Abstract
Background/Purpose: The rates and outcomes of primary biliary cholangitis (PBC) in Taiwan remain unclear. Methods: A nationwide population-based cohort study (Taiwan National Health Insurance Research Database, 2002–2015) was conducted. Data from four PBC cohorts with various definitions were compared (cohort 1 (C1): ICD-9-CM (571.6); C2: alkaline phosphatase (Alk-P) and antimitochondrial antibody (AMA) measurements; C3: Alk-p and AMA measurements and ursodeoxycholic acid (UDCA) treatment; C4: ICD-9-CM (571.6), Alk-p and AMA measurements and UDCA treatment). Results: The average prevalence rate ranged from 9.419/105 (C4) to 307.658/105 (C2), and the female-to-male ratio ranged from 1.192 (C1) to 3.66 (C4). Prevalence rates increased over time in all cohorts. The average incidence rates ranged from 1.456/105 (C4) to 66.386/105 (C2). Incidence rates decreased over time in C1 (−9.09%, p < 0.0001) and C4 (−6.68%, p < 0.0001) and remained steady in the others. C4 had the lowest prevalence and incidence rates and highest female-to-male ratio. Cirrhosis rates ranged from 7.21% (C2) to 39.34% (C4), hepatoma rates ranged from 2.77%(C2) to 6.66%(C1), liver transplantation (LT) rates ranged from 1.07% (C2) to 6.77% (C4), and mortality rates ranged from 18.24% (C2) to 47.36% (C1). C4 had the highest LT (6.77%), osteoporosis (13.87%) and dyslipidemia rates (17.21%). Conclusions: Based on the reported ranges of reasonable rates, female predominance and characteristic outcomes, C4 was the most representative Taiwanese PBC cohort, with average prevalence and incidence rates of 9.419/105 and 1.456/105, respectively, and a female-to-male ratio of 3.66. In a 14-year period, cirrhosis, hepatoma, LT, and mortality were noted in 39.34%, 5.52%, 6.77%, and 34.22% of C4 patients, respectively.
1. Introduction
Primary biliary cholangitis (PBC), formerly called primary biliary cirrhosis [1], is an idiopathic chronic autoimmune liver disease that predominantly affects middle-aged women [2]. Without treatment, most PBC patients eventually develop hepatic fibrosis and may require liver transplantation during the late disease stage [3]. Ursodeoxycholic acid (UDCA) and obeticholic acid (OCA) are currently the two licensed therapies for PBC [4]. However, some patients treated with UDCA or OCA do not exhibit adequate responses, as only 60–70% of PBC patients treated with UDCA exhibit complete responses [5], and up to 60% of UDCA suboptimal responders do not have a therapeutic response to OCA [6]. The early detection and treatment of PBC is therefore important to prevent complications [7].
Most of the information on PBC has been derived from studies conducted in Europe [4] or the USA [2]. Nationwide epidemiological data on PBC are lacking in Taiwan, an Asian country where OCA is still not available for the treatment of PBC. Several population-based studies have defined PBC based on administrative data on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM:571.6), or ICD-10-CM, codes and used those data to determine the prevalence or incidence rates of PBC in various countries [8,9,10,11,12,13]. However, in addition to PBC, the ICD-9-CM code 571.6 represents secondary biliary cirrhosis, which is not uncommon in Taiwan, where hepatolithiasis is prevalent [14]. Therefore, the number of true PBC cases might be less than the reported number, and biases may arise from differences in coding practices across different clusters [10]. While the ICD-10-CM code K74.3 is specific for PBC, it was not used in Taiwan until 2016; thus, the precise annual incidence rates for PBC in Taiwan cannot be estimated based on this ICD-10-CM code as the washout time for surveying incidence rates, which should be more than 4 years, has not yet been reached [15].
Accordingly, we conducted a retrospective study with data from the Taiwan National Health Insurance Research Database (TNHIRD). Four PBC cohorts defined with various combinations of the ICD-9-CM code, biochemistry measurements and/or UDCA administration were assessed. The associated prevalence and incidence rates and outcomes were compared among the four PBC cohorts to identify the most accurately representative PBC cohort in Taiwan.
