Ras, TrkB, and ShcA Protein Expression Patterns in Pediatric Brain Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
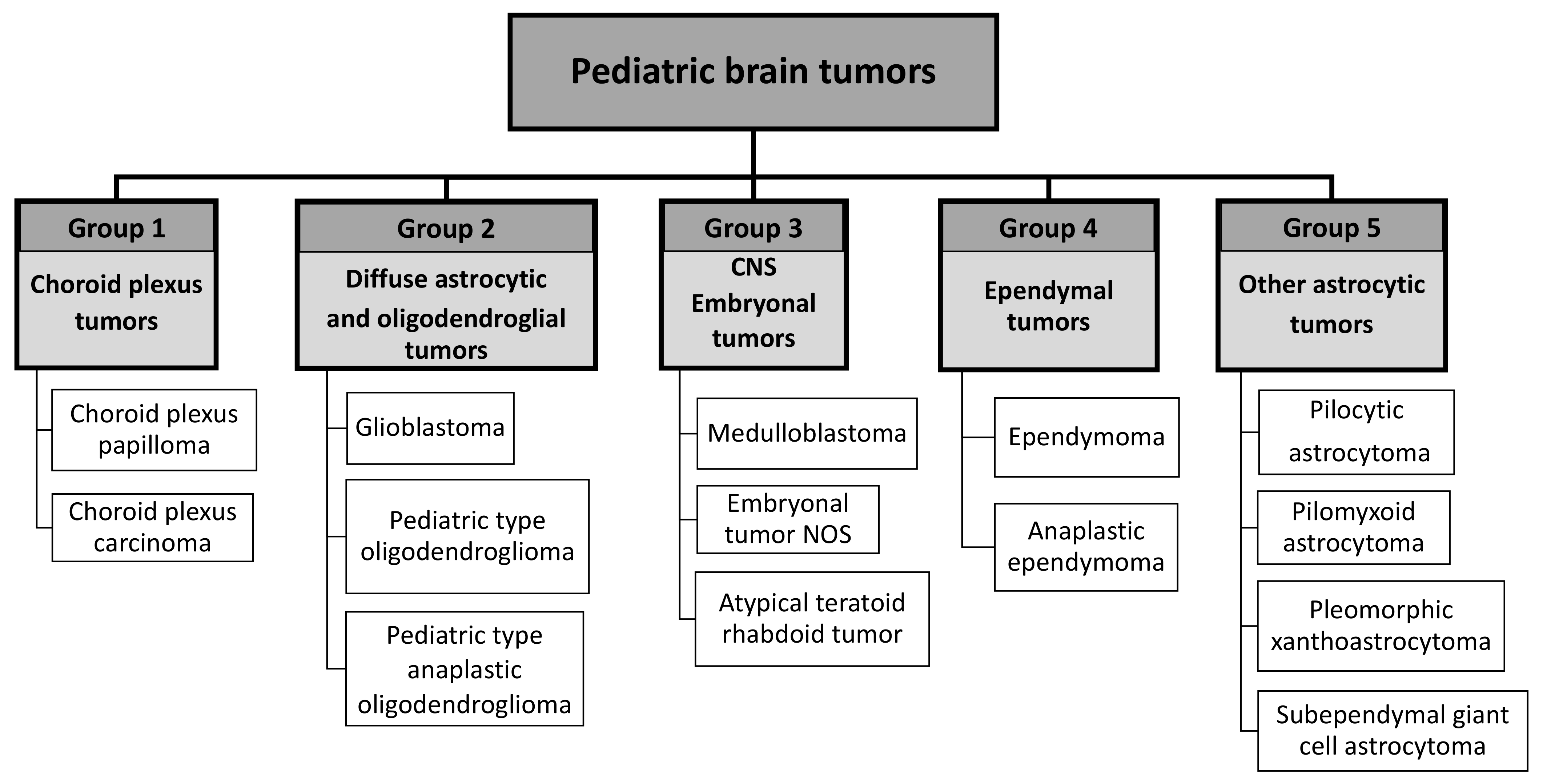
2.2. Tumor Classification
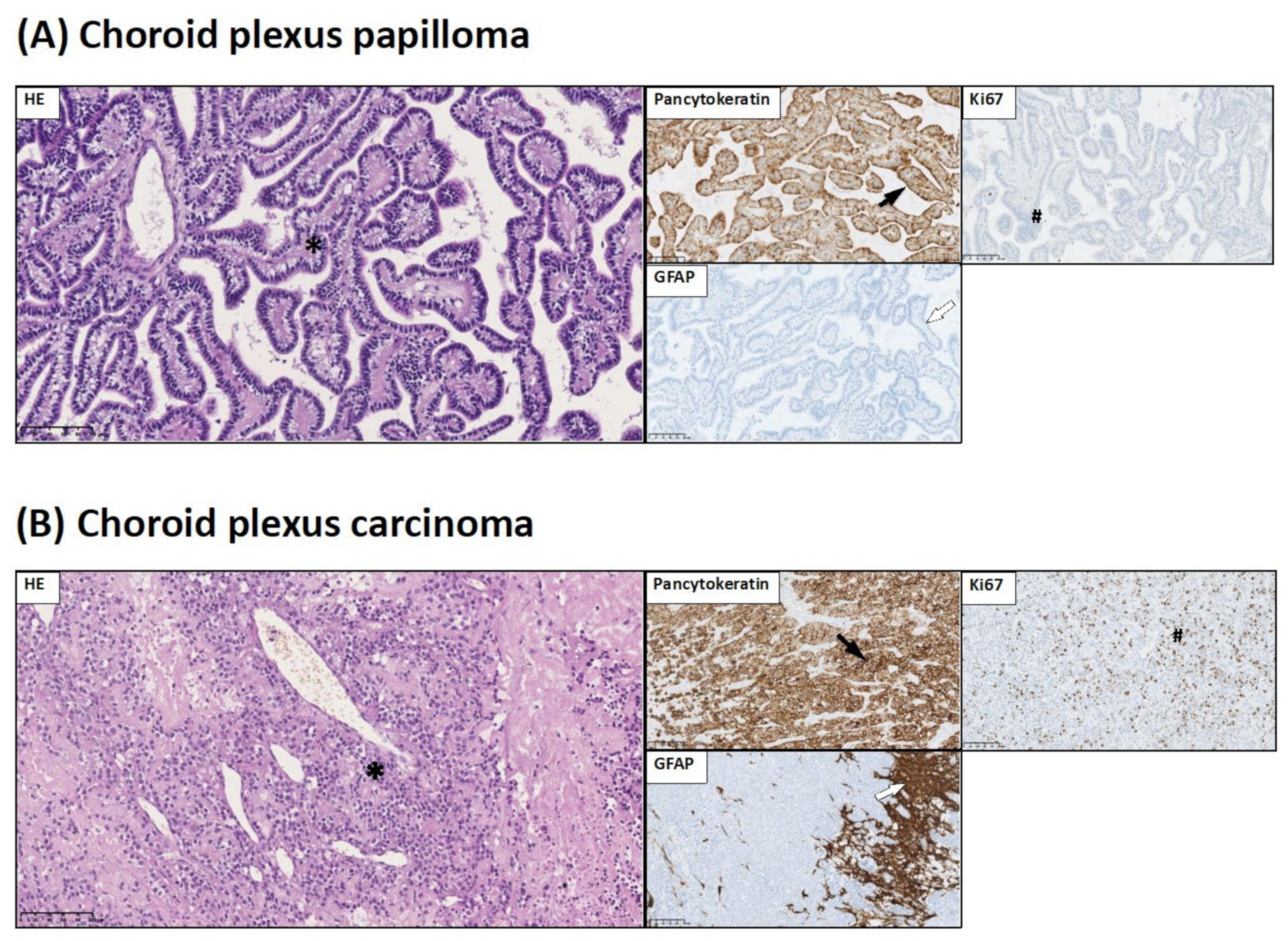
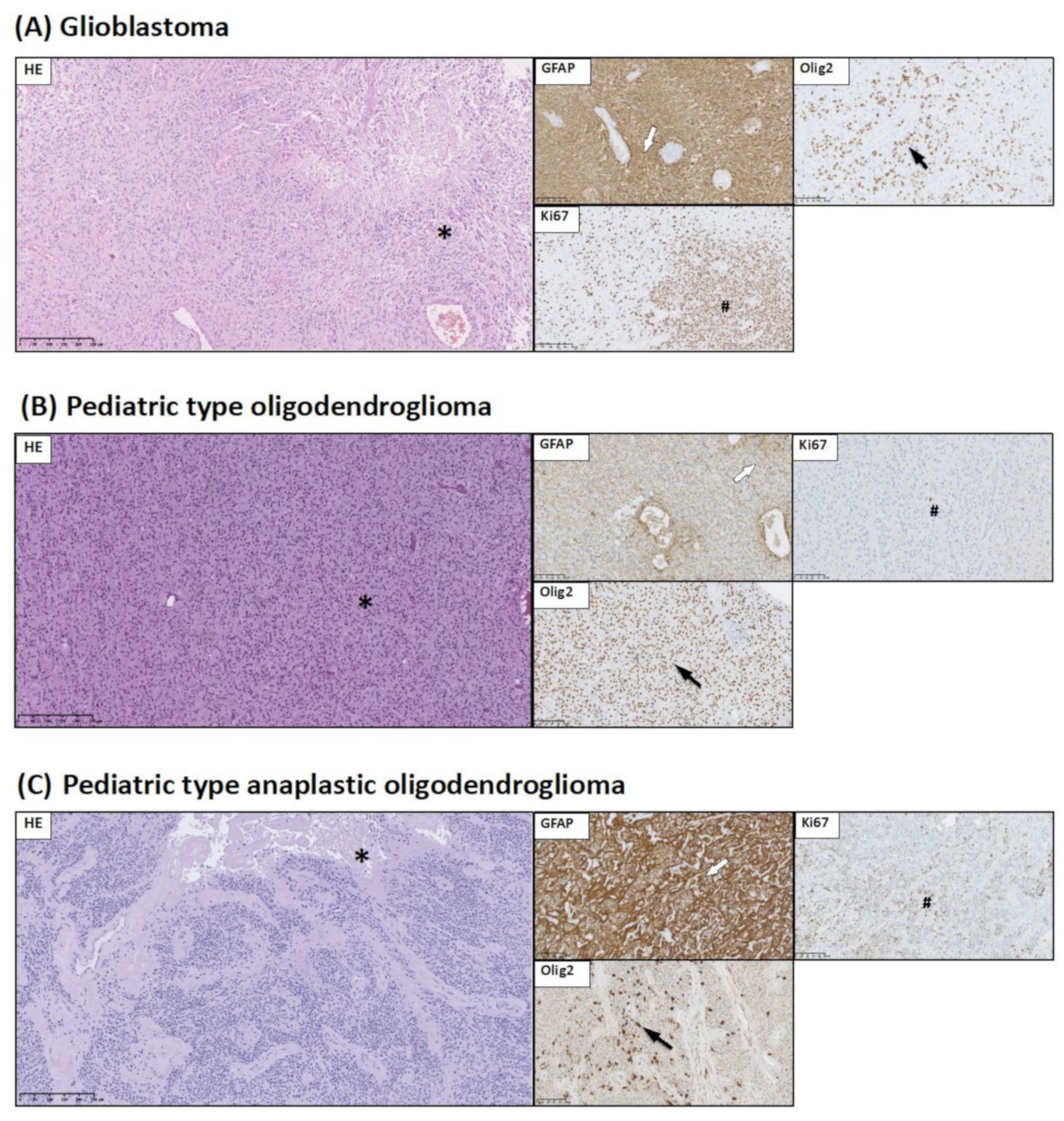
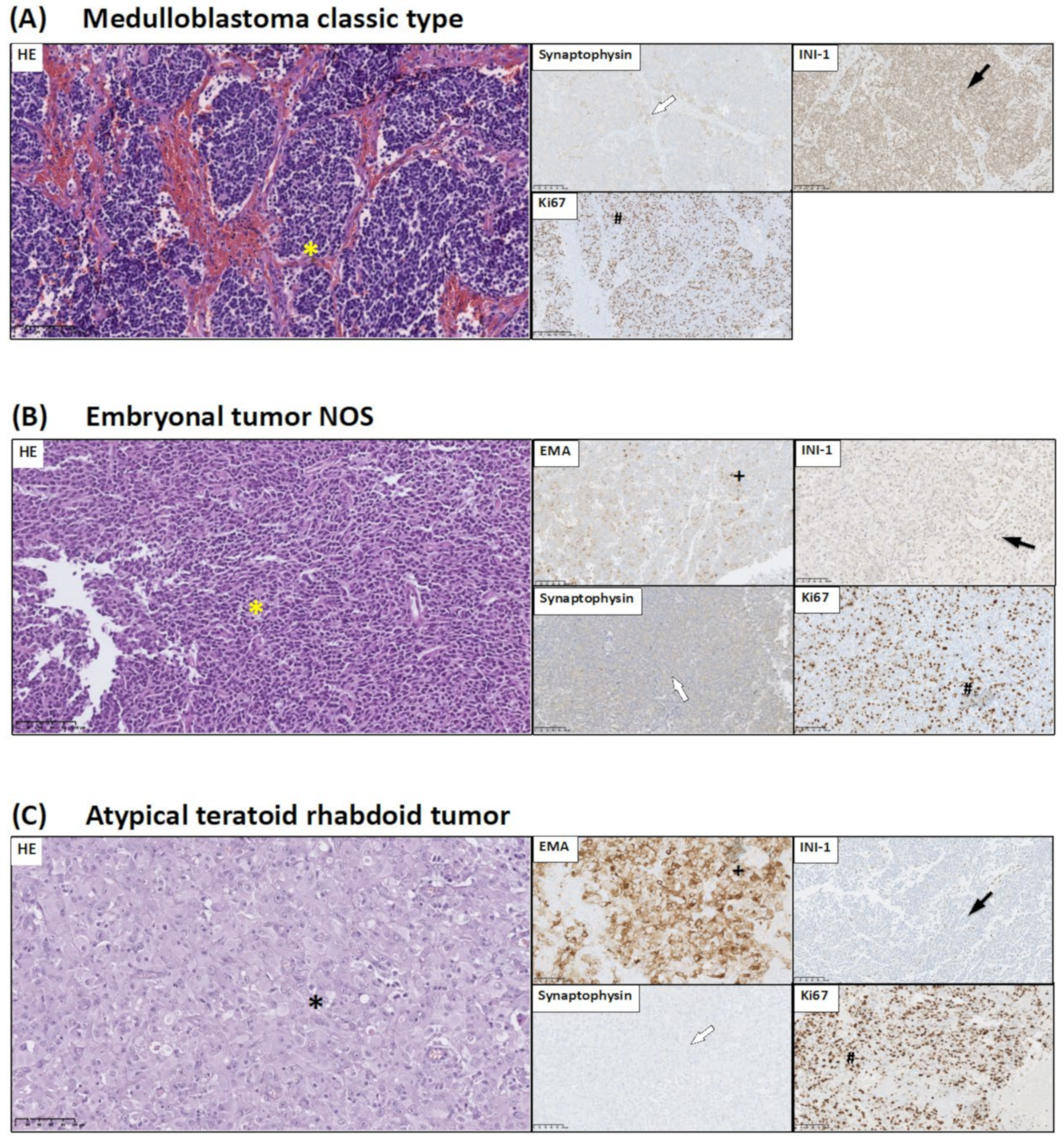
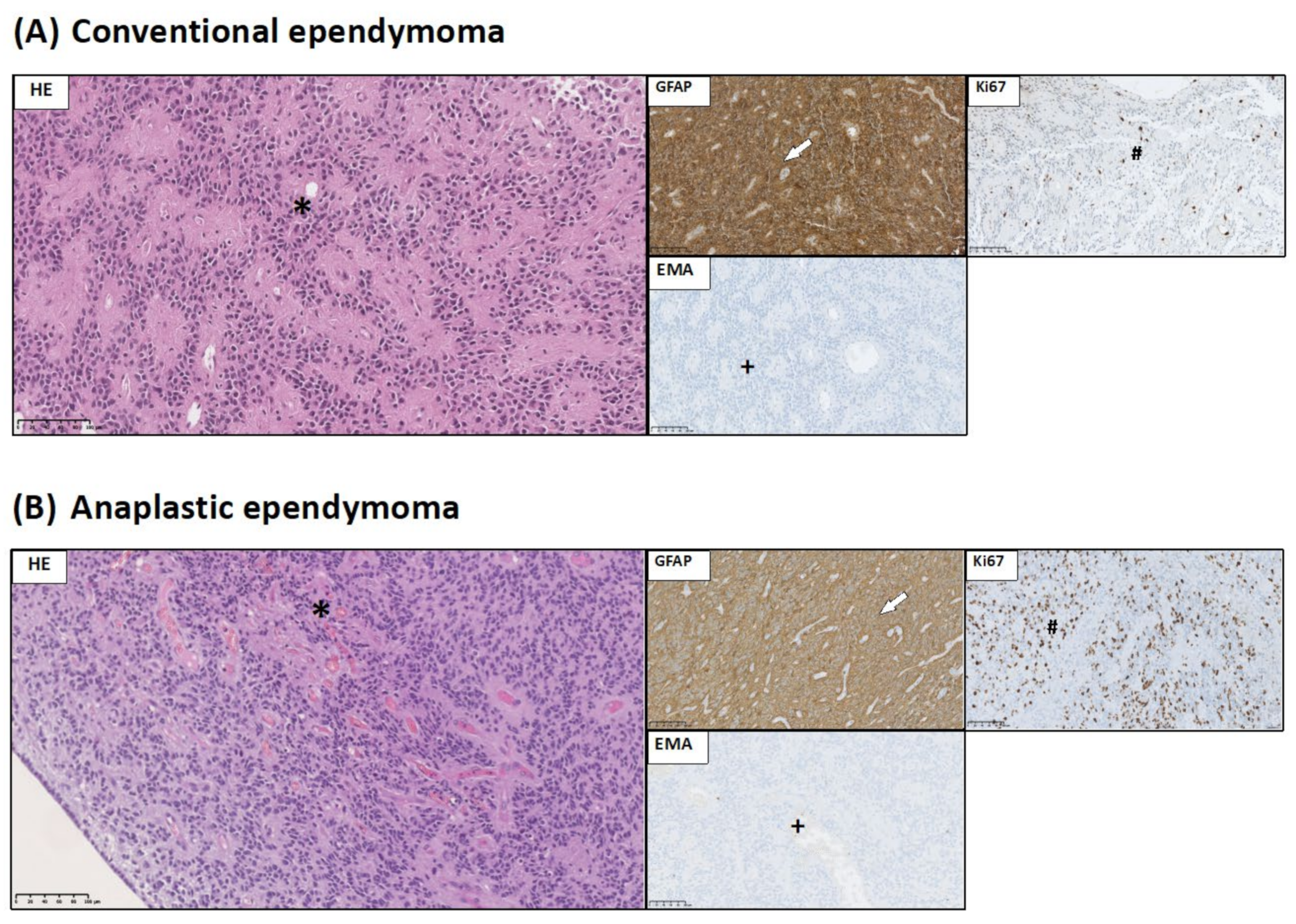
2.3. Brain Tumor Histology and Immunohistochemistry
- Ki67, which is considered to be malignancy marker;
- GFAP expression allows identification of glial origin of neoplastic cells;
- Pancytokeratin expression is considered to be a marker of epithelial origin of choroid plexus tumors;
- Olig2 expression is considered to be a marker of gliomas: pilocytic astrocytoma, diffuse-type astrocytic tumors, and pediatric type of oligodendroglioma;
- INI-1 is used in the diagnosis of CNS atypical teratoid/rhabdoid tumors. INI-1 expression picture allows to distinguish atypical teratoid/rhabdoid tumor (loss of INI-1) from choroid plexus carcinoma (INI-1+);
- Synaptophysin expression allows for the identification of the neuronal origin of neoplastic cells;
- EMA staining might serve as sensitive and specific markers of ependymal differentiation in glial tumors; and
- S100 is a characteristic marker for glial tumors as well as choroid plexus tumors.
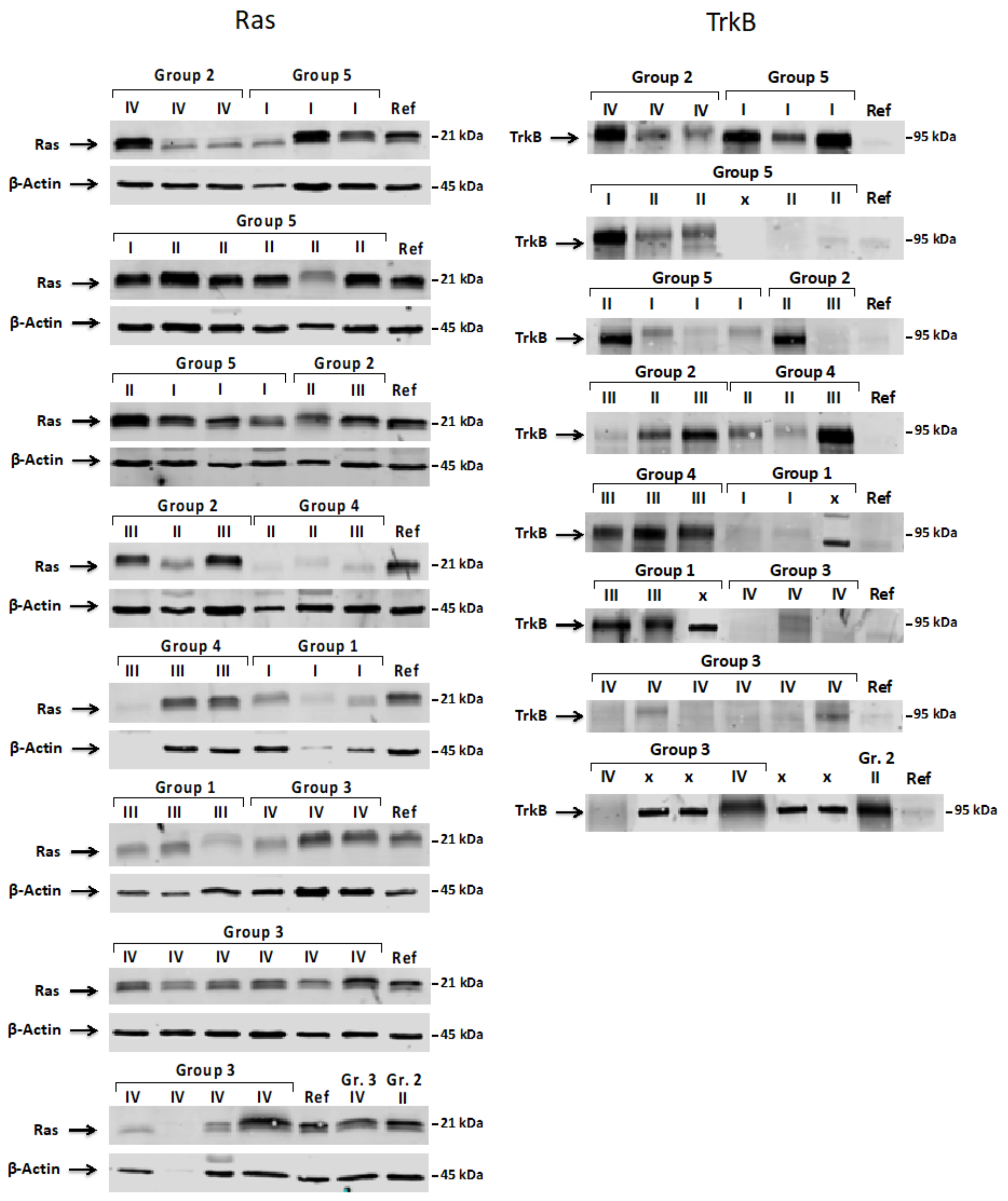
2.4. Western Blot Analysis
2.5. Statistical Analysis
3. Results
3.1. Histopathological and Immunohistochemical Studies
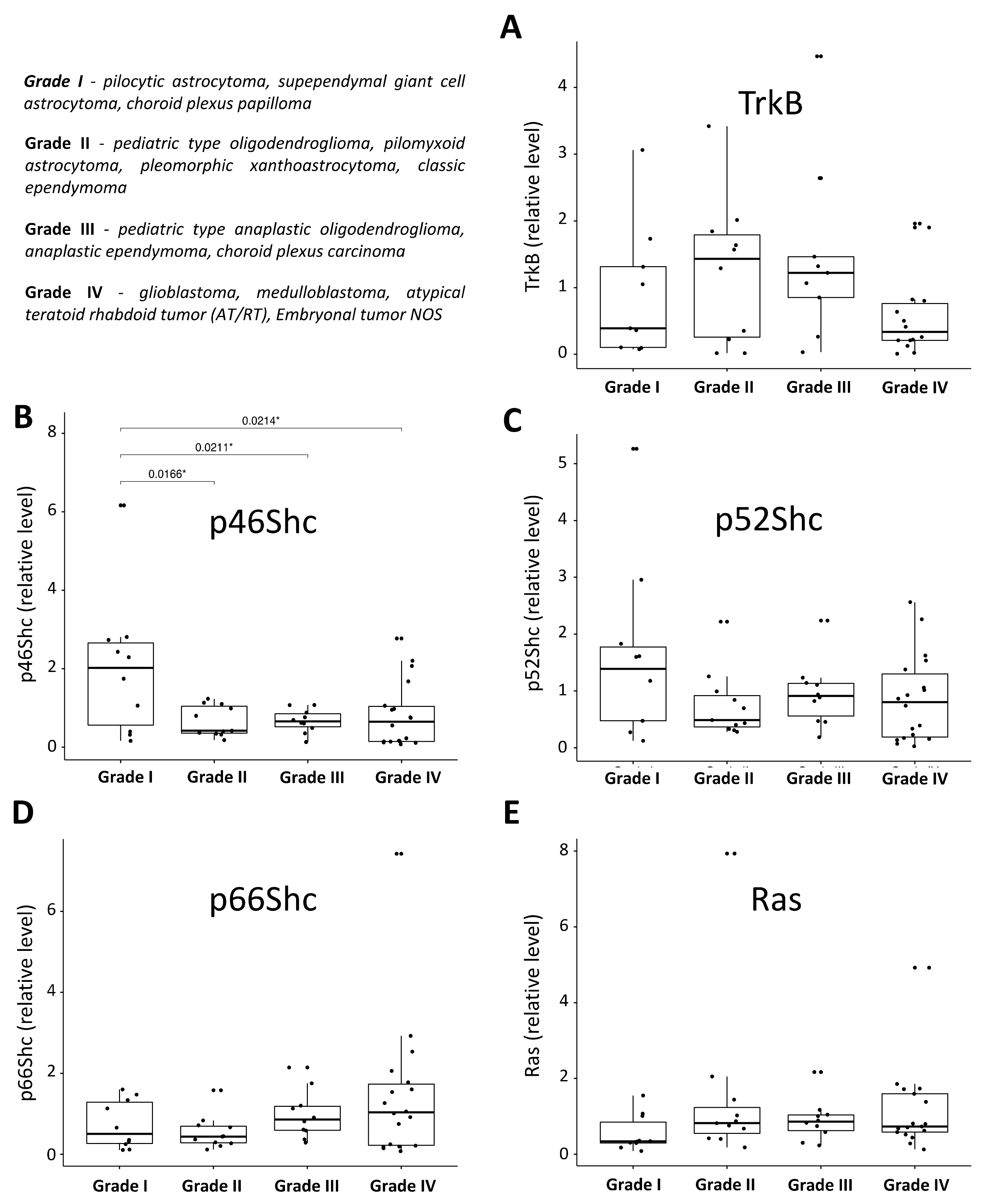
3.2. Expression Pattern of ShcA, Ras and TrkB Proteins in Pediatric Brain Tumors and Their Levels in a Function of Tumor Malignancy Grade
4. Discussion
4.1. Expression Pattern of ShcA, Ras, and TrkB Proteins in Studied Pediatric Brain Tumors
4.2. Pediatric Brain Tumor Malignancy Grade and the Pattern of ShcA, Ras, and TrkB Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pelicci, G.; Dente, L.; de Giuseppe, A.; Verducci-Galletti, B.; Giuli, S.; Mele, S.; Vetriani, C.; Giorgio, M.; Pandolfi, P.P.; Cesareni, G.; et al. A family of Shc related proteins with conserved PTB, CH1 and SH2 regions. Oncogene 1996, 13, 633–641. [Google Scholar] [PubMed]
