4.5:1 | 50 ± 15 | DCM | [31] |
| 1990 | 201 | 163:38 | 4.2:1 | 48 ± 11 | DCM | [32] |
| 1992 | 225 | 163:62 | 2.6:1 | 41 ± 12.3 | DCM | [33] |
| 1993 | 303 | 238:65 | 3.6:1 | Idiopathic DCM | [34] | |
| 1994 | 128 | 68:60 | 1.1:1 | 59 | DCM | [35] |
| 1995 | 441 | 309:132 | 2.3:1 | 43 ± 13 | DCM | [36] |
| 1996 | 144 | 118:26 | 4.5:1 | 39 ± 10.4 | Nonischemic DCM | [37] |
| 2000 | 131 | 108:23 | 4.7:1 | 52 ± 12 | DCM | [38] |
| 2004 | 458 | 326:132 | 2.4:1 | 58.3 | Nonischemic DCM | [39] |
| 2004 | 56 | 42:14 | 3:1 | 50.3 ± 2.2 | DCM | [40] |
| 2005 | 20 | 14:6 | 2.3:1 | 46.5 ± 10 | Recent onset CM | [10] |
| 2008 | 54 | 38:16 | 2.3:1 | – | DCM in elderly (65–83 years of age) | [41] |
| 2010 | 43 | 29:13 | 2.2:1 | – | Idiopathic DCM with new onset HF | [42] |
| 2010 | 115 | 100:35 | 1.5:1 | – | DCM | [43] |
| 2011 | 373 | 230:143 | 1.6:1 | 45 ± 14 | [44] | |
| 2012 | 95 | 52:43 | 1.2:1 | – | 95 DCM patients vs. 95 healthy subjects | [45] |
| 2013 c | 603 | 440:163 | 2.7:1 | – | Idiopathic DCM | [46] |
| 2013 | 96 | 66:30 | 2.2:1 | 53 ± 11.6 | DCM | [47] |
| 2014 | 373 | 230:143 | 1.6:1 | 45 ± 14 | DCM | [48] |
| 2014 | 853 | 614:239 | 2.5:1 | 45 ± 15 | Idiopathic DCM | [49] |
| 2015 | 213 | 128:85 | 1.5:1 | – | DCM | [50] |
| 2015 | 639 | 405:212 | 1.9:1 | – | DCM | [51] |
| 2015 | 16,091 | 11,059:5032 | 2.2:1 | 48.3 ± 12.6 | DCM UNOS Database | [52] |
| 2018 | 881 | 591:290 | 2:1 | 52 ± 15 | DCM | [53] |
| 2018 | 52 | 40:12 | 3.3:1 | 57.2 ± 7 | Non-ischemic DCM | [54] |
| 2018 | 1260 | 935:325 | 2.8:1 | – | CM Registry-DCM | [55] |
| 2019 | 35 | 24:11 | 2.1:1 | – | DCM with/without PH | [56] |
| Gene | Encoding Protein | Function of the Protein | References |
|---|---|---|---|
| TTN | Titin | Forms the structure of the sarcomere | [61] |
| LMNA | Lamin A/C | Nuclear membrane envelope | [71] |
| MYH7 | Beta-myosin heavy chain | Sarcomere | [57] |
| MYH6 | Alpha myosin heavy chain | Sarcomere | [57] |
| BAG3 | BAG family molecular chaperone regulator | Chaperone-assisted selective autophagy | [72,73] |
| MYPN | Myopalladin | Z-disc in the sarcomere | [74] |
| DSP | Desmoplakin | Desmosome | [75] |
| FLNC | Filamin C | Functions at Z discs | [76] |
| RBM20 | RNA- binding protein 20 | Spliceosome, RNA-binding protein | [77] |
| TTNT2 | Cardiac Troponin T | Sarcomere | [57] |
| SCN5A | Sodium ion channel | Ion channel | [78,79] |
| TTNC1 | Cardiac Troponin C | Sarcomere | [57] |
| TTNI3 | Cardiac Troponin I | Sarcomere | [57] |
| TPM1 | Alpha- Tropomyosin | Sarcomere | [58,80] |
| Year of Publication | Patients (n) | Male:Female | Sex Ratio (M:F) | Mean Age of Patients | Mode of Inheritance | References |
|---|---|---|---|---|---|---|
| 1989 | 45 | 33:12 | 2.7:1 | 54 | [26] | |
| 1999 b | 345 | 169:176 | 0.97:1 | Adults (38.3 ± 14) and children (<16 years of age) | AD | [85] |
| 2006 | 304 | 173:131 | 1.3:1 | – | AD (most common) | [86] |
| 2012 | 312 | 208:104 | 2:1 | 48.6 ± 13.0 | [61] | |
| 2013 | 418 | 233:185 | 1.3:1 | 46 ± 13 | [87] | |
| 2015 | 492 | 311:181 | 1.7:1 | 42.1 ± 13.1 | [52] | |
| 2017 | 72 | 48:24 | 2:1 | 34 | AD (TTN +/−) | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, A.; Norton, N.; Bruno, K.A.; Cooper, L.T., Jr.; Atwal, P.S.; Fairweather, D. Sex Differences, Genetic and Environmental Influences on Dilated Cardiomyopathy. J. Clin. Med. 2021, 10, 2289. https://doi.org/10.3390/jcm10112289
Jain A, Norton N, Bruno KA, Cooper LT Jr., Atwal PS, Fairweather D. Sex Differences, Genetic and Environmental Influences on Dilated Cardiomyopathy. Journal of Clinical Medicine. 2021; 10(11):2289. https://doi.org/10.3390/jcm10112289
Chicago/Turabian StyleJain, Angita, Nadine Norton, Katelyn A. Bruno, Leslie T. Cooper, Jr., Paldeep S. Atwal, and DeLisa Fairweather. 2021. "Sex Differences, Genetic and Environmental Influences on Dilated Cardiomyopathy" Journal of Clinical Medicine 10, no. 11: 2289. https://doi.org/10.3390/jcm10112289
APA StyleJain, A., Norton, N., Bruno, K. A., Cooper, L. T., Jr., Atwal, P. S., & Fairweather, D. (2021). Sex Differences, Genetic and Environmental Influences on Dilated Cardiomyopathy. Journal of Clinical Medicine, 10(11), 2289. https://doi.org/10.3390/jcm10112289