2. Methods
2.1. TNHIRD Samples and Measurements
Given that positivity for the antimitochondrial antibody (AMA) with elevated alkaline phosphatase (Alk-p) levels are used to establish the diagnosis of PBC in most cases [16], UDCA is the first-line therapy for PBC [17] and interventional therapy but not UDCA is the main treatment for most patients with secondary biliary cholangitis [18], therefore the epidemiological characteristics of PBC in Taiwan might be comprehensively examined by using the ICD-9-CM code, biochemical parameters and treatment data to define patients with PBC in the TNHIRD, which provides the medical information of a nationwide population of 26,573,661 persons, including the National Health Insurance (NHI) administrative database, the Cancer Registry Database, and the Death Registry Database. The mandatory, single-payer NHI program provides comprehensive coverage including ambulatory care, hospital services, laboratory tests, and prescription drugs. More than 99% of the population is enrolled in the program, and approximately 92% of healthcare organizations are contracted with the NHI Administration. However, the use of the ICD-9-CM code is not a prerequisite for the prescription of UDCA for PBC in Taiwan. Some patients might have never been assigned the ICD-9-CM code, but were diagnosed with PBC based on their results for Alk-p and AMA, then treated with UDCA if it was not contraindicated. These patients could also have complete records of their biochemical results and medications in the TNHIRD despite the fact that they were never assigned the ICD-9-CM code. Thus, four PBC cohorts were defined as follows:
PBC cohort 1 (C1): ICD-9-CM code (571.6)
PBC cohort 2 (C2): Alk-p and AMA measurements
PBC cohort 3 (C3): Alk-p and AMA measurements and UDCA treatment
PBC cohort 4 (C4): ICD-9-CM code (571.6), Alk-p and AMA measurements, and UDCA treatment
For these PBC cohorts, the date a patient was first assigned the ICD-9-CM code or underwent the first AMA test was assumed to be the index date of diagnosis, and the data from this date were considered the baseline data. Only those patients who met the definitions for C1, C2, C3, or C4 for the first time when they were ≥18 years old were enrolled. The outcomes, including liver cirrhosis, hepatocellular carcinoma (HCC), liver transplantation (LT), dyslipidemia, osteoporosis, and mortality, were recorded. Information on HCC diagnosis was retrieved from the Cancer Registry Database. Subjects were observeduntil the date of death or the end of follow-up (31 December 2015), whichever came first.
2.2. Statistics
All statistical analyses were performed using the Statistical Analysis Software (SAS version 9.4, SAS Institute Inc., Cary, NC, USA). Continuous variables are summarized as the mean ± standard deviations, and categorical variables are summarized as numbers and percentages (%). To compare variables in different groups, continuous variables were analyzed using Student’s t-tests, whereas categorical variables were analyzed using chi-squared or Fisher’s exact tests, as appropriate. Data were analyzed by one-way analysis of variance (ANOVA) when comparing variables among multiple groups. The annual national prevalence rate of PBC was determined as the number of PBC cases divided by the number of adult NHI enrollees in the corresponding year from 2002 to 2015. We used a 4-year washout period (2002–2005) [15] to calculate the annual incidence rate of PBC from 2006 to 2015 while excluding prevalent cases due to the data recording beginning in 2002. The annual incidence rates of PBC were calculated as the number of new cases of PBC per 100,000 people per year. Age- and sex-adjusted rates were calculated by dividing the expected number of cases in each age and sex group by the age- and sex-specific standard population. For the standard population, we used the population of inhabitants at the end of 2010 from the Department of Household Registration Affairs, Ministry of the Interior of Taiwan, as the NHI covers >99% of the population in Taiwan. The average annual percentage change, analyzed by the JoinPoint regression program (Version 4.7.0.0), was adopted to evaluate trends in the annual prevalence and incidence rates. Data were analyzed by ANOVA when comparing variables among multiple groups. Post hoc analyses were performed with the least significant difference multiple comparison test. Statistical significance was defined at the 5% level.