- Simanshu, D.K.; Nissley, D.V.; Mccormick, F. RAS Proteins and Their Regulators in Human Disease. Cell 2017, 170, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.S.; Anand, D.; Khanday, F.A. p66Shc as a switch in bringing about contrasting responses in cell growth: Implications on cell proliferation and apoptosis. Mol. Cancer 2015, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Arias-Romero, L.E.; Villamar-Cruz, O.; Pacheco, A.; Kosoff, R.; Huang, M.; Muthuswamy, S.K.; Chernoff, J. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene 2010, 29, 5839–5849. [Google Scholar] [CrossRef]
- Samuel, M.S.; Lourenço, F.C.; Olson, M.F. K-Ras mediated murine epidermal tumorigenesis is dependent upon and associated with elevated Rac1 activity. PLoS ONE 2011, 6, e17143. [Google Scholar] [CrossRef] [PubMed]
- Menges, C.W.; Sementino, E.; Talarchek, J.; Xu, J.; Chernoff, J.; Peterson, J.R.; Testa, J.R. Group I p21-activated kinases (PAKs) promote tumor cell proliferation and survival through the AKT1 and Raf-MAPK pathways. Mol. Cancer Res. 2012, 10, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.S. Signaling via Shc family adapter proteins. Oncogene 2001, 20, 6322–6330. [Google Scholar] [CrossRef] [PubMed]
- Lotti, L.V.; Lanfrancone, L.; Migliaccio, E.; Zompetta, C.; Pelicci, G.; Salcini, A.E.; Falini, B.; Pelicci, P.G.; Torrisi, M.R. Shc proteins are localized on endoplasmic reticulum membranes and are redistributed after tyrosine kinase receptor activation. Mol. Cell. Biol. 1996, 16, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, E.; Mele, S.; Salcini, A.E.; Pelicci, G.; Venus Lai, K.-M.; Superti-Furga, G.; Pawson, T.; di Fiore, P.P.; Lanfrancone, L.; Pelicci, P.G. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 1997, 16, 706–716. [Google Scholar] [CrossRef]
- Migliaccio, E.; Giorgio, M.; Mele, S.; Pelicci, G.; Reboldi, P.; Pandolfi, P.P.; Lanfrancone, L.; Pelicci, P.G. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 1999, 402, 309–313. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Chao, J.; Chen, C.; Lv, L.L.; Han, Y.C.; Liu, B.C. The PKCβ-p66shc-NADPH oxidase pathway plays a crucial role in diabetic nephropathy. J. Pharm. Pharmacol. 2019, 71, 338–347. [Google Scholar] [CrossRef]
