Why do We Use the Concepts of Adult Anesthesia Pharmacology in Developing Brains? Will It Have an Impact on Outcomes? Challenges in Neuromonitoring and Pharmacology in Pediatric Anesthesia
Abstract
:1. Introduction
2. The Core of the Current Debate
3. Neural Development, EEG, and the Emergence of Consciousness
4. Pharmacology
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, D.; Flick, R.P.; Zaccariello, M.J.; Colligan, R.C.; Katusic, S.K.; Schroeder, D.R.; Hanson, A.C.; Buenvenida, S.L.; Gleich, S.J.; Wilder, R.T.; et al. Association between Exposure of Young Children to Procedures Requiring General Anesthesia and Learning and Behavioral Outcomes in a Population-based Birth Cohort. Anesthesiology 2017, 127, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Jevtovic-Todorovic, V.; Absalom, A.R.; Blomgren, K.; Brambrink, A.; Crosby, G.; Culley, D.J.; Fiskum, G.; Giffard, R.G.; Herold, K.F.; Loepke, A.W.; et al. Anaesthetic neurotoxicity and neuroplasticity: An expert group report and statement based on the BJA Salzburg Seminar. Br. J. Anaesth. 2013, 111, 143–151. [Google Scholar] [CrossRef] [Green Version]
- FDA Drug Safety Communication (12-14-2016): FDA Review Results in New Warnings about Using General Anesthetics and Sedation Drugs in Young Children and Pregnant Women. Available online: http://www.fda.gov/Drugs/DrugSafety/ucm532356.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery (accessed on 18 May 2021).
- Sun, L.S.; Li, G.; Miller, T.L.K.; Salorio, C.; Byrne, M.W.; Bellinger, D.C.; Ing, C.; Park, R.; Radcliffe, J.; Hays, S.R.; et al. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA 2016, 315, 2312–2320. [Google Scholar] [CrossRef]
- Warner, O.D.; Zaccariello, J.M.; Katusic, K.S. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: The mayo anesthesia safety in kids (MASK) study. Anesthesiology 2018, 129, 89–105. [Google Scholar] [CrossRef]
- McCann, M.E.; de Graaff, J.C.; Dorris, L. Neurodevelopmental outcome at 5 years of age after general an-aesthesia or awake-regional anaesthesia in infancy (GAS): An international, multicentre, randomised, con-trolled equivalence trial. Lancet 2019, 393, 664–677. [Google Scholar] [CrossRef]
- De Heer, I.J.; Bouman, S.J.M.; Weber, F. Electroencephalographic (EEG) density spectral array monitoring in children during sevoflurane anaesthesia: A prospective observational study. Anaesthesia 2018, 74, 45–50. [Google Scholar] [CrossRef]
- Anderson, B.; Bagshaw, O. Practicalities of Total Intravenous Anesthesia and Target-controlled Infusion in Children. Anesthesiology 2019, 131, 164–185. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H. Understanding Dosing: Children are Small Adults, Neonates are Immature Children. Arch. Dis. Child. 2013, 98, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Glover, V.; Fisk, N.M. Fetal pain: Implications for research and practice. Br. J. Obstet. Gynaecol. 1999, 106, 881–886. [Google Scholar] [CrossRef] [Green Version]
- Slater, R.; Cantarella, A.; Gallella, S.; Worley, A.; Boyd, S.; Meek, J.; Fitzgerald, M. Cortical Pain Responses in Human Infants. J. Neurosci. 2006, 26, 3662–3666. [Google Scholar] [CrossRef] [Green Version]
