Alcohol Septal Ablation: An Option on the Rise in Hypertrophic Obstructive Cardiomyopathy
Abstract
1. Introduction
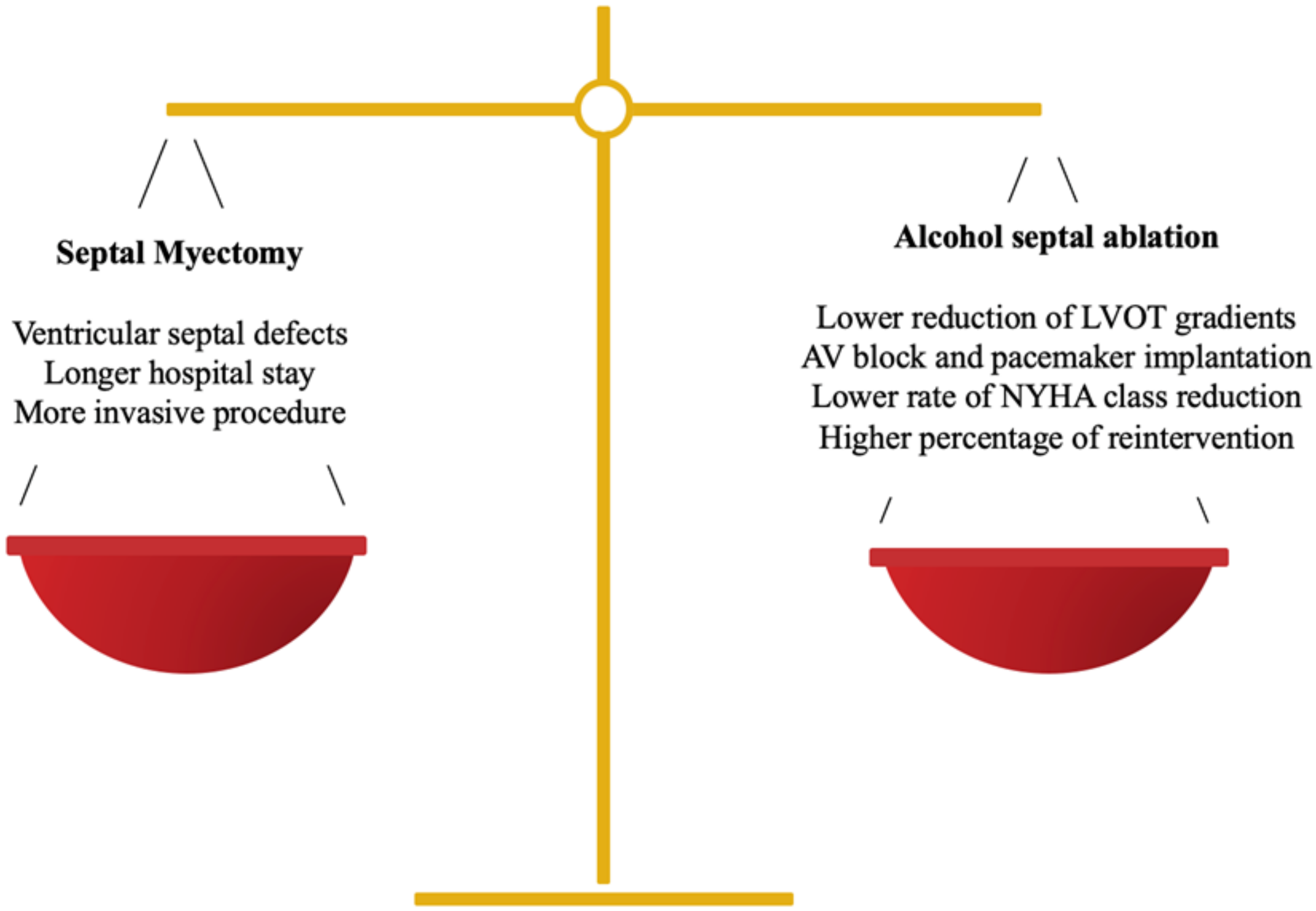
2. Septal Reduction Therapy
2.1. Surgical Approach
2.2. Percutaneous Approach
3. ASA Technique and Considerations
3.1. Anatomical Considerations
3.2. Role of Echocardiography in ASA
3.3. Step-by-Step and Technical Considerations
- (1)
- The first step is to obtain an arterial access with a 6 or 7 F sheath, radial or femoral. At the beginning, femoral was the most common access, with a change in the approach in recent years. Radial access was proposed to achieve less patient discomfort, early ambulation, and less vascular complications [35]. Depending on the preferences of the center, two accesses could be performed, one for the catheter used for ablation, and the other for an invasive monitoring of the gradient during the procedure.