2.3. Primary and Secondary Outcomes
The primary outcomes were the prevalence and incidence rates in the most representative PBC cohort in Taiwan; the secondary outcomes were the hepatic and extrahepatic complications in that representative cohort.
2.4. Institutional Review Board
The study was conducted in accordance with good clinical practice and all applicable regulations, including the Declaration of Helsinki and local regulatory requirements, and was approved by the institutional ethics committee. The need to obtain consent was waived due to the national data used in this study being deidentified by encrypting any personally identifying information.
3. Results
3.1. Baseline Characteristics
As shown in Figure 1 and Table 1, 12,843 (females: 6235 (48.55%)), 130,093 (females: 74,191(57.03%)), 43,883 (females: 23,959 (54.60%)) and 3396 (females: 2533 (74.59%)) individuals met the criteria for C1, C2, C3 and C4, respectively. The mean age ranged from 53.73 years (C2) to 57.61 years (C4). The female-to-male ratio ranged from 0.943 (C1) to 2.93 (C4). Among the four cohorts, C4 had the highest female-to-male ratio and mean age, and C2 had the lowest mean age.
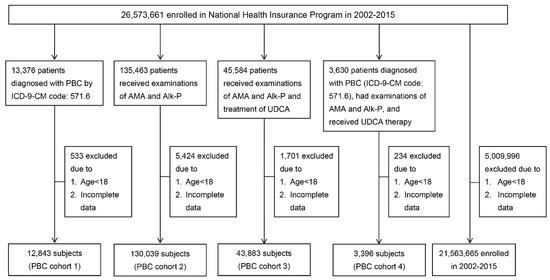
Figure 1.
A schematic flowchart of patient enrollment. PBC: primary biliary cholangitis;ICD-9-CM: International Classification of Disease, Ninth Revision, Clinical Modification; AMA: antimitochondrial antibodies; Alk-P: alkaline phosphatase; UDCA: ursodeoxycholic acid; HBV: hepatitis B virus; HCV: hepatitis C virus; YO: years old.

Table 1.
Baseline characteristics of the 4 PBC cohorts.
3.2. Prevalence Rates
As shown in Table 2, in the 21,536,665 subjects (Figure 1) enrolled from 2002 to 2015, the average annual age- and sex-adjusted prevalence rates ranged from 9.419/105 (C4) to 307.658/105 (C2). The average age-adjusted prevalence rate of PBC ranged from 14.74/105 (C4) to 366.428/105 (C2) for females and from 4.028/105 (C4) to 248.106/105 (C2) for males, with the female-to-male ratio ranging from 1.192 (C1) to 3.66 (C4). C4 had the lowest prevalence rate and highest female-to-male ratio among the four cohorts. There were increasing trends in the age- and sex-adjusted prevalence rates for all four cohorts (Figure 2A–D, Table 2).

Table 2.
Prevalence rates of the 4 PBC cohorts.
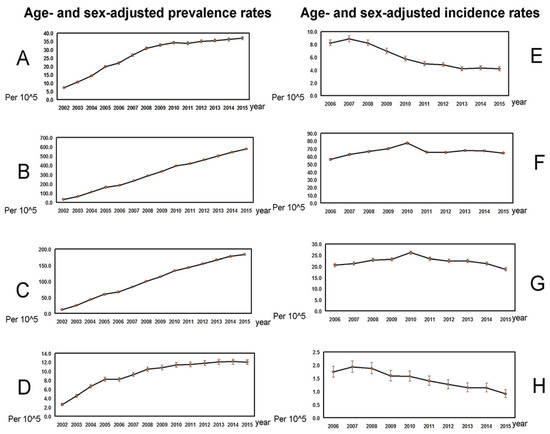
Figure 2.
The trends in age- and sex-adjusted prevalence (A–D) and incidence (E–H) rates (per 105; mean ± 95% confidence interval of prevalence rates). (A), Prevalence rates in PBC cohort 1. (B), Prevalence rates in PBC cohort 2. (C), Prevalence rates in PBC cohort 3. (D), Prevalence rates in PBC cohort 4. (E), Incidence rates in PBC cohort 1. (F), Incidence rates in PBC cohort 2. (G), Incidence rates in PBC cohort 3. (H), Incidence rates in PBC cohort 4.