- Bartocci, M.; Bergqvist, L.L.; Lagercrantz, H.; Anand, K.J. Pain activates cortical areas in the preterm newborn brain. Pain 2006, 122, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Sonner, J.; Antognini, J.; Dutton, R.; Flood, P.; Gray, A.; Eger, E. Inhaled Anesthetics and Immobility: Mecha-nisms, Mysteries, and Minimum Alveolar Anesthetic Concentration. Anesth. Analg. 2003, 97, 718–740. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.K.; Park, R.; Sun, L. Report on the Fifth PANDA Symposium on “Anesthesia and Neurodevelop-ment in Children”. J. Neurosurg. Anesth. 2016, 28, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F.E.; Silverstein, F.S. Neonatal Seizures. Swaiman’s Pediatric Neurol. 2012, 62, 112–120. [Google Scholar] [CrossRef]
- Rakhade, S.N.; Jensen, F.E. Epileptogenesis in the immature brain: Emerging mechanisms. Nat. Rev. Neurol. 2009, 5, 380–391. [Google Scholar] [CrossRef] [Green Version]
- Sanders, R.D.; Hassell, J.; Davidson, A.J.; Robertson, N.J.; Ma, D. Impact of anaesthetics and surgery on neuro-development: An update. Br. J. Anaesth. 2013, 110, i53–i72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, T.G.; Pedersen, J.K.; Henneberg, S.W.; Pedersen, D.A.; Murray, J.C.; Morton, N.S.; Christensen, K. Aca-demic performance in adolescence after inguinal hernia repair in infancy: A nationwide cohort study. Anesthesiology 2011, 114, 1076–1085. [Google Scholar] [CrossRef] [Green Version]
- Ing, C.; DiMaggio, C.; Whitehouse, A.; Hegarty, M.K.; Brady, J.; Von Ungern-Sternberg, B.S.; Davidson, A.; Wood, A.J.; Li, G.; Sun, L.S. Long-term Differences in Language and Cognitive Function After Childhood Exposure to Anesthesia. Pediatrics 2012, 130, e476–e485. [Google Scholar] [CrossRef] [Green Version]
- Davidson, A.J.; Disma, N.; De Graaff, J.C.; Withington, D.E.; Dorris, L.; Bell, G.; Stargatt, R.; Bellinger, D.C.; Schuster, T.; Arnup, S.J.; et al. GAS consortium: Neurode-velopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): An international multicentre, randomised controlled trial. Lancet 2016, 387, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.H. Functional brain development in humans. Nat. Rev. Neurosci. 2001, 2, 475–483. [Google Scholar] [CrossRef]
- Kinnala, A.; Suhonen-Polvi, H.; Aarimaa, T.; Kero, P.; Korvenranta, H.; Ruotsalainen, U.; Bergman, J.; Haaparanta, M.; Solin, O.; Nuutila, P.; et al. Cerebral metabolic rate for glucose during the first six months of life: An FDG positron emission tomography study. Arch. Dis. Child. Fetal Neonatal Ed. 1996, 74, F153–F157. [Google Scholar] [CrossRef] [Green Version]
- Kanold, P.O.; Luhmann, H.J. The Subplate and Early Cortical Circuits. Annu. Rev. Neurosci. 2010, 33, 23–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagercrantz, H. Stress, Arousal, and Gene Activation at Birth. News Physiol. Sci. 1996, 11, 214–218. [Google Scholar] [CrossRef]
- Sanders, R.D.; Xu, J.; Shu, Y.; Januszewski, A.; Halder, S.; Fidalgo, A.; Sun, P.; Hossain, M.; Ma, D.; Maze, M. Dex-medetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology 2009, 110, 1077–1085. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zeng, M.; Chen, W.; Liu, C.; Wnag, F.; Han, F.; Zuo, Z.; Peng, S. Dexmedetomidine reduces isoflu-rane-induced neuroapoptosis partly by preserving PI3K/Akt pathway in the hippocampus of neonatal rats. PLoS ONE 2014, 9, e93639. [Google Scholar]
- Suvisto, S. Effects of Dexmedetomidine on the Cortical Activity of Neonates. 2018. Available online: https://aaltodoc.aalto.fi/handle/123456789/34735 (accessed on 18 May 2021).