- (2)
- The common femoral vein (or internal jugular vein) is punctured and a temporary pacemaker (PM) is placed on the right ventricle. This step is important to ensure a fast response in case of complete atrio-ventricular (AV) block [36].
- (3)
- After vascular accesses are obtained, 100 units per 1 kg of weight of unfractionated heparin should be administrated.
- (4)
- LM ostium should be engaged with standard guide catheters 6–7 F (Judkins left, Amplatz left or Extra Back-Up). If invasive monitoring is performed during the procedure, a pigtail catheter is placed into the left ventricle (LV) via a second arterial access.
- (5)
- The gradient present through the LVOT must be measured using TTE and/or the catheters placed in the aorta and LV.
- (6)
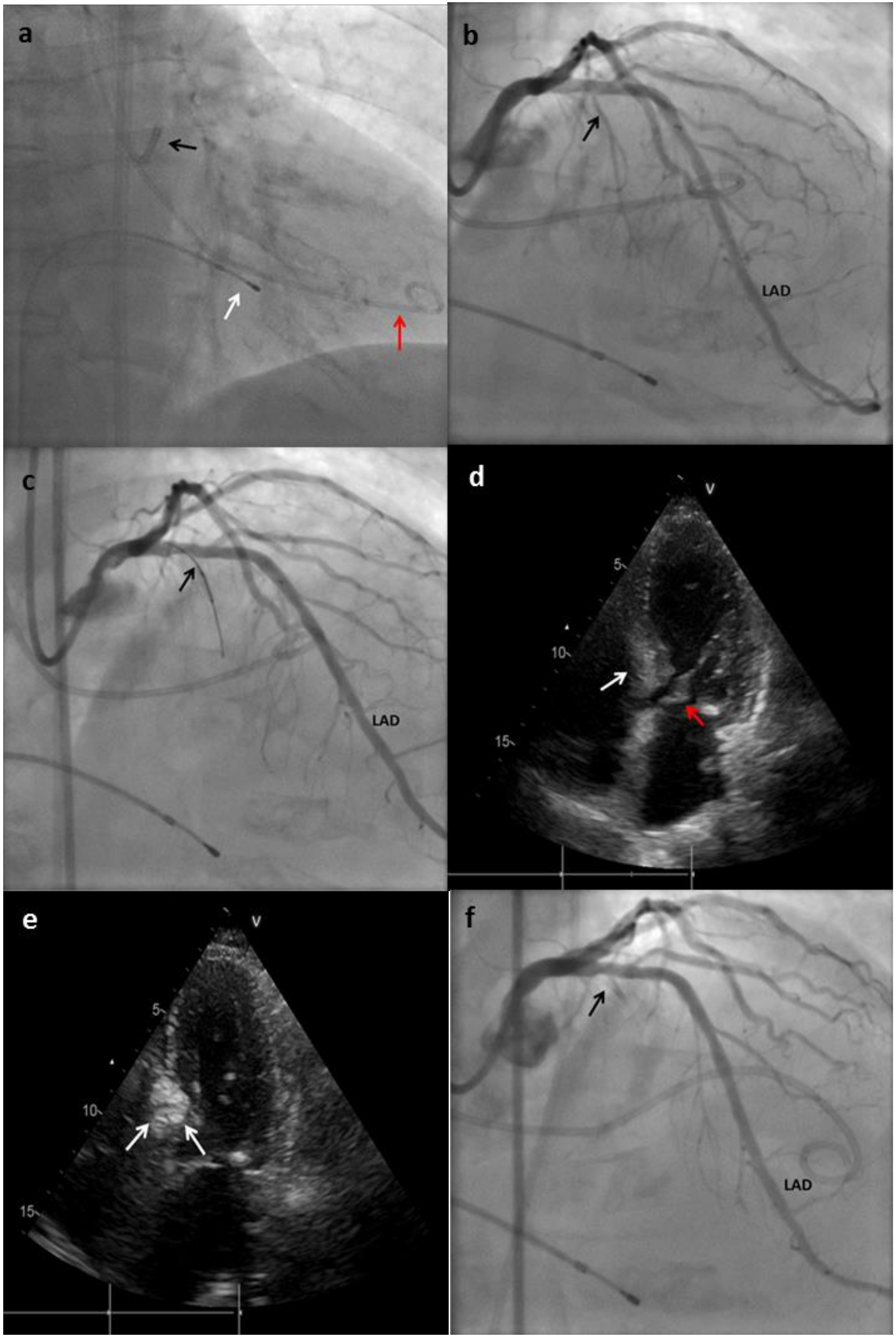
- Coronary angiography is performed to identify a suitable septal branch. The trajectory of the septal arteries can be visualized through right anterior oblique or postero-anterior cranial projections, while the left anterior oblique view allows differentiating whether the septal branches run on the right or left side of the septum.