3.3. Incidence Rates
As shown in Table 3, from 2006 to 2015, the average annual age- and sex-adjusted incidence rates ranged from 1.456/105 (C4) to 66.386/105 (C2), the average age-adjusted incidence rates ranged from 2.093/105 (C4) to 74.045/105 (C2) for females and from 0.810/105 (C4) to 58.634/105 (C2) for males. The female-to-male ratio ranged from 0.850 (C1) to 2.583 (C4). C4 had the lowest incidence rate and highest female-to-male ratio among the four cohorts. There were decreasing trends in the annual incidence rates in C1 (−9.09%, p < 0.0001) and C4 (−6.68%, p < 0.0001), while the incidence rates remained steady in C2 and C3 (Figure 2E–H, Table 3).

Table 3.
Incidence rates of the 4 PBC cohorts.
3.4. Hepatic and Extrahepatic Outcomes
The prevalence rates of incident outcomes in PBC patients are listed in Table 4. The cirrhosis rates ranged from 7.21% (C2) to 39.34% (C4), the HCC rates ranged from 2.77% (C2) to 6.66% (C1), and the LT rates ranged from 1.07% (C2) to 6.77% (C4). The osteoporosis rates ranged from 7.06% (C2) to 13.87% (C4), and the dyslipidemia rates ranged from 10.82% (C1) to 17.21% (C4). The mortality rates ranged from 18.24% (C2) to 47.36% (C1). Among the four cohorts, C4 had the highest rates of cirrhosis, LT, osteoporosis and dyslipidemia; C1 had the highest rates of HCC and mortality; and C2 had the lowest rates of cirrhosis, HCC, LT, osteoporosis and mortality.

Table 4.
Prevalence rates of various complications of 4 PBC cohorts.
4. Discussion
Consistent with most studies [13,19], the prevalence rates increased over time in all PBC cohorts, reflecting the fact that most patients diagnosed with PBC, especially those treated with UDCA, have an expected lifespan that does not differ from that of the general population [1]. The inclusion criteria for the various PBC cohorts accounted for the differences in the numbers of enrolled patients: C2 and C4 had the highest and lowest numbers (and prevalence and incidence rates), respectively, as they had the least and most strict definitions of PBC, respectively. The prevalence rates of PBC thus ranged from 9.419/105 (C4) to 307.658/105 (C2), and the incidence rates ranged from 1.456/105 (C4) to 66.386/105 (C2) in Taiwan. Given that the range of prevalence rates for PBC was 1.91–40.2/105 [20,21], and the range of incidence rates was 0.33–5.8/105 [20], only C1 (prevalence rate: 25.8/105; incidence rate: 6.062/105) and C4 were within the reported ranges; thus, C1 and C4 might be the most accurately representative PBC cohorts in Taiwan based on the prevalence and incidence rates. With regard to the female-to-male ratio, which was 1.192, 1.477, 1.30 and 3.66 in C1, C2, C3 and C4, respectively, the ratio of 3.66 in C4 is the most accurate representation of the degree of female predominance in the population of patients with PBC in Taiwan. The female-to-male ratio was found to be 4:1 in Hong Kong [10], which is also located in Asia and has a population of the same ethnicity as that of Taiwan. The female-to-male ratio in the PBC cohort in Hong Kong was based on the definition of PBC according to the ICD-9-CM code [10]. Although the median female-to-male ratio in the population with PBC is generally accepted to be 10:1 [4], a declining trend in the female-to-male ratio in patients with PBC had been reported recently [15], and it has been suggested that PBC may have been under-diagnosed in men in early studies.