- Boengler, K.; Bornbaum, J.; Schlüter, K.D.; Schulz, R. P66shc and its role in ischemic cardiovascular diseases. Basic Res. Cardiol. 2019, 114, 29. [Google Scholar] [CrossRef]
- Savino, C.; Pelicci, P.; Giorgio, M. The P66Shc/mitochondrial permeability transition pore pathway determines neurodegeneration. Oxid. Med. Cell. Longev. 2013, 2013, 719407. [Google Scholar] [CrossRef]
- Lebiedzinska, M.; Karkucinska-Wieckowska, A.; Giorgi, C.; Karczmarewicz, E.; Pronicka, E.; Pinton, P.; Duszynski, J.; Pronicki, M.; Wieckowski, M.R. Oxidative stress-dependent p66Shc phosphorylation in skin fibroblasts of children with mitochondrial disorders. Biochim. Biophys. Acta 2010, 1797, 952–960. [Google Scholar] [CrossRef]
- Lebiedzinska, M.; Duszynski, J.; Rizzuto, R.; Pinton, P.; Wieckowski, M.R. Age-related changes in levels of p66Shc and serine 36-phosphorylated p66Shc in organs and mouse tissues. Arch. Biochem. Biophys. 2009, 486, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Veeramani, S.; Yuan, T.C.; Lin, F.F.; Lin, M.F. Mitochondrial redox signaling by p66Shc is involved in regulating androgenic growth stimulation of human prostate cancer cells. Oncogene 2008, 27, 5057–5068. [Google Scholar] [CrossRef]
- Lee, M.S.; Igawa, T.; Chen, S.J.; Van Bemmel, D.; Lin, J.S.; Lin, F.F.; Johansson, S.L.; Christman, J.K.; Lin, M. p66Shc protein is upregulated by steroid hormones in hormone-sensitive cancer cells and in primary prostate carcinomas. Int. J. Cancer 2004, 108, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, M.; Thomes, P.; Zhang, L.; Veeramani, S.; Lin, M.F. p66Shc—A longevity redox protein in human prostate cancer progression and metastasis: p66Shc in cancer progression and metastasis. Cancer Metastasis Rev. 2010, 29, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.M.; Rajendran, M.; Ouyang, S.; Veeramani, S.; Zhang, L.; Lin, M.F. A novel role of Shc adaptor proteins in steroid hormone-regulated cancers. Endocr. Relat. Cancer 2009, 16, 1–16. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Z.; Wu, R.F.; Terada, L.S. p66(Shc) restrains Ras hyperactivation and suppresses metastatic behavior. Oncogene 2010, 29, 5559–5567. [Google Scholar] [CrossRef]
