Machine Learning Models to Predict 30-Day Mortality in Mechanically Ventilated Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Data Collection and Definitions
2.3. Machine Learning Algorithms
2.4. Variable Importance
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Model Performance
3.3. Variable Importance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esteban, A.; Anzueto, A.; Frutos, F.; Alía, I.; Brochard, L.; Stewart, T.E.; Benito, S.; Epstein, S.K.; Apezteguía, C.; Nightingale, P.; et al. Characteristics and Outcomes in Adult Patients Receiving Mechanical VentilationA 28-Day International Study. JAMA 2002, 287, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in In-tensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Behrendt, C.E. Acute respiratory failure in the United States: Incidence and 31-day survival. Chest 2000, 118, 1100–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, S.S.; Kahn, J.M.; Hough, C.L.; Seeley, E.J.; White, D.B.; Douglas, I.S.; Cox, C.E.; Caldwell, E.; Bangdiwala, S.I.; Garrett, J.M.; et al. A multicenter mortality prediction model for patients receiving prolonged mechanical ventilation*. Crit. Care Med. 2012, 40, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, R.; Voicu, S.; Jaber, S.; Chousterman, B.; Paugam-Burtz, C.; Oueslati, H.; Damoisel, C.; Caillard, A.; Roquilly, A.; Feuillet, F.; et al. Tracheostomy and long-term mortality in ICU patients undergoing prolonged mechanical ventila-tion. PLoS ONE 2019, 14, e0220399. [Google Scholar] [CrossRef] [Green Version]
- Kalanuria, A.; Zai, W.; Mirski, M. Ventilator-associated pneumonia in the ICU. Crit. Care 2014, 18, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hough, C.L.; Caldwell, E.S.; Cox, C.E.; Douglas, I.S.; Kahn, J.M.; White, D.B.; Seeley, E.J.; Bangdiwala, S.I.; Rubenfeld, G.D.; Angus, D.C.; et al. Development and Validation of a Mortality Prediction Model for Patients Receiving 14 Days of Mechanical Ventilation. Crit. Care Med. 2015, 43, 2339–2345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; Sheng, Y.; Zhu, N.; Tan, Y.; Xie, X.; Wang, S.; Cai, J. The v-DECAF score can predict 90-day all-cause mortality in patients with COPD exacerbation requiring invasive mechanical ventilation. Clin. Respir. J. 2019, 13, 438–445. [Google Scholar] [CrossRef]
- Udeh, C.I.; Hadder, B.; Udeh, B.L. Validation and Extension of the Prolonged Mechanical Ventilation Prognostic Model (ProVent) Score for Predicting 1-Year Mortality after Prolonged Mechanical Ventilation. Ann. Am. Thorac. Soc. 2015, 12, 1845–1851. [Google Scholar] [CrossRef]
- Aydoğdu, M.; Ozyilmaz, E.; Aksoy, H.; Gürsel, G.; Ekim, N. Mortality prediction in community-acquired pneumonia requiring mechanical ventilation; values of pneumonia and intensive care unit severity scores. Tuberk. ve Toraks 2010, 58, 25–34. [Google Scholar]
- Özlü, T.; Küçük, M.P.; Kaya, A.; Yarar, E.; Kirakli, S.C.; Dikiş, Ö.S.; Çelik, H.K.; Özkan, S.; Aksoy, H.B.; Küçük, A.O. Can we predict patients that will not benefit from invasive mechanical ventilation? A novel scoring system in intensive care: The IMV Mortality Prediction Score (IMPRES). Turk. J. Med Sci. 2019, 49, 1662–1673. [Google Scholar] [PubMed]