- (7)
- A 0.014-inch coronary guidewire is advanced to the first septal artery and, through this, an over-the-wire (OTW) balloon is placed in this vessel. In some cases, the takeoff of the septal vessel is extremely angulated, making it difficult to advance the balloon; when this situation occurs, an extra support wire can be used. The size of the balloon should generally be short (1.5–2.5 mm in diameter, 6–10 mm in length, and with a balloon-artery ratio of 1.3:1 approximately or usually 0.25 mm greater than the vessel diameter). Short balloons are recommended to avoid hyperselectivity in the presence of a septal branch with early bifurcation [26,37]. After placing the balloon, the guidewire is removed.
- (8)
- The balloon is inflated at low pressure (5 or 10 atm), and then slow injection of 1–2 mL of angiographic contrast should be performed to test the correct occlusion of the septal, absence of contrast reflux into the LAD, and to rule out the presence of collateral flow from the septal branch toward another branch of the left or right coronary system [26,29]. During balloon inflation, continuous monitoring of the gradient could show a significant reduction, indicating a favorable target vessel and generally predicts a good response to ASA [27].
- (9)
- Echocardiographic contrast is injected through the OTW balloon lumen. The possible area affected by the ablation is evaluated. If the contrast medium enhances an inadequate territory for ASA, the balloon must be deflated and repositioned in another branch. In some cases, it is not possible to identify a suitable vessel that perfuses the base of the hypertrophied septum at the point where maximal systolic anterior motion occurs, in which case the procedure should be discontinued [27]. Inability to identify a satisfactory septal branch occurs in approximately 10% of the candidates.
- (10)
- Having selected the septal target vessel via the prior angiographic and echocardiographic assessment, ˃94° ethanol is injected through the OTW balloon lumen into the branch. The amount of ethanol should be of 1 to 3 mL [1]. Some authors describe an efficient and safe way to measure the quantity of ethanol as 0.1 mL per 1 mm of septal thickness. Higher doses were associated with higher rates of complications and postprocedural mortality [38]. Potential explanations could lie in the more extended infarct scar due to the higher alcohol dose. Ethanol must be injected slowly, usually 1 min per ml. A slow injection, rather than a bolus administration was demonstrated to be safer [27,39].
- (11)
- Analgesics (i.e., morphine) can be administrated to avoid the pain caused by the iatrogenic myocardial infarction.
- (12)
- After instillation, balloon occlusion should be maintained for at least 3 to 5 min. The catheter is flushed with saline before the balloon is deflated and removed from the septal branch to prevent spillage of alcohol into the LAD circulation [27].
- (13)
- The balloon is removed, and a coronary angiogram is performed to ensure septal branch no-reflow and to rule out any unexpected complication.
- (14)
- Continual monitoring is used to measure the effects of ASA in gradient values. A gradient reduction ≥ 50% from baseline is considered successful (by echocardiography or invasive hemodynamics). If the ablation fails to achieve this improvement, a second septal or subseptal branch should be explored [28].
- (15)
- Once the objective of gradient reduction has been achieved, or there are no additional septal branches perfusing the area that need ablation, the procedure is terminated. The arterial accesses are removed and subjected to hemostasis, and the temporary PM is secured.