- Pelicci, G.; Lanfrancone, L.; Salcini, A.E.; Romano, A.; Mele, S.; Grazia Borrello, M.; Segatto, O.; Di Fiore, P.P.; Pelicci, P.G. Constitutive phosphorylation of Shc proteins in human tumors. Oncogene 1995, 11, 899–907. [Google Scholar] [PubMed]
- Cox, A.D.; Der, C.J. Ras history: The saga continues. Small GTPases 2010, 1, 2–27. [Google Scholar] [CrossRef]
- Milburn, M.V.; Tong, L.; Devos, A.M.; Brünger, A.; Yamaizumi, Z.; Nishimura, S.; Kim, S.H. Molecular switch for signal transduction: Structural differences between active and inactive forms of protooncogenic ras proteins. Science 1990, 247, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Counter, C.M. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/AKT pathway during tumor maintenance. Cancer Cell 2005, 8, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.A.; Beare, D.; Boutselakis, H.; Bamford, S.; Bindal, N.; Tate, J.; Cole, C.G.; Ward, S.; Dawson, E.; Ponting, L.; et al. COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017, 45, D777–D783. [Google Scholar] [CrossRef]
- Bos, J.L. Ras oncogenes in human cancer: A review. Cancer Res. 1989, 49, 4682–4689. [Google Scholar] [PubMed]
- Davol, P.A.; Bagdasaryan, R.; Elfenbein, G.J.; Maizel, A.L.; Frackelton, A.R.J.R. Shc proteins are strong, independent prognostic markers for both node-negative and node-positive primary breast cancer. Cancer Res. 2003, 63, 6772–6783. [Google Scholar]
- Frackelton, A.R.J.R.; Lu, L.; Davol, P.A.; Bagdasaryan, R.; Hafer, L.J.; Sgroi, D.C. p66 Shc and tyrosine-phosphorylated Shc in primary breast tumors identify patients likely to relapse despite tamoxifen therapy. Breast Cancer Res. 2006, 8, R73. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.R.; Lyle, S.; Resnick, M.B.; Sabo, E.; Lis, R.T.; Rosinha, E.; Liu, Q.; Hsieh, C.-H.; Bhat, G.; Frackelton, A.R., Jr.; et al. p66 Shc tumor levels show a strong prognostic correlation with disease outcome in stage IIA colon cancer. Clin. Cancer Res. 2007, 13, 5798–5804. [Google Scholar] [CrossRef]