- Easley, R.B.; Tobias, J.D. Pro: Dexmedetomidine Should Be Used for Infants and Children Undergoing Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2008, 22, 147–151. [Google Scholar] [CrossRef]
- Hammer, G.B. Con: Dexmedetomidine Should Not Be Used for Infants and Children During Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2008, 22, 152–154. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Zhang, L.; Wang, G.; Zhang, M.; Yu, Y. Neuroprotection of Dexmedetomidine against Cerebral Ischemia-Reperfusion Injury in Rats: Involved in Inhibition of NF-κB and Inflammation Response. Biomol. Ther. 2017, 25, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Changeux, J.-P. Climbing Brain Levels of Organisation from Genes to Consciousness. Trends Cogn. Sci. 2017, 21, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Malk, K.; Metsäranta, M.; Vanhatalo, S. Drug effects on endogenous brain activity in preterm babies. Brain Dev. 2014, 36, 116–123. [Google Scholar] [CrossRef]
- Kostović, J.; Jovanov-Milošević, N. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin. Fetal Neonatal Med. 2006, 11, 415–422. [Google Scholar] [CrossRef]
- Niedermeyer, E. Maturation of the eeg: Development of waking and sleep patterns. Electroencephalogr. Basic Princ. Clin. Appl. Relat. Fields 2005, 167, 209–234. [Google Scholar]