Notably, most PBC studies have shown that the incidence rates either remained steady [13,19] or increased over time [22]. In contrast, although C4 had reasonable prevalence and incidence rates [20,21] and a similarly reasonable female-to-male ratio [10,15], it showed a clear decreasing trend in the incidence rate. As the risk of PBC has increased in areas with high levels of socioeconomic deprivation [23], the decrease in the incidence rate might reflect the profound socioeconomic improvement in the Taiwanese population. On the other hand, it might also indicate that the criteria used to establish the diagnosis of PBC in this cohort were too strict to enroll new cases. Future prospective studies based on comprehensive ICD-10-CM codes to survey the precise trends in the annual incidence rates of PBC in Taiwan are needed.
The fact that C2 had the lowest rates of cirrhosis, HCC, and LT among the four cohorts suggests that C2 was composed of patients with early liver disease rather than PBC; thus, the prognosis was the best in that cohort even though no UDCA was prescribed. The fact that C2 had the lowest mean age also supports the findings of the least severe outcomes in this cohort. Interestingly, C4, the candidate cohort that most accurately represented Taiwanese PBC patients, yielded the highest rate of cirrhosis, 39.34%, which reflects the fact that only 60–70% of patients with PBC treated with UDCA exhibit complete responses [5]. C4 also had the highest mean age of the four cohorts. However, the highest rates of HCC and mortality were noted in C1, rather than in C4. This phenomenon indicates that a substantial proportion of patients with secondary biliary cirrhosis might have been included into C1, leading to the relatively worse prognosis in C1 than in any of the other cohorts. This might partially account for the higher LT rate in C4 than in C1, as LT is not recommended for most cases of secondary biliary cirrhosis, while up to 40% of PBC patients with suboptimal responses to UDCA require LT, or second-line treatment [24] with OCA [6] which is not yet available in Taiwan. These differences in outcomes between C1 and C4 suggest that defining PBC with biochemistry measurements and UDCA treatment, in addition to the ICD-9-CM code, increases the representativeness of C4 for PBC patients in Taiwan. Consistent with this finding, C4 had the highest rates of osteoporosis and dyslipidemia, both of which are common extrahepatic complications of PBC [7]. Moreover, the mortality of C4 (34.22%) was within the reported range of mortality in PBC patients [25] and supported the reliability of C4 as the representative PBC corhort in Taiwan. As mentioned above, some unregistered (i.e., lacking ICD-9-CM (571.6) data) patients might be diagnosed with PBC based on biochemistry data, and treated with UDCA. Those patients were categorized into C3. Although C3 was not the most representative PBC cohort for Taiwan in all aspects, the inclusion of some unregistered PBC patients in C3 might account for the underestimations of the prevalence and incidence rates in C4. Thus, C3 provides the extreme upper limits of the prevalence (104.254/105) and incidence (22.217/105) rates of PBC in Taiwan.
There are limitations of the current study. First, as previously mentioned, the ICD-9-CM code 571.6 includes cases of secondary biliary cirrhosis, which might bias the data in C1 and C4, although we attempted to reduce this bias by adding the Alk-p and AMA measurements and UDCA treatment as enrollment criteria for C4. Given that the TNHIRD does not contain direct laboratory results, and UDCA treatment is not specific to PBC, even subjects who met the criteria for C4 are not necessarily true cases of PBC. Second, hepatitis B virus (HBV) infection is hyperendemic, and hepatitis C virus (HCV) infection is wide spread in Taiwan [26]. Both HBV [27] and HCV infections [28,29] are associated with many hepatic and extrahepatic complications, which might bias the outcomes in all four PBC cohorts. Third, some primary sclerosing cholangitis (PSC) patients with cirrhosis might have been categorized into C4, while the portion of PSC in C4 should be negligible, since PSC is very rare in Taiwan [30]. Fourth, PBC-specific antinuclear antibodies (ANAs), including anti-sp100 and anti-gp210, might aid to diagnose AMA-negative PBC [4]. However, these autoantibodies were not assessed in most labs in Taiwan, and the associated data thus are not available in TNHIRD. A future prospective study of PBC patients who were all registered with the ICD-10-CM code K74.3,with comprehensive surveys of anti-sp100 and anti-gp210, and are not infected with HBV or HCV, is needed to precisely verify the prevalence, incidence and outcomes of PBC in Taiwan.
A summary of the characteristics of the 4 cohorts, particularly C4, is shown in Figure 3.