- Jackson, J.G.; Yoneda, T.; Clark, G.M.; Yee, D. Elevated levels of p66 Shc are found in breast cancer cell lines and primary tumors with high metastatic potential. Clin. Cancer Res. 2000, 6, 1135–1139. [Google Scholar]
- Haines, E.; Saucier, C.; Claing, A. The adaptor proteins p66Shc and Grb2 regulate the activation of the GTPases ARF1 and ARF6 in invasive breast cancer cells. J. Biol. Chem. 2014, 289, 5687–5703. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Henderson, J.T.; O’bryan, J.P.; Elia, A.J.; Saxton, T.M.; Pawson, T. The mammalian ShcB and ShcC phosphotyrosine docking proteins function in the maturation of sensory and sympathetic neurons. Neuron 2000, 28, 819–833. [Google Scholar] [CrossRef]
- Conti, L.; Sipione, S.; Magrassi, L.; Bonfanti, L.; Rigamonti, D.; Pettirossi, V.; Peschanski, M.; Haddad, B.; Pelicci, P.; Milanesi, G.; et al. Shc signaling in differentiating neural progenitor cells. Nat. Neurosci. 2001, 4, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Shimura, T.; Kitajima, T.; Kondo, S.; Ide, S.; Okugawa, Y.; Saigusa, S.; Toiyama, Y.; Inoue, Y.; Araki, K.T.; et al. Tropomyosin-related receptor kinase B at the invasive front and tumour cell dedifferentiation in gastric cancer. Br. J. Cancer 2014, 110, 2923–2934. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Tanaka, K.; Inoue, Y.; Kawamura, M.; Kawamoto, A.; Hiro, J.; Saigusa, S.; Toiyama, Y.; Ohi, M.; Uchida, K.; et al. Brain-derived neurotrophic factor/tropomyosin-related kinase B pathway in gastric cancer. Br. J. Cancer 2013, 108, 121–130. [Google Scholar] [CrossRef]
- Zhang, Y.; Fujiwara, Y.; Doki, Y.; Takiguchi, S.; Yasuda, T.; Miyata, H.; Yamazaki, M.; Ngan, C.Y.; Yamamoto, H.; Ma, Q.; et al. Overexpression of tyrosine kinase B protein as a predictor for distant metastases and prognosis in gastric carcinoma. Oncology 2008, 75, 17–26. [Google Scholar] [CrossRef]