3.4. Postprocedural Care
4. Periprocedural Complications
5. Limitations of the Technique
6. Long-Term Results
7. Myosin Inhibitors: Perspectives of a Future Option
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliott, P.M.; Uk, C.; Anastasakis, A.; Germany, M.A.B.; Germany, M.B.; Cecchi, F.; France, P.C.; Alain, A.; France, H.; Lafont, A.; et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy the task force for the diagnosis and management of hypertrophic cardiomyopathy of the european society of cardiology (ESC). European 2014, 35, 2733–2779. [Google Scholar] [CrossRef]
- Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Kimmelstiel, C.; Kittleson, M.; Link, M.S.; Maron, M.S.; et al. Circulation AHA/ACC CLINICAL PRACTICE GUIDELINE 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy association joint committee on clinical practice guidelines. Circulation 2020, 142, 558–631. [Google Scholar] [CrossRef]
- Veselka, J.; Faber, L.; Liebregts, M.; Cooper, R.; Januska, J.; Kashtanov, M.; Dabrowski, M.; Hansen, P.R.; Seggewiss, H.; Hansvenclova, E.; et al. Short- And long-term outcomes of alcohol septal ablation for hypertrophic obstructive cardiomyopathy in patients with mild left ventricular hypertrophy: A propensity score matching analysis. Eur. Heart J. 2019, 40, 1681–1687. [Google Scholar] [CrossRef]
- Richard, P.; Charron, P.; Carrier, L.; Ledeuil, C.; Cheav, T.; Pichereau, C.; Benaiche, A.; Isnard, R.; Dubourg, O.; Burban, M.; et al. Hypertrophic cardiomyopathy: Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 2003, 107, 2227–2232. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Basso, C.; Schiavon, M.; Thiene, G. Does sports activity enhance the risk of sudden cardiac death? J. Cardiovasc. Med. 2006, 7, 228–233. [Google Scholar] [CrossRef]
- Sherrid, M.V.; Barac, I.; McKenna, W.J.; Elliott, P.M.; Dickie, S.; Chojnowska, L.; Casey, S.; Maron, B.J. Multicenter Study of the Efficacy and Safety of Disopyramide in Obstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2005, 45, 1251–1258. [Google Scholar] [CrossRef]
- Rigopoulos, A.G.; Seggewiss, H. A Decade of percutaneous septal ablation in hypertrophic cardiomyopathy. Circ. J. 2011, 75, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.K.; Swaminathan, R.V.; Looser, P.; Minutello, R.M.; Wong, S.C.; Bergman, G.; Naidu, S.S.; Gade, C.L.F.; Charitakis, K.; Singh, H.S.; et al. Hospital volume outcomes after septal myectomy and alcohol septal ablation for treatment of obstructive hypertrophic cardiomyopathy: US nationwide inpatient database, 2003–2011. JAMA Cardiol. 2016, 1, 324–332. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.G.; Brockengbrough, E.C. Surgical treatment of idiopathic hypertrophic subaortic stenosis: Technic and hemodynamic results of subaortic ventriculomyotomy. Ann. Surg. 1961, 154, 181–189. [Google Scholar] [CrossRef]
- Kotkar, K.D.; Said, S.M.; Dearani, J.A.; Schaff, H.V. Hypertrophic obstructive cardiomyopathy: The mayo clinic experience. Ann. Cardiothorac. Surg. 2017, 6, 329–336. [Google Scholar] [CrossRef]
- Ferrazzi, P.; Spirito, P.; Iacovoni, A.; Calabrese, A.; Migliorati, K.; Simon, C.; Pentiricci, S.; Poggio, D.; Grillo, M.; Amigoni, P.; et al. Transaortic chordal cutting mitral valve repair for obstructive hypertrophic cardiomyopathy with mild septal hypertrophy. J. Am. Coll. Cardiol. 2015, 66, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Sherrid, M.V.; Chaudhry, F.A.; Swistel, D.G. Obstructive hypertrophic cardiomyopathy: Echocardiography, pathophysiology, and the continuing evolution of surgery for obstruction. Ann. Thorac. Surg. 2003, 75, 1684. [Google Scholar] [CrossRef]
- Swaminathan, M.; DeBruijn, N.P.; Glower, D.D.; Mathew, J.P. Unexpected transesophageal echocardiographic finding after septal myectomy. J. Cardiothorac. Vasc. Anesth. 2002, 16, 384–385. [Google Scholar] [CrossRef]