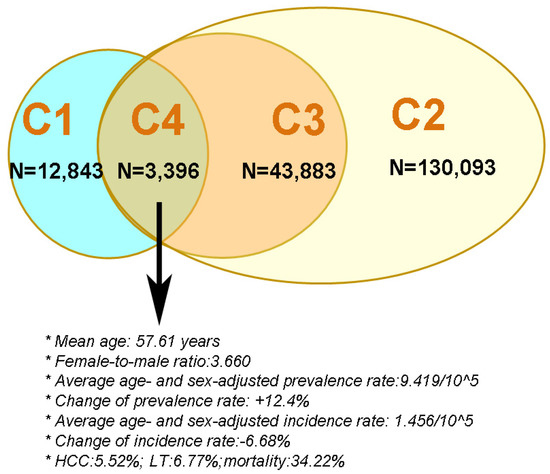
Figure 3.
The criteria for and sample size of the 4 primary biliary cholangitis cohorts in Taiwan. C: cohort; blue circle: PBC cohort 1; green oval; PBC cohort 4; brown circle: PBC cohort 3; yellow oval: PBC cohort 2; HCC: hepatocellular carcinoma; LT: liver transplantation.
Taken together, the cohort that most accurately represented PBC patients in Taiwan was C4, with average age- and sex-adjusted prevalence and incidence rates of 9.419/105 and 1.456/105, respectively. Both rates were within the ranges reported worldwide, and the female-to-male ratio was 3.66, which is close to what was reported in other Asian countries. The prevalence rates increased over time, while the incidence rates decreased. In this PBC cohort, 39.34% had cirrhosis, 5.52% had HCC, 6.77% underwent LT, and 34.22% died within 14 years.
Author Contributions
C.-J.C.: data collection, statistics and manuscript writing; J.-S.C., H.-E.W., C.-W.H., J.-H.H., W.-T.C., M.-Y.C., C.-Y.L., and R.-N.C.: data collection and manuscript writing; H.-P.K.: statistics and manuscript writing; M.-L.C.: study design and implementation, manuscript drafting, and critical revision of the manuscript for important intellectual content. All authors have read and agreedto the published version of the manuscript.
Funding
This study was supported by grants from the Chang Gung Medical Research Program (CMRPG3I0412, CMRPG3K0721 and CMRPG1K0111) and the National Science Council (MOST 108-2314-B-182-051-, 109-2314-B-182-024- and 109-2629-B-182-002-) to MLC. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest.
Institutional Review Board Statement
The study was conducted in accordance with good clinical practice and all applicable regulations, including the Declaration of Helsinki and local regulatory requirements, and was approved by the institutional ethics committee of Chang Gung Memorial Hospital (5 October 2016; No. 104-7005B).
Informed Consent Statement
The need to obtain consent was waived due to the national data used in this study being deidentified by encrypting any personally identifying information.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank Shu-Chun Chen, Chia-Hui Tsai, Chun-Kai Liang and Shuen-Shian Shiau from the Liver Research Center, Chang Gung Memorial Hospital, Taiwan, for their assistance with data mining.
Conflicts of Interest
The authors declare that there are no conflict of interest.