- Fan, M.; Sun, J.; Wang, W.; Fan, J.; Wang, L.; Zhang, X.; Yang, A.; Wang, W.; Zhang, R.; Li, J. Tropomyosin-related kinase B promotes distant metastasis of colorectal cancer through protein kinase B-mediated anoikis suppression and correlates with poor prognosis. Apoptosis 2014, 19, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.; Grundmann, S.; Koelzer, V.H.; Galván, J.A.; Kirsch, R.; Karamitopoulou, E.; Lugli, A.; Inderbitzin, D.; Zlobec, I. Tyrosine kinase receptor B (TrkB) expression in colorectal cancers highlights anoikis resistance as a survival mechanism of tumour budding cells. Histopathology 2015, 66, 715–725. [Google Scholar] [CrossRef]
- Okamura, K.; Harada, T.; Wang, S.; Ijichi, K.; Furuyama, K.; Koga, T.; Okamoto, T.; Takayama, K.; Yano, T.; Nakanishi, Y. Expression of TrkB and BDNF is associated with poor prognosis in non-small cell lung cancer. Lung Cancer 2012, 78, 100–106. [Google Scholar] [CrossRef]
- Au, C.W.; Siu, M.K.; Liao, X.; Wong, E.S.; Ngan, H.Y.; Tam, K.F.; Chan, D.C.W.; Chan, Q.K.Y.; Cheung, A.N.Y. Tyrosine kinase B receptor and BDNF expression in ovarian cancers—Effect on cell migration, angiogenesis and clinical outcome. Cancer Lett. 2009, 281, 151–161. [Google Scholar] [CrossRef]
- Li, S.S.; Liu, J.J.; Wang, S.; Tang, Q.L.; Liu, B.B.; Yang, X.M. Clinical significance of TrkB expression in nasopharyngeal carcinoma. Oncol. Rep. 2014, 31, 665–672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sasahira, T.; Ueda, N.; Yamamoto, K.; Bhawal, U.K.; Kurihara, M.; Kirita, T.; Kuniyasu, H. Trks are novel oncogenes involved in the induction of neovascularization, tumor progression, and nodal metastasis in oral squamous cell carcinoma. Clin. Exp. Metastasis 2013, 30, 165–176. [Google Scholar] [CrossRef]
- Li, L.; Zhu, L. Expression and clinical significance of TrkB in sinonasal squamous cell carcinoma: A pilot study. Int. J. Oral. Maxillofac. Surg. 2017, 46, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, X.; Gao, D.; Ruan, H.; Lin, Z.; Li, X.; Liu, G.; Ma, Z.; Li, X. The prognostic value of over-expressed TrkB in solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 99394–99401. [Google Scholar] [CrossRef][Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, N.; Chao, M.V. Mechanisms of neurotrophin receptor signalling. Biochem. Soc. Trans. 2006, 34, 607–611. [Google Scholar] [CrossRef]