- Maron, B.J.; Dearani, J.A.; Ommen, S.R.; Maron, M.S.; Schaff, H.V.; Nishimura, R.A.; Ralph-Edwards, A.; Rakowski, H.; Sherrid, M.V.; Swistel, D.G.; et al. Low operative mortality achieved with surgical septal myectomy role of dedicated hypertrophic cardiomyopathy centers in the management of dynamic subaortic obstruction. J. Am. Coll. Cardiol. 2015, 66, 1307–1308. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.; Rakowski, H. Does myectomy convey survival benefit in hypertrophic cardiomyopathy? Heart Fail. Clin. 2007, 3, 275–288. [Google Scholar] [CrossRef]
- Panaich, S.S.; Badheka, A.O.; Chothani, A.; Mehta, K.; Patel, N.J.; Deshmukh, A.; Singh, V.; Savani, G.T.; Arora, S.; Patel, N.; et al. Results of ventricular septal myectomy and hypertrophic cardiomyopathy (from nationwide inpatient sample [1998–2010]). Am. J. Cardiol. 2014, 114, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Ommen, S.R.; Maron, B.J.; Olivotto, I.; Maron, M.S.; Cecchi, F.; Betocchi, S.; Gersh, B.J.; Ackerman, M.J.; McCully, R.B.; Dearani, J.A.; et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Rowin, E.J.; Udelson, J.E.; Maron, M.S. Clinical spectrum and management of heart failure in hypertrophic cardiomyopathy. JACC Hear. Fail. 2018, 6, 353–363. [Google Scholar] [CrossRef]
- Sorajja, P. Alcohol septal ablation for obstructive hypertrophic cardiomyopathy: A word of balance. J. Am. Coll. Cardiol. 2017, 70, 489–494. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Rosenberg, M.A.; Fifer, M.A.; Palacios, I.F.; Lowry, P.A.; Ruskin, J.N.; Sanborn, D.M.; Picard, M.H.; Vlahakes, G.J.; Mela, T.; et al. Ventricular arrhythmia following alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 2009, 104, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Veselka, J.; Faber, L.; Liebregts, M.; Cooper, R.; Januska, J.; Krejci, J.; Bartel, T.; Dabrowski, M.; Hansen, P.R.; Almaas, V.M.; et al. Outcome of alcohol septal ablation in mildly symptomatic patients with hypertrophic obstructive cardiomyopathy: A long-term follow-up study based on the euro-alcohol septal ablation registry. J. Am. Heart Assoc. 2017, 6, 1–6. [Google Scholar] [CrossRef]
- Sorajja, P.; Valeti, U.; Nishimura, R.A.; Ommen, S.R.; Rihal, C.S.; Gersh, B.J.; Hodge, D.O.; Schaff, H.V.; Holmes, D.R. Outcome of alcohol septal ablation for obstructive. Circulation 2015, 118, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Seggewiss, H.; Schaff, H.V. Hypertrophic obstructive cardiomyopathy: Surgical myectomy and septal ablation. Circ. Res. 2017, 121, 771–783. [Google Scholar] [CrossRef]
- Bytyçi, I.; Nistri, S.; Mörner, S.; Henein, M.Y. Alcohol septal ablation versus septal myectomy treatment of obstructive hypertrophic cardiomyopathy: A systematic review and meta-analysis. J. Clin. Med. 2020, 9, 3062. [Google Scholar] [CrossRef]
- Pelliccia, F.; Niccoli, G.; Gragnano, F.; Limongelli, G.; Moscarella, E.; Andò, G.; Esposito, A.; Stabile, E.; Ussia, G.P.; Tarantini, G.; et al. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: A contemporary reappraisal. EuroIntervention 2019, 15, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Valeti, U.S.; Nishimura, R.A. Featured topics alcohol septal ablation for hypertrophic cardiomyopathy: Indications and technique. Catheter. Cardiovasc. Interv. 2005, 389, 375–389. [Google Scholar] [CrossRef]
- Spaziano, M.; Sawaya, F.J.; Lefèvre, T. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: Indications, technical aspects, and clinical outcomes. J. Invasive Cardiol. 2017, 29, 404–410. [Google Scholar] [CrossRef]
- El Masry, H.; Breall, J.A. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Curr. Cardiol. Rev. 2014, 4, 193. [Google Scholar] [CrossRef] [PubMed]
- Savarimuthu, S.; Harky, A. Alcohol septal ablation: A useful tool in our arsenal against hypertrophic obstructive cardiomyopathy. J. Cardiac Surg. 2020, 35, 2017–2024. [Google Scholar] [CrossRef]