- Lagercrantz, H.; Changeaux, J.P. The Emergence of Human Consciousness: From Fetal to Neonatal Life. Pediatr. Res. 2009, 65, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.N.; Solt, K.; Purdon, P.L.; Akeju, O. Monitoring brain state during general anesthesia and sedation. In Miller’s Anesthesia, 8th ed.; Elsevier Health Sciences: Cambridge, UK, 2015; Volume 1, p. 50. [Google Scholar]
- Cornelissen, L.; Kim, S.-E.; Purdon, P.L.; Brown, E.N.; Berde, C.B. Age-dependent electroencephalogram (EEG) patterns during sevoflurane general anesthesia in infants. eLife 2015, 4, e06513. [Google Scholar] [CrossRef] [Green Version]
- Davidson, A.J.; Sale, S.M.; Wong, C.; McKeever, S.; Sheppard, S.; Chan, Z.; Williams, C. The electroencephalo-graph during anesthesia and emergence in infants and children. Paediatr. Anaesth. 2008, 18, 60–70. [Google Scholar]
- Constant, I.; Sabourdin, N. The EEG signal: A window on the cortical brain activity. Pediatr. Anesth. 2012, 22, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Sury, M.; Worley, A.; Boyd, S. Age-related changes in EEG power spectra in infants during sevoflurane wash-out. Br. J. Anaesth. 2014, 112, 686–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasui, Y.; Masaki, E.; Kato, F. Sevoflurane Directly Excites Locus Coeruleus Neurons of Rats. Anesthesiology 2007, 107, 992–1002. [Google Scholar] [CrossRef] [Green Version]
- Anderson, B.J.; Holford, N.H. Tips and traps analyzing pediatric PK data. Pediatr. Anesth. 2011, 21, 222–237. [Google Scholar] [CrossRef]