References
- Beuers, U.; Gershwin, M.E.; Gish, R.G.; Invernizzi, P.; Jones, D.E.; Lindor, K.; Ma, X.; Mackay, I.R.; Parés, A.; Tanaka, A.; et al. Changing nomenclature for PBC: From ‘cirrhosis’ to ‘cholangitis’. Hepatology 2015, 62, 1620–1622. [Google Scholar] [CrossRef]
- Samur, S.; Klebanoff, M.; Banken, R.; Pratt, D.S.; Chapman, R.; Ollendorf, D.A.; Loos, A.M.; Corey, K.; Hur, C.; Chhatwal, J. Long-term clinical impact and cost-effectiveness of obeticholic acid for the treatment of primary biliary cholangitis. Hepatology 2017, 65, 920–928. [Google Scholar] [CrossRef]
- John, B.V.; Aitcheson, G.; Schwartz, K.B.; Shabbir Khakoo, N.; Dahman, B.; Deng, Y.; Goldberg, D.; Martin, P.; Taddei, T.; Levy, C.; et al. Male Gender is Associated with Higher Rates of Liver-related Mortality in Primary Biliary Cholangitis and Cirrhosis. Hepatology 2021. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Poupon, R. Primary biliary cirrhosis: A 2010 update. J. Hepatol. 2010, 52, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Mason, A.; Luketic, V.; Lindor, K.; Gordon, S.C.; Mayo, M.; Kowdley, K.V.; Vincent, C.; Bodhenheimer, H.C., Jr.; Parés, A.; et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology 2015, 148, 751–761.e8. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Cheng, Y.T.; Chang, M.L.; Chien, R.N. The extrahepatic events of Asian patients with primary biliary cholangitis: A 30-year cohort study. Sci. Rep. 2019, 9, 7577. [Google Scholar] [CrossRef]
- Sayiner, M.; Golabi, P.; Stepanova, M.; Younossi, I.; Nader, F.; Racila, A.; Younossi, Z.M. Primary Biliary Cholangitis in Medicare Population: The Impact on Mortality and Resource Use. Hepatology 2019, 69, 237–244. [Google Scholar] [CrossRef]
- Kim, K.A.; Ki, M.; Choi, H.Y.; Kim, B.H.; Jang, E.S.; Jeong, S.H. Population-based epidemiology of primary biliary cirrhosis in South Korea. Aliment. Pharmacol. Ther. 2016, 43, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Seto, W.K.; Fung, J.; Lai, C.L.; Yuen, M.F. Epidemiology and Natural History of Primary Biliary Cholangitis in the Chinese: A Territory-Based Study in Hong Kong between 2000 and 2015. Clin. Transl. Gastroenterol. 2017, 8, e116. [Google Scholar] [CrossRef]
- Shahab, O.; Sayiner, M.; Paik, J.; Felix, S.; Golabi, P.; Younossi, Z.M. Burden of Primary Biliary Cholangitis Among Inpatient Population in the United States. Hepatol. Commun. 2019, 3, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.M.; Mason, A.; Peltekian, K.M.; Shah, H.; Thiele, S.; Borrelli, R.; Fischer, A. Epidemiology and liver transplantation burden of primary biliary cholangitis: A retrospective cohort study. CMAJ Open 2018, 6, E664–E670. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Myers, R.P.; Shaheen, A.A.; Fong, A.; Burak, K.W.; Wan, A.; Swain, M.G.; Hilsden, R.J.; Sutherland, L.; Quan, H. Epidemiology and natural history of primary biliary cirrhosis in a Canadian health region: A population-based study. Hepatology 2009, 50, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Sheen-Chen, S.M.; Cheng, Y.F.; Chen, F.C.; Chou, F.F.; Lee, T.Y. Ductal dilatation and stenting for residual hepatolithiasis: A promising treatment strategy. Gut 1998, 42, 708–710. [Google Scholar] [CrossRef]
- Lleo, A.; Jepsen, P.; Morenghi, E.; Carbone, M.; Moroni, L.; Battezzati, P.M.; Podda, M.; Mackay, I.R.; Gershwin, M.E.; Invernizzi, P. Evolving Trends in Female to Male Incidence and Male Mortality of Primary Biliary Cholangitis. Sci. Rep. 2016, 6, 25906. [Google Scholar] [CrossRef] [PubMed]
- Lammers, W.J.; van Buuren, H.R.; Hirschfield, G.M.; Janssen, H.L.; Invernizzi, P.; Mason, A.L.; Ponsioen, C.Y.; Floreani, A.; Corpechot, C.; Mayo, M.J.; et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: An international follow-up study. Gastroenterology 2014, 147, 1338–1349.e5. [Google Scholar] [CrossRef]