- Monakier, D.; Woo, A.; Puri, T.; Schwartz, L.; Ross, J.; Jamorski, M.; Yang, H.; Liu, Z. Usefulness of myocardial contrast echocardiographic quantification of risk area for predicting postprocedural complications in patients undergoing septal ethanol ablation for obstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 2004, 94, 1515–1522. [Google Scholar] [CrossRef]
- Faber, L.; Seggewiss, H.; Ziemssen, P.; Gleichmann, U. Intraprocedural myocardial contrast echocardiography as a routine procedure in percutaneous transluminal septal myocardial ablation: Detection of threatening myocardial necrosis distant from the septal target area. Catheter. Cardiovasc. Interv. 1999, 47, 462–466. [Google Scholar] [CrossRef]
- Faber, L.; Seggewiss, H.; Gleichmann, U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy. results with respect to intraprocedural myocardial. Differences 1998, 98, 2415–2421. [Google Scholar]
- Luis, J.; Mur, M.; Tahoces, L.S.; Luis, J.; Barceló, M.; Muñoz, D.R.; Hernández, R.; Fernández-golfín, C.; Luis, J.; Gómez, Z. Alcohol septal ablation in hypertrophic cardiomyopathy. 3d contrast echocardiography allows localization and quanti fi cation of the extension of intraprocedural vascular recruitment. Int. J. Cardiol. 2014, 174, 761–762. [Google Scholar] [CrossRef]
- Sawaya, F.J.; Louvard, Y.; Spaziano, M.; Morice, M.C.; Hage, F.; El-Khoury, C.; Roy, A.; Garot, P.; Hovasse, T.; Benamer, H.; et al. Short and long-term outcomes of alcohol septal ablation with the trans-radial versus the trans-femoral approach a single center-experience. Int. J. Cardiol. 2016, 220, 7–13. [Google Scholar] [CrossRef] [PubMed]
- El-Jack, S.S.; Nasif, M.; Blake, J.W.; Dixon, S.R.; Grines, C.L.; O’Neill, W.W. Predictors of complete heart block after alcohol septal ablation for hypertrophic cardiomyopathy and the timing of pacemaker implantation. J. Interv. Cardiol. 2007, 20, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Afanasyev, A.V.; Bogachev-prokophiev, A.V.; Kashtanov, M.G.; Astapov, D.A.; Zalesov, A.S.; Budagaev, S.A.; Sharifulin, R.M.; Idov, E.M.; Zheleznev, S.I. Myectomy versus alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.M.; Nagueh, S.F.; Spencer, W.H.; Lakkis, N.M. Complete heart block: Determinants and clinical impact in patients with hypertrophic obstructive cardiomyopathy undergoing nonsurgical septal reduction therapy. J. Am. Coll. Cardiol. 2003, 42, 296–300. [Google Scholar] [CrossRef]
- Kuhn, H.; Strunk-mueller, C.; Bartelsmeier, M. Survival after transcoronary ablation of septal hypertrophy in hypertrophic obstructive cardiomyopathy (TASH): A 10 year experience. Clin. Res. Cardiol. 2008, 243, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Veselka, J.; Procházková, Š.; Duchoňová, R.; Bolomová-Homolová, I.; Páleníčková, J.; Tesař, D.; Červinka, P.; Honěk, T. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: Lower alcohol dose reduces size of infarction and has comparable hemodynamic and clinical outcome. Catheter. Cardiovasc. Interv. 2004, 235, 231–235. [Google Scholar] [CrossRef]
- Fitzgerald, P.; Kusumoto, F. The effects of septal myectomy and alcohol septal ablation for hypertrophic cardiomyopathy on the cardiac conduction system. J. Interv. Card. Electrophysiol. 2018, 52, 403–408. [Google Scholar] [CrossRef]
- Valeti, U.S.; Nishimura, R.A.; Holmes, D.R.; Araoz, P.A.; Glockner, J.F.; Breen, J.F.; Ommen, S.R.; Gersh, B.J.; Tajik, A.J.; Rihal, C.S.; et al. Comparison of surgical septal myectomy and alcohol septal ablation with cardiac magnetic resonance imaging in patients with hypertrophic obstructive cardiomyopathy. J. Am. Coll. Cardiol. 2007, 49, 350–357. [Google Scholar] [CrossRef]