- Schuttler, J.; Ihmsen, H. Population pharmacokinetics of propofol: A multicenter study. Anesthesiology 2000, 92, 727–738. [Google Scholar] [CrossRef]
- Davison, J.; Wong, A.; Kottenbelt, G.; Frawley, G. MAC-awake of sevoflurane in children. Pediatr. Anesth. 2008, 18, 702–707. [Google Scholar] [CrossRef]
- Petroz, G.C.; Sikich, N.; James, M.; Van Dyk, H.; Shafer, S.L.; Schily, M.; Lerman, J. A Phase I, Two-center Study of the Pharmacokinetics and Pharmacodynamics of Dexmedetomidine in Children. Anesthesiology 2006, 105, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, C.; Schulman, S.; Herrera Castellanos, M.; Cofer, B.; Mitra, S.; Garcia da Rocha, M.; Wisemandle, W.; Gramlich, L. A Phase II/III, Multicenter, Safety, Efficacy, and Pharmacokinetic Study of Dexmedetomi-dine in Preterm and Term Neonates. J. Pediatr. 2014, 164, 276–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potts, A.L.; Anderson, B.J.; Warman, G.R.; Lerman, J.; Diaz, S.M.; Vilo, S. Dexmedetomidine pharmacokinetics in pediatric intensive care—A pooled analysis. Pediatr. Anesth. 2009, 19, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Kataria, B.K.; Ved, S.A.; Nicodemus, H.F. The pharmacokinetics of propofol in children using three dif-ferent data analysis approaches. Anesthesiology 1994, 80, 104–122. [Google Scholar] [CrossRef]
- Marsh, B.; White, M.; Morton, N. Kenny GNC. Pharmacokinetic model driven infusion of propofol in children. Br. J. Anaest. 1991, 67, 41–48. [Google Scholar] [CrossRef]