- Alvaro, D.; Carpino, G.; Craxi, A.; Floreani, A.; Moschetta, A.; Invernizzi, P. Primary biliary cholangitis management: Controversies, perspectives and daily practice implications from an expert panel. Liver Int. 2020, 40, 2590–2601. [Google Scholar] [CrossRef]
- Hu, B.; Sun, B.; Cai, Q.; Wong Lau, J.Y.; Ma, S.; Itoi, T.; Moon, J.H.; Yasuda, I.; Zhang, X.; Wang, H.-P.; et al. Asia-Pacific consensus guidelines for endoscopic management of benign biliary strictures. Gastrointest. Endosc. 2017, 86, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhou, Y.; Haller, I.V.; Romanelli, R.J.; VanWormer, J.J.; Rodriguez, C.V.; Anderson, H.; Boscarino, J.A.; Schmidt, M.A.; Daida, Y.G.; et al. Increasing Prevalence of Primary Biliary Cholangitis and Reduced Mortality With Treatment. Clin. Gastroenterol. Hepatol. 2018, 16, 1342–1350.e1. [Google Scholar] [CrossRef]
- Podda, M.; Selmi, C.; Lleo, A.; Moroni, L.; Invernizzi, P. The limitations and hidden gems of the epidemiology of primary biliary cirrhosis. J. Autoimmun. 2013, 46, 81–87. [Google Scholar] [CrossRef]
- Zeng, N.; Duan, W.; Chen, S.; Wu, S.; Ma, H.; Ou, X.; You, H.; Kong, Y.; Jia, J. Epidemiology and clinical course of primary biliary cholangitis in the Asia-Pacific region: A systematic review and meta-analysis. Hepatol. Int. 2019, 13, 788–799. [Google Scholar] [CrossRef]
- Tanaka, A.; Mori, M.; Matsumoto, K.; Ohira, H.; Tazuma, S.; Takikawa, H. Increase trend in the prevalence and male-to-female ratio of primary biliary cholangitis, autoimmune hepatitis, and primary sclerosing cholangitis in Japan. Hepatol. Res. 2019, 49, 881–889. [Google Scholar] [CrossRef]
- McNally, R.J.; James, P.W.; Ducker, S.; Norman, P.D.; James, O.F. No rise in incidence but geographical heterogeneity in the occurrence of primary biliary cirrhosis in North East England. Am. J. Epidemiol. 2014, 179, 492–498. [Google Scholar] [CrossRef]
- Aguilar, M.T.; Carey, E.J. Current Status of Liver Transplantation for Primary Biliary Cholangitis. Clin. Liver Dis. 2018, 22, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.L.; Jones, D.E.; Metcalf, J.V.; Park, J.B.; Burt, A.D.; Bassendine, M.F.; James, O.F. Presentation and mortality of primary biliary cirrhosis in older patients. Age Ageing 2000, 29, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Chen, M.Y.; Yeh, C.T.; Lin, H.S.; Lin, M.S.; Huang, T.J.; Chang, M.L. Sexual Dimorphic Metabolic Alterations in Hepatitis C Virus-infected Patients: A Community-Based Study in a Hepatitis B/Hepatitis C Virus Hyperendemic Area. Medicine 2016, 95, e3546. [Google Scholar] [CrossRef]
- Chang, M.L.; Cheng, J.S.; Chien, R.N.; Liaw, Y.F. Hepatitis Flares Are Associated with Better Outcomes Than No Flare in Patients With Decompensated Cirrhosis and Chronic Hepatitis B Virus Infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2064–2072.e2. [Google Scholar] [CrossRef]
- Chang, M.L.; Lin, Y.S.; Chang, M.Y.; Hsu, C.L.; Chien, R.N.; Fann, C.S. Accelerated cardiovascular risk after viral clearance in hepatitis C patients with the NAMPT-rs61330082 TT genotype: An 8-year prospective cohort study. Virulence 2021, 12, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.L.; Chen, W.T.; Hu, J.H.; Chen, S.C.; Gu, P.W.; Chien, R.N. Altering retinol binding protein 4 levels in hepatitis C: Inflammation and steatosis matter. Virulence 2020, 11, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.L.; Lin, H.Y.; Lin, W.H.; Lai, H.S. Choledochal cyst originating from primary sclerosing cholangitis in a child. Int. Surg. 2011, 96, 316–319. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).