- Talreja, D.R.; Nishimura, R.A.; Edwards, W.D.; Valeti, U.S.; Ommen, S.R.; Tajik, A.J.; Dearani, J.A.; Schaff, H.V.; Holmes, D.R. Alcohol septal ablation versus surgical septal myectomy: Comparison of effects on atrioventricular conduction tissue. J. Am. Coll. Cardiol. 2004, 44, 2329–2332. [Google Scholar] [CrossRef] [PubMed]
- Rigopoulos, A.G.; Panou, F.; Kremastinos, D.T.; Seggewiss, H. Alcohol septal ablation in hypertrophic obstructive cardiomyopathy. Hell. J. Cardiol. 2009, 50, 511–522. [Google Scholar] [CrossRef]
- Cuisset, T.; Lefèvre, T. Contemporary techniques for catheter-based intervention for hypertrophic obstructive cardiomyopathy. EuroIntervention 2016, 12, X44–X47. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Ueda, M.; Nakayama, T.; Saegusa, N.; Uehara, M.; Lee, K.; Sekine, T.; Daimon, M.; Kobayashi, Y.; Funabashi, N.; et al. Occurrence of de novo sustained monomorphic ventricular tachycardia induced after percutaneous transluminal alcohol septal myocardial ablation for hypertrophic obstructive cardiomyopathy. Int. J. Cardiol. 2007, 119, 403–407. [Google Scholar] [CrossRef]
- Rigopoulos, A.; Sepp, R.; Palinkas, A.; Ungi, I. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: Collateral vessel communication between septal branches. J. Interv. Cardiol. 2006, 113, 67–69. [Google Scholar] [CrossRef]
- Veselka, J.; Kvistholm, M.; Liebregts, M.; Januska, J.; Krejci, J.; Bartel, T.; Dabrowski, M.; Riis, P.; Bundgaard, H.; Steggerda, R.; et al. Low procedure-related mortality achieved with alcohol septal ablation in european patients. Int. J. Cardiol. 2016, 209, 194–195. [Google Scholar] [CrossRef]
- Sum, S.; Field, M.; Gupta, D.; Cameron, D. Surgical septal myectomy or alcohol septal ablation: Which approach offers better outcomes for patients with hypertrophic obstructive cardiomyopathy ? Interact. Cardiovasc. Thorac. Surg. 2017, 24, 951–961. [Google Scholar] [CrossRef]
- Batzner, A.; Pfeiffer, B.; Neugebauer, A.; Aicha, D.; Blank, C.; Seggewiss, H. Survival after alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. J. Am. Coll. Cardiol. 2018, 72, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- An, S.Y.; Yang, Y.J.; Hang, F.; Wang, Z.M.; Fan, C.M. Procedural complication and long term outcomes after alcohol septal ablation in patients with obstructive hypertrophic cardiomyopathy: Data from China. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De la Torre Hernandez, J.M.; Centol, M.M.; Saenz, L.; Rodriguez, D.F.; Carren, M.S.; de Carlos, F.G.; Pin, P.; Tenas, M.S.; Zueco, J. Effectiveness and safety beyond 10 years of percutaneous transluminal septal ablation in hypertrophic obstructive cardiomyopathy. Rev. Española Cardiol. 2014, 67, 353–358. [Google Scholar] [CrossRef]
- Kashtanov, M.; Rzhannikova, A.; Chernyshev, S.; Kardapoltsev, L.; Idov, E.; Berdnikov, S. Results of ten-year follow-up of alcohol septal ablation in patients with obstructive hypertrophic cardiomyopathy. Int. J. Angiol. 2018, 27, 202–207. [Google Scholar] [CrossRef]
- Veselka, J.; Jensen, M.K.; Liebregts, M.; Januska, J.; Krejci, J.; Bartel, T.; Dabrowski, M.; Hansen, P.R.; Almaas, V.M.; Seggewiss, H.; et al. Long-term clinical outcome after alcohol septal ablation for obstructive hypertrophic cardiomyopathy: Results from the Euro-ASA registry. Eur. Heart J. 2016, 37, 1517–1523. [Google Scholar] [CrossRef]
- Jahnlová, D.; Tomašov, P.; Adlová, R.; Januška, J.; Krejčí, J.; Dabrowski, M.; Veselka, J. Outcome of patients ≥ 60 years of age after alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Arch. Med. Sci. 2019, 15, 650–655. [Google Scholar] [CrossRef]