- Short, T.G.; Aun, C.S.; Tan, P.; Wong, J.; Tam, Y.H.; Oh, T.E. A prospective evaluation of pharmacokinetic mod-el-controlled infusion of propofol in paediatric patients. Br. J. Anaesth. 1994, 72, 302–306. [Google Scholar] [CrossRef]
- Absalom, A.; Kenny, G. ‘Paedfusor’ pharmacokinetic data set. Br. J. Anaesth. 2005, 95, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepúlveda, P.; Cortínez, L.; Sáez, C.; Penna, A.; Solari, S.; Guerra, I.; Absalom, A. Performance evaluation of paediatric propofol pharmacokinetic models in healthy young children. Br. J. Anaesth. 2011, 107, 593–600. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, T.; McCheyne, A.J.; Morton, N.; Karsli, C.; Luginbuehl, I.; Adeli, K.; Walsh, W.; Bissonnette, B. Clinical adaptation of a pharmacokinetic model of Propofol plasma concentrations in children. Pediatr. Anesth. 2008, 18, 235–239. [Google Scholar] [CrossRef]
- Sepúlveda, P.O.; Carrasco, E.; Tapia, L.F.; Ramos, M.; Cruz, F.; Conget, P.; Olivares, Q.F.B.; Cortínez, I. Evidence of hysteresis in propofol pharmacodynamics. Anaesthesia 2018, 73, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Sepúlveda, P.O.; Tapia, L.F.; Monsalves, S. Neural inertia and differences between loss of and recovery from consciousness during total intravenous anaesthesia: A narrative review. Anaesthesia 2019, 74, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, H.R.; León, P.J.; Fuentes, R.S.; Echevarría, G.C.; Cortínez, L.I. Prospective evaluation of the time to peak effect of propofol to target the effect site in children. Acta Anaesthesiol. Scand. 