- Terui, E.; Matsunaga, T.; Yoshida, H.; Kouchi, K.; Kuroda, H.; Hishiki, T.; Saito, T.; Yamada, S.-I.; Shirasawa, H.; Ohnuma, N. Shc family expression in neuroblastoma: High expression of shcC is associated with a poor prognosis in advanced neuroblastoma. Clin. Cancer Res. 2005, 11, 3280–3287. [Google Scholar] [CrossRef][Green Version]
- Nakagawara, A.; Arima-Nakagawara, M.; Scavarda, N.J.; Azar, C.G.; Cantor, A.B.; Brodeur, G.M. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N. Engl. J. Med. 1993, 328, 847–854. [Google Scholar] [CrossRef]
- Jaboin, J.; Kim, C.J.; Kaplan, D.R.; Thiele, C.J. Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3’-kinase pathway. Cancer Res. 2002, 62, 6756–6763. [Google Scholar]
- Muñoz-Maldonado, C.; Zimmer, Y.; Medová, M. A Comparative Analysis of Individual RAS Mutations in Cancer Biology. Front. Oncol. 2019, 9, 1088. [Google Scholar] [CrossRef]
- Pollack, I.F.; Agnihotri, S.; Broniscer, A. Childhood brain tumors: Current management, biological insights, and future directions. J. Neurosurg. Pediatr. 2019, 23, 261–273. [Google Scholar] [CrossRef]
- Salles, D.; Laviola, G.; Malinverni, A.C.M.; Stavale, J.N. Pilocytic Astrocytoma: A Review of General, Clinical, and Molecular Characteristics. J. Child Neurol. 2020, 35, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Kline, C.N.; Packer, R.J.; Hwang, E.I.; Raleigh, D.R.; Braunstein, S.; Raffel, C.; Bandopadhayay, P.; Solomon, D.A.; Aboian, M.; Cha, S.; et al. Case-based review: Pediatric medulloblastoma. Neurooncol. Pract. 2017, 4, 138–150. [Google Scholar] [CrossRef]
- Juraschka, K.; Taylor, M.D. Medulloblastoma in the age of molecular subgroups: A review. J. Neurosurg. Pediatr. 2019, 24, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Erlandsson, S.; Herrero-Vidal, P.; Fernandez-Alfara, M.; Hernandez-Garcia, S.; Gonzalo-Flores, S.; Mudarra-Rubio, A.; Fresno, M.; Cubelos, B. R-RAS2 overexpression in tumors of the human central nervous system. Mol. Cancer 2013, 12, 127. [Google Scholar] [CrossRef] [PubMed]