- Fernandes, V.L.; Nagueh, S.F.; Franklin, J.; Wang, W.; Roberts, R.; Spencer, W.H. A prospective follow-up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy-the baylor experience (1996–2002). Clin. Cardiol. 2005, 28, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Sohns, C.; Sossalla, S.; Schmitto, J.D.; Jacobshagen, C.; Raab, B.W.; Obenauer, S.; Maier, L.S. Visualization of transcoronary ablation of septal hypertrophy in patients with hypertrophic obstructive cardiomyopathy: A comparison between cardiac MRI, invasive measurements and echocardiography. Clin. Res. Cardiol. 2010, 99, 359–368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akita, K.; Tsuruta, H.; Yuasa, S.; Murata, M.; Fukuda, K.; Maekawa, Y. Prognostic significance of repeated brain natriuretic peptide measurements after percutaneous transluminal septal myocardial ablation in patients with drug-refractory hypertrophic obstructive cardiomyopathy. Open Hear. 2018, 5, e000786. [Google Scholar] [CrossRef]
- Anderson, R.L.; Trivedi, D.V.; Sarkar, S.S.; Henze, M.; Ma, W.; Gong, H.; Rogers, C.S.; Gorham, J.M.; Wong, F.L.; Morck, M.M.; et al. Deciphering the super relaxed state of human β-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc. Natl. Acad. Sci. USA 2018, 115, E8143–E8152. [Google Scholar] [CrossRef]
- Olivotto, I.; Oreziak, A.; Barriales-Villa, R.; Abraham, T.P.; Masri, A.; Garcia-Pavia, P.; Saberi, S.; Lakdawala, N.K.; Wheeler, M.T.; Owens, A.; et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 396, 759–769. [Google Scholar] [CrossRef]
| Favors SM | Favors ASA | |
|---|---|---|
| Clinical factors | ||
| Young age | + | |
| Advanced age | + | |
| High surgical risk/severe comorbidity | + | |
| Frailty | + | |
| Cardiac conditions | ||
| Previous cardiac surgery | + | |
| Previous pacemaker or defibrillator | + | |
| Right bundle branch block | + | |
| Left bundle branch block | + | |
| Mid-ventricular obstruction | + | |
| Operator related factors | ||
| Local operator experience in SM | + | |
| Local operator experience in ASA | + | |
| Patient’s preference | ± | ± |
| Indications | Contraindications |
|---|---|
| Severe symptoms (NYHA III-IV, CCS III-IV angina, presyncope, or recurrent syncope) despite GDMT. | Presence of a supra or subvalvular aortic membrane |
| LVOT gradient ≥ 50 mm Hg at rest or with provocation despite maximum-tolerated medical treatment | Severe coronary artery disease requiring coronary artery bypass graft surgery |
| At least one septal artery supplying the target septal area (left ventricular outflow tract obstruction zone) | Severe aortic stenosis requiring surgical valve replacement |
| Life expectancy > 1 year, absence of comorbidities that would compromise clinical improvement (i.e., severe dementia) | Severe valvular or mitral valve abnormality requiring surgical treatment |
| Septal thickness > 30 mm or ≤16 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arévalos, V.; Rodríguez-Arias, J.J.; Brugaletta, S.; Micari, A.; Costa, F.; Freixa, X.; Masotti, M.; Sabaté, M.; Regueiro, A. Alcohol Septal Ablation: An Option on the Rise in Hypertrophic Obstructive Cardiomyopathy. J. Clin. Med. 2021, 10, 2276. https://doi.org/10.3390/jcm10112276
Arévalos V, Rodríguez-Arias JJ, Brugaletta S, Micari A, Costa F, Freixa X, Masotti M, Sabaté M, Regueiro A. Alcohol Septal Ablation: An Option on the Rise in Hypertrophic Obstructive Cardiomyopathy. Journal of Clinical Medicine. 2021; 10(11):2276. https://doi.org/10.3390/jcm10112276
Chicago/Turabian StyleArévalos, Victor, Juan José Rodríguez-Arias, Salvatore Brugaletta, Antonio Micari, Francesco Costa, Xavier Freixa, Mónica Masotti, Manel Sabaté, and Ander Regueiro. 2021. "Alcohol Septal Ablation: An Option on the Rise in Hypertrophic Obstructive Cardiomyopathy" Journal of Clinical Medicine 10, no. 11: 2276. https://doi.org/10.3390/jcm10112276
APA StyleArévalos, V., Rodríguez-Arias, J. J., Brugaletta, S., Micari, A., Costa, F., Freixa, X., Masotti, M., Sabaté, M., & Regueiro, A. (2021). Alcohol Septal Ablation: An Option on the Rise in Hypertrophic Obstructive Cardiomyopathy. Journal of Clinical Medicine, 10(11), 2276. https://doi.org/10.3390/jcm10112276