- Sudarsanam, T.D.; Jeyaseelan, L.; Thomas, K.; John, G. Predictors of mortality in mechanically ventilated patients. Postgrad. Med. J. 2005, 81, 780–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajic, O.; Afessa, B.; Thompson, B.T.; Frutos-Vivar, F.; Malinchoc, M.; Rubenfeld, G.D.; Esteban, A.; Anzueto, A.; Hub-Mayr, R.D.; Second International Study of Mechanical, V.; et al. Prediction of death and prolonged mechanical ventila-tion in acute lung injury. Crit. Care 2007, 11, R53. [Google Scholar] [CrossRef] [Green Version]
- Hersch, M.; Izbicki, G.; Dahan, D.; Breuer, G.S.; Nesher, G.; Einav, S. Predictors of mortality of mechanically ventilated patients in internal medicine wards. J. Crit. Care 2012, 27, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Parreco, J.; Hidalgo, A.; Parks, J.J.; Kozol, R.; Rattan, R. Using artificial intelligence to predict prolonged mechanical ventilation and tracheostomy placement. J. Surg. Res. 2018, 228, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Shashikumar, S.P.; Wardi, G.; Paul, P.; Carlile, M.; Brenner, L.N.; Hibbert, K.A.; North, C.M.; Mukerji, S.S.; Robbins, G.K.; Shao, Y.-P.; et al. Development and Prospective Validation of a Deep Learning Algorithm for Predicting Need for Mechanical Ventilation. Chest 2020. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Hsieh, M.-J.; Chen, C.-M.; Hsieh, C.-C.; Chao, C.-M.; Lai, C.-C. An Artificial Neural Network Model for Predicting Successful Extubation in Intensive Care Units. J. Clin. Med. 2018, 7, 240. [Google Scholar] [CrossRef] [Green Version]
- Kwong, M.T.; Colopy, G.W.; Weber, A.M.; Ercole, A.; Bergmann, J.H.M. The efficacy and effectiveness of machine learning for weaning in mechanically ventilated patients at the intensive care unit: A systematic review. Bio-Design Manuf. 2019, 2, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Hezarjaribi, N.; Dutta, R.; Xing, T.; Murdoch, G.K.; Mazrouee, S.; Mortazavi, B.J.; Ghasemzadeh, H. Monitoring Lung Mechanics during Mechanical Ventilation using Machine Learning Algorithms. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; Volume 2018, pp. 1160–1163. [Google Scholar]
- Subbe, C.P.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified Early Warning Score in medical admis-sions. QJM Mon. J. Assoc. Physicians 2001, 94, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longi-tudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, Y.; Lee, Y.; Lee, S.; Park, J. An Algorithm Based on Deep Learning for Predicting In-Hospital Cardiac Arrest. J. Am. Hear. Assoc. 2018, 7, e008678. [Google Scholar] [CrossRef] [Green Version]
- Baum, E.B. On the capabilities of multilayer perceptrons. J. Complex. 1988, 4, 193–215. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, W. A Comparative Performance Assessment of Ensemble Learning for Credit Scoring. Mathematics 2020, 8, 1756. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Permutation Importance. Available online: https://eli5.readthedocs.io/en/latest/blackbox/permutation_importance.html (accessed on 1 March 2021).