| Antibody | Clone | Host | Supplier |
|---|---|---|---|
| EMA | E29 | Mouse | VENATANA/ROCHE |
| Vimentin | Vim 3B4 | Mouse | VENATANA/ROCHE |
| Pancytokeratin | AE1/AE3 & PCK26 | Mouse | VENATANA/ROCHE |
| Glial Fibrillary Acidic Protein (GFAP) | EP672Y | Rabbit | CELL MARQUE |
| Neurofilament | 2F11 | Mouse | CELL MARQUE |
| Ki67 | 30.9 | Rabbit | VENATANA/ROCHE |
| Olig2 | EP356 | Rabbit | CELL MARQUE |
| S-100 | Polyclonal | Rabbit | VENATANA/ROCHE |
| Synaptophysin | MRQ-40 | Rabbit | CELL MARQUE |
| INI-1 | MRQ-27 | Mouse | CELL MARQUE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prill, M.; Karkucinska-Wieckowska, A.; Lebiedzinska-Arciszewska, M.; Morciano, G.; Charzynska, A.; Dabrowski, M.; Pronicki, M.; Pinton, P.; Grajkowska, W.; Wieckowski, M.R. Ras, TrkB, and ShcA Protein Expression Patterns in Pediatric Brain Tumors. J. Clin. Med. 2021, 10, 2219. https://doi.org/10.3390/jcm10102219
Prill M, Karkucinska-Wieckowska A, Lebiedzinska-Arciszewska M, Morciano G, Charzynska A, Dabrowski M, Pronicki M, Pinton P, Grajkowska W, Wieckowski MR. Ras, TrkB, and ShcA Protein Expression Patterns in Pediatric Brain Tumors. Journal of Clinical Medicine. 2021; 10(10):2219. https://doi.org/10.3390/jcm10102219
Chicago/Turabian StylePrill, Monika, Agnieszka Karkucinska-Wieckowska, Magdalena Lebiedzinska-Arciszewska, Giampaolo Morciano, Agata Charzynska, Michal Dabrowski, Maciej Pronicki, Paolo Pinton, Wieslawa Grajkowska, and Mariusz R. Wieckowski. 2021. "Ras, TrkB, and ShcA Protein Expression Patterns in Pediatric Brain Tumors" Journal of Clinical Medicine 10, no. 10: 2219. https://doi.org/10.3390/jcm10102219
APA StylePrill, M., Karkucinska-Wieckowska, A., Lebiedzinska-Arciszewska, M., Morciano, G., Charzynska, A., Dabrowski, M., Pronicki, M., Pinton, P., Grajkowska, W., & Wieckowski, M. R. (2021). Ras, TrkB, and ShcA Protein Expression Patterns in Pediatric Brain Tumors. Journal of Clinical Medicine, 10(10), 2219. https://doi.org/10.3390/jcm10102219