2009, 53, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Jeleazcov, C.; Ihmsen, H.; Schmidt, J.; Ammon, C.; Schwilden, H.; Schüttler, J.; Fechner, J. Pharmacodynamic modelling of the bispectral index response to propofol-based anaesthesia during general surgery in children. Br. J. Anaesth. 2008, 100, 509–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tononi, G. Integrated information theory of consciousness: An updated account. Arch. Ital. Biol. 2012, 150, 290–326. [Google Scholar]
- Douglass, L.M.; Wu, J.Y.; Rosman, N.P.; Stafstrom, C.E. Burst suppression electroencephalogram pattern in the newborn: Predicting the outcome. J. Child Neurol. 2002, 17, 403–408. [Google Scholar] [CrossRef]
- Yuan, I.; Landis, W.P.; Topjian, A.A.; Abend, N.S.; Lang, S.S.; Huh, J.W.; Kirschen, M.P.; Mensinger, J.L.; Zhang, B.; Kurth, C.D. Prevalence of Isoelectric Electroencephalography Events in Infants and Young Children Un-dergoing General Anesthesia. Anesth. Analg. 2020, 130, 462–471. [Google Scholar] [CrossRef]
- Davidson, A.J.; Sun, L.S. Clinical evidence for any effect of anesthesia on the developing brain. Anesthesiology 2018, 128, 840–853. [Google Scholar] [CrossRef] [Green Version]
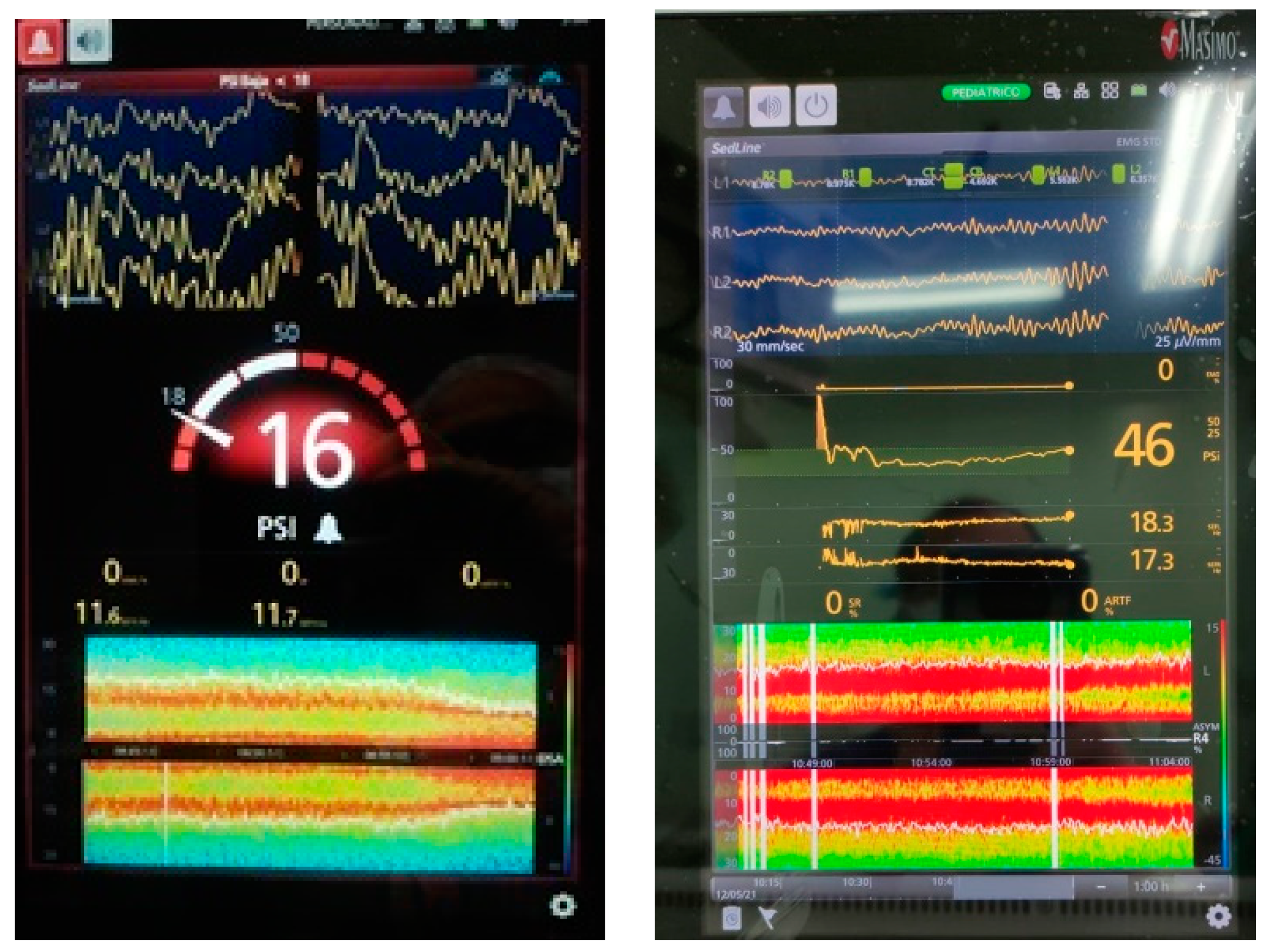
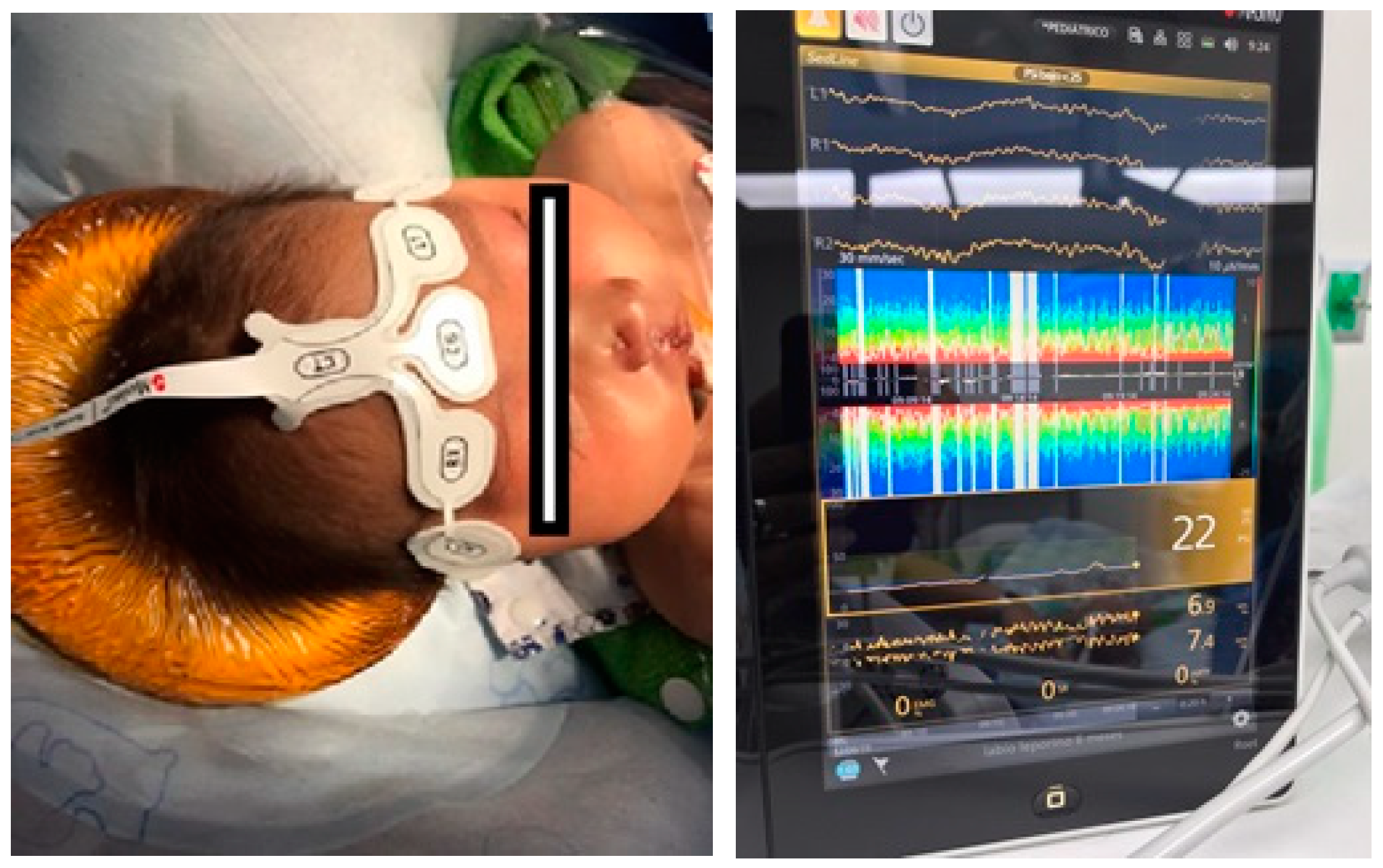