- Kang, M.W.; Kim, J.; Kim, D.K.; Oh, K.H.; Joo, K.W.; Kim, Y.S.; Han, S.S. Machine learning algorithm to predict mortal-ity in patients undergoing continuous renal replacement therapy. Crit. Care 2020, 24, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serviá, L.; Montserrat, N.; Badia, M.; Llompart-Pou, J.A.; Barea-Mendoza, J.A.; Chico-Fernández, M.; Sánchez-Casado, M.; Jiménez, J.M.; Mayor, D.M.; Trujillano, J. Machine learning techniques for mortality prediction in critical traumatic patients: Anatomic and physiologic variables from the RETRAUCI study. BMC Med. Res. Methodol. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Abujaber, A.; Fadlalla, A.; Gammoh, D.; Abdelrahman, H.; Mollazehi, M.; El-Menyar, A. Prediction of in-hospital mor-tality in patients on mechanical ventilation post traumatic brain injury: Machine learning approach. BMC Med. Inform. Decis. Mak. 2020, 20, 336. [Google Scholar] [CrossRef]
- Thorsen-Meyer, H.-C.; Nielsen, A.B.; Nielsen, A.P.; Kaas-Hansen, B.S.; Toft, P.; Schierbeck, J.; Strøm, T.; Chmura, P.J.; Heimann, M.; Dybdahl, L.; et al. Dynamic and explainable machine learning prediction of mortality in patients in the intensive care unit: A retrospective study of high-frequency data in electronic patient records. Lancet Digit. Heal. 2020, 2, e179–e191. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.; Cho, J.; Kim, Y.S.; Sol, I.S.; Sung, Y.; Cho, I.; Park, M.; Jang, H.; Kim, Y.H.; et al. A deep learning model for real-time mortality prediction in critically ill children. Crit. Care 2019, 23, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.E.; Mark, R.G. Real-time mortality prediction in the Intensive Care Unit. In AMIA Annual Symposium Proceedings; AMIA Symposium: Boston, WA, USA, 2018; Volume 2017, pp. 994–1003. [Google Scholar]
- Kaier, K.; Heister, T.; Wolff, J.; Wolkewitz, M. Mechanical ventilation and the daily cost of ICU care. BMC Heal. Serv. Res. 2020, 20, 267. [Google Scholar] [CrossRef]
- Dar, M.; Swamy, L.; Gavin, D.; Theodore, A. Mechanical-Ventilation Supply and Options for the COVID-19 Pandemic. Leveraging All Available Resources for a Limited Resource in a Crisis. Ann. Am. Thorac. Soc. 2021, 18, 408–416. [Google Scholar] [CrossRef]
- The Lancet Respiratory, M. Opening the black box of machine learning. Lancet. Respir. Med. 2018, 6, 801. [Google Scholar] [CrossRef]
