Current Therapy of the Patients with MDS: Walking towards Personalized Therapy
Abstract