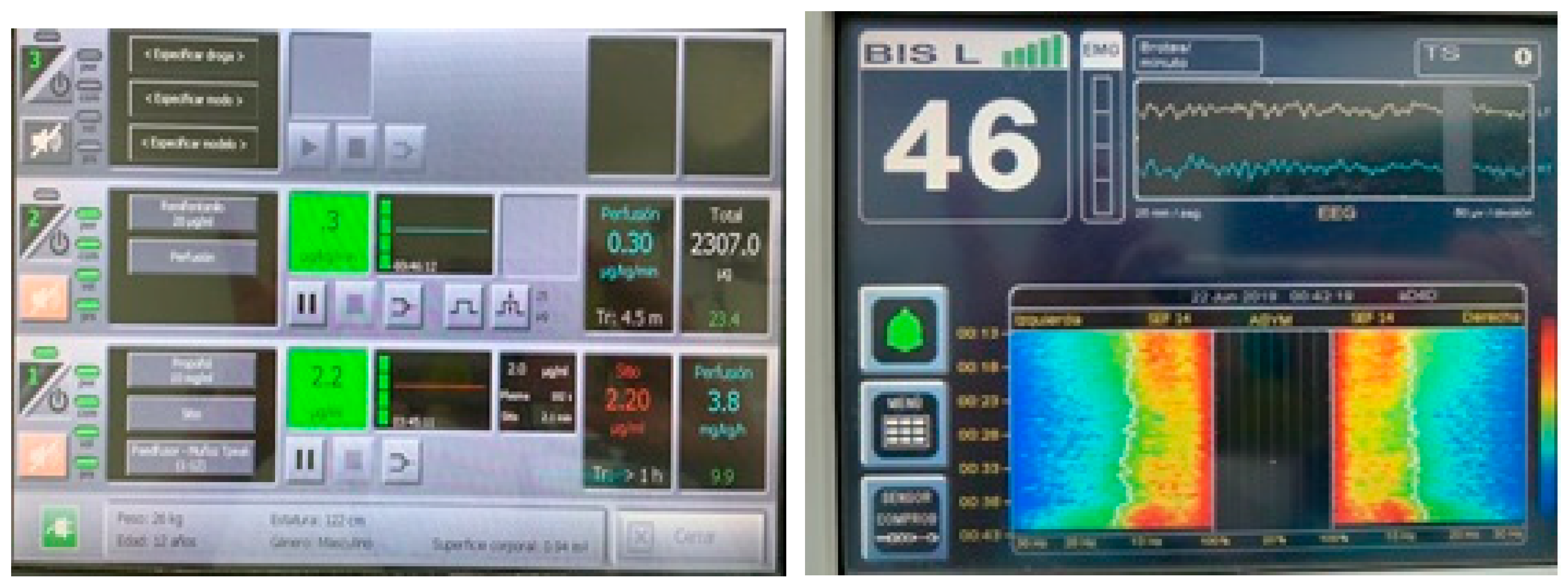
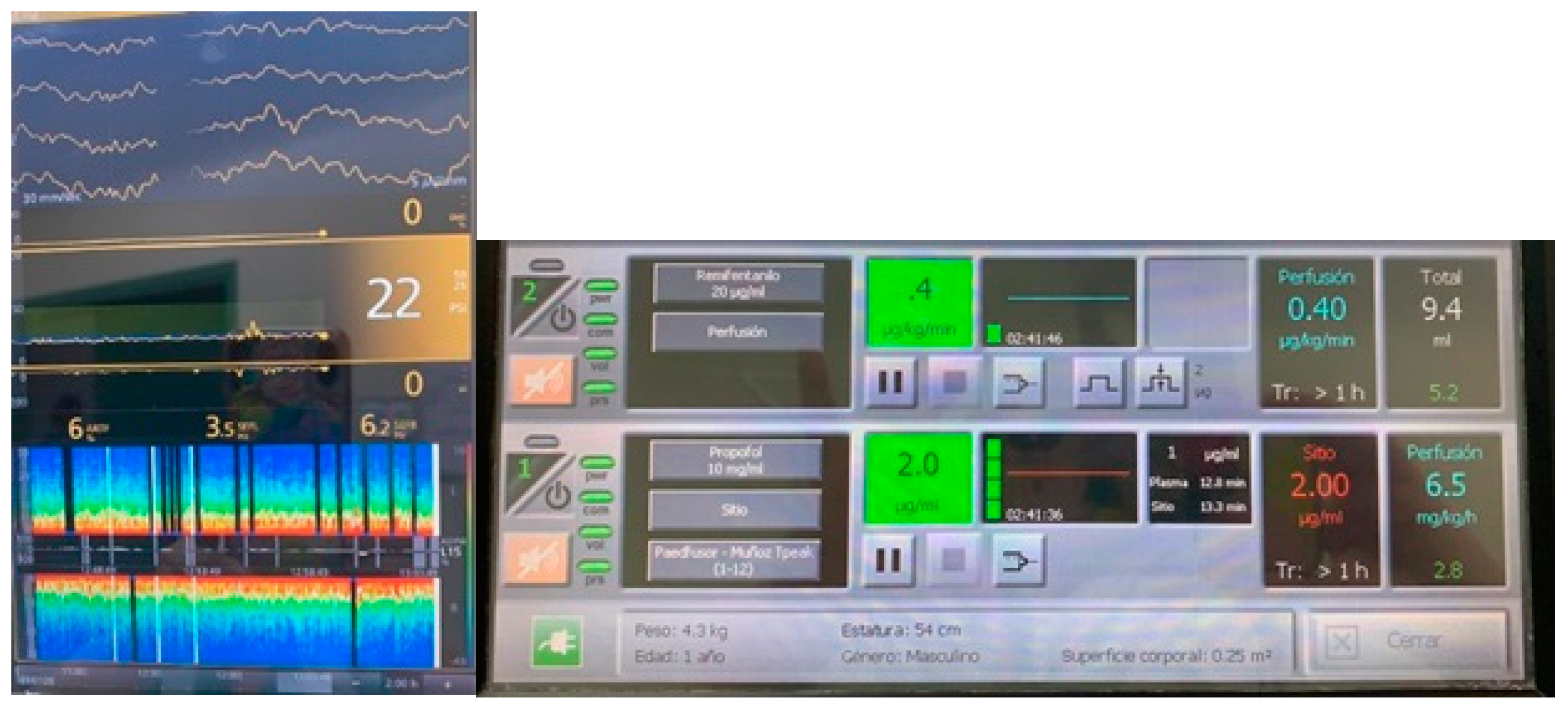
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepúlveda, P.O.; Epulef, V.; Campos, G. Why do We Use the Concepts of Adult Anesthesia Pharmacology in Developing Brains? Will It Have an Impact on Outcomes? Challenges in Neuromonitoring and Pharmacology in Pediatric Anesthesia. J. Clin. Med. 2021, 10, 2175. https://doi.org/10.3390/jcm10102175
Sepúlveda PO, Epulef V, Campos G. Why do We Use the Concepts of Adult Anesthesia Pharmacology in Developing Brains? Will It Have an Impact on Outcomes? Challenges in Neuromonitoring and Pharmacology in Pediatric Anesthesia. Journal of Clinical Medicine. 2021; 10(10):2175. https://doi.org/10.3390/jcm10102175
Chicago/Turabian StyleSepúlveda, Pablo O., Valeria Epulef, and Gustavo Campos. 2021. "Why do We Use the Concepts of Adult Anesthesia Pharmacology in Developing Brains? Will It Have an Impact on Outcomes? Challenges in Neuromonitoring and Pharmacology in Pediatric Anesthesia" Journal of Clinical Medicine 10, no. 10: 2175. https://doi.org/10.3390/jcm10102175
APA StyleSepúlveda, P. O., Epulef, V., & Campos, G. (2021). Why do We Use the Concepts of Adult Anesthesia Pharmacology in Developing Brains? Will It Have an Impact on Outcomes? Challenges in Neuromonitoring and Pharmacology in Pediatric Anesthesia. Journal of Clinical Medicine, 10(10), 2175. https://doi.org/10.3390/jcm10102175