- Cressoni, M.; Cadringher, P.; Chiurazzi, C.; Amini, M.; Gallazzi, E.; Marino, A.; Brioni, M.; Carlesso, E.; Chiumello, D.A.; Quintel, M.; et al. Lung Inhomogeneity in Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2013, 189, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, C.; Lopes, A.; Copot, D.; Machado, J.; Bates, J. The role of fractional calculus in modeling biological phenomena: A review. Commun. Nonlinear Sci. Numer. Simul. 2017, 51, 141–159. [Google Scholar] [CrossRef]
- Copot, D.; De Keyser, R.; Derom, E.; Ionescu, C.-M. Structural changes in the COPD lung and related heterogeneity. PLoS ONE 2017, 12, e0177969. [Google Scholar] [CrossRef] [PubMed]
- Copot, D.; De Keyser, R.; Derom, E.; Ortigueira, M.; Ionescu, C.M. Reducing bias in fractional order impedance esti-mation for lung function evaluation. Biomed. Signal Process. Control 2018, 39, 74–80. [Google Scholar] [CrossRef]

| Variables | Total (n = 16,940) | Survivors (n = 11,879) | Non-Survivors (n = 5061) | p Value |
|---|---|---|---|---|
| Age (years) | 67 ± 15 | 66 ± 15 | 69 ± 14 | <0.001 |
| Male sex (%) | 61.5 | 61.3 | 61.8 | 0.567 |
| Interval between hospitalization and ICU admission (days) | 2 ± 7 | 2 ± 6 | 3 ± 9 | <0.001 |
| Interval between hospitalization and MV initiation (days) | 1 ± 6 | 1 ± 6 | 2 ± 7 | <0.001 |
| APACHE II | 23 ± 4 | 22 ± 7 | 26 ± 7 | <0.001 |
| ProVent score | 3 ± 1 | 3 ± 1 | 4 ± 1 | <0.001 |
| Modified early warning score | 5 ± 2 | 4 ± 2 | 6 ± 2 | <0.001 |
| Transfer from skilled nursing facility (%) | 9.2 | 8.8 | 10.1 | 0.007 |
| Charlson comorbidity index | 4 ± 3 | 4 ± 3 | 5 ± 2 | 0.006 |
| Comorbidities a (%) | ||||
| Diabetes | 20.5 | 22.2 | 16.4 | <0.001 |
| Congestive heart failure | 18.1 | 19.8 | 14.0 | <0.001 |
| Myocardial infarction | 8.5 | 8.8 | 7.8 | 0.037 |
| Chronic pulmonary disease | 16.5 | 18.4 | 12.1 | <0.001 |
| Liver disease | 9.3 | 8.5 | 11.4 | <0.001 |
| Moderate to severe CKD | 12.6 | 12.6 | 12.6 | 0.998 |
| Any malignancy | 20.1 | 19.2 | 22.0 | <0.001 |
| Rheumatic disease | 1.6 | 1.4 | 2.2 | <0.001 |
| Dementia | 7.0 | 7.6 | 5.4 | <0.001 |
| Cerebrovascular disease | 26.6 | 27.6 | 24.2 | <0.001 |
| Continuous renal replacement therapy (%) | 14.6 | 10.1 | 25.1 | <0.001 |
| Transfusion (%) | 27.3 | 24.6 | 33.6 | <0.001 |
| Medications (%) | ||||
| Vasopressors and inotropes | 50.9 | 44.3 | 66.3 | <0.001 |
| Corticosteroids | 16.4 | 15.1 | 19.4 | <0.001 |
| Opioids | 33.7 | 33.2 | 34.6 | 0.077 |
| Sedatives | 20.8 | 22.1 | 17.8 | <0.001 |
| Neuromuscular blockades | 12.4 | 11.9 | 13.8 | <0.001 |
| PaO2/FiO2 ratio | 246 ± 177 | 262 ± 176 | 207 ± 173 | <0.001 |
| Length of stay (day) | 29 ± 36 | 29 ± 36 | 28 ± 36 | 0.175 |
| ICU stay (day) | 16 ± 27 | 16 ± 27 | 17 ± 26 | 0.767 |
| Duration of MV (day) | 11 ± 23 | 11 ± 23 | 11 ± 22 | 0.592 |
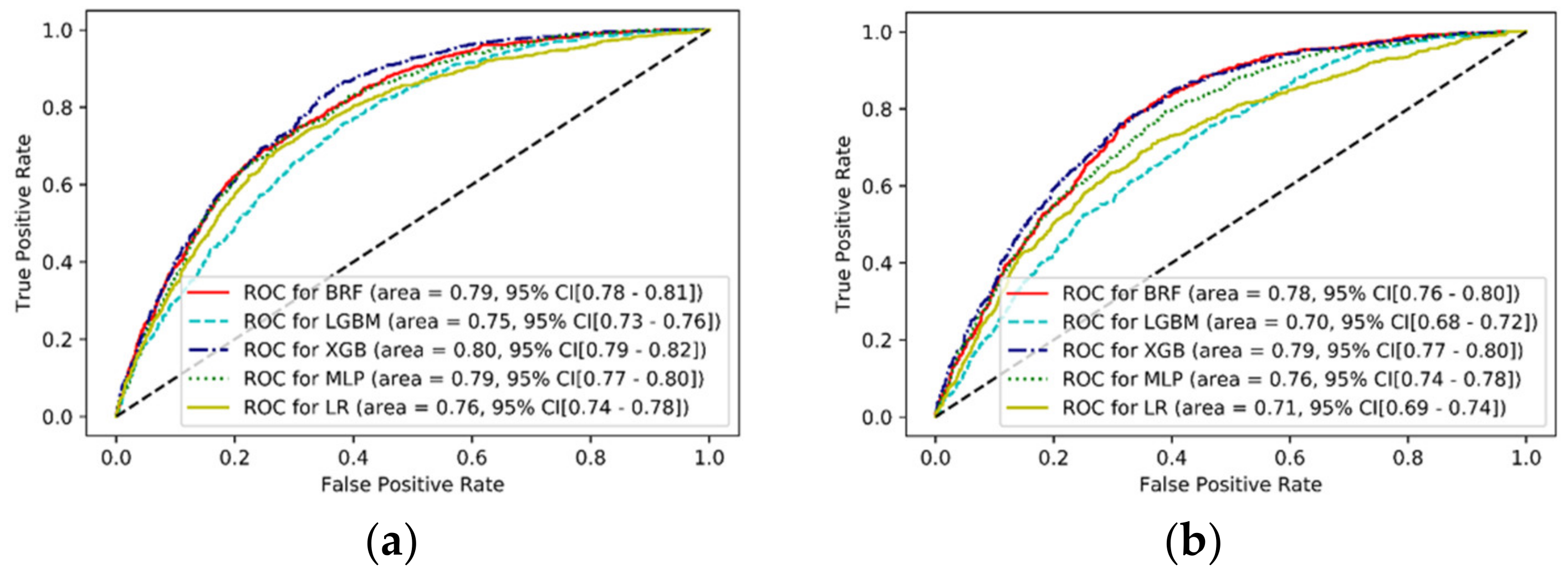
| Models | AUC | Positive Predictive Value | Sensitivity | Accuracy |
|---|---|---|---|---|
| BRF | 0.78 | 0.37 | 0.84 | 0.65 |
| LGBM | 0.70 | 0.37 | 0.52 | 0.70 |
| XGB | 0.79 | 0.46 | 0.58 | 0.76 |
| MLP | 0.76 | 0.41 | 0.62 | 0.72 |
| LR | 0.71 | 0.40 | 0.55 | 0.72 |
| Machine Learning Models | |||||
|---|---|---|---|---|---|
| Ranking | BRF | LGBM | XGB | MLP | LR |
| 1 | APACHE II | APACHE II | Norepinephrine | CCI | CCI |
| 2 | Base excess | SpO2 | CHF | APACHE II | APACHE II |
| 3 | HCO3 | Respiratory rate | Chronic pulmonary disease | CHF | Age |
| 4 | Platelet | Chronic pulmonary disease | Diabetes | Chronic pulmonary disease | CHF |
| 5 | Norepinephrine | Midazolam | APACHE II | Diabetes | Chronic pulmonary disease |
| 6 | pH | CHF | SpO2 | Norepinephrine | Diabetes |
| 7 | PaO2/FiO2 | Norepinephrine | Midazolam | Age | Age group of CCI |
| 8 | Blood urea nitrogen | Age | Disease of the nervous system | Age group of CCI | Malignancy |
| 9 | eGFR | HCO3 | Endocrine, nutritional, and metabolic disease | Transfer from skilled nursing facility | Remifentanil |
| 10 | FiO2 | Diabetes | Mental and behavioral disorders | Malignancy | Norepinephrine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Kwon, Y.S.; Baek, M.S. Machine Learning Models to Predict 30-Day Mortality in Mechanically Ventilated Patients. J. Clin. Med. 2021, 10, 2172. https://doi.org/10.3390/jcm10102172
Kim JH, Kwon YS, Baek MS. Machine Learning Models to Predict 30-Day Mortality in Mechanically Ventilated Patients. Journal of Clinical Medicine. 2021; 10(10):2172. https://doi.org/10.3390/jcm10102172
Chicago/Turabian StyleKim, Jong Ho, Young Suk Kwon, and Moon Seong Baek. 2021. "Machine Learning Models to Predict 30-Day Mortality in Mechanically Ventilated Patients" Journal of Clinical Medicine 10, no. 10: 2172. https://doi.org/10.3390/jcm10102172
APA StyleKim, J. H., Kwon, Y. S., & Baek, M. S. (2021). Machine Learning Models to Predict 30-Day Mortality in Mechanically Ventilated Patients. Journal of Clinical Medicine, 10(10), 2172. https://doi.org/10.3390/jcm10102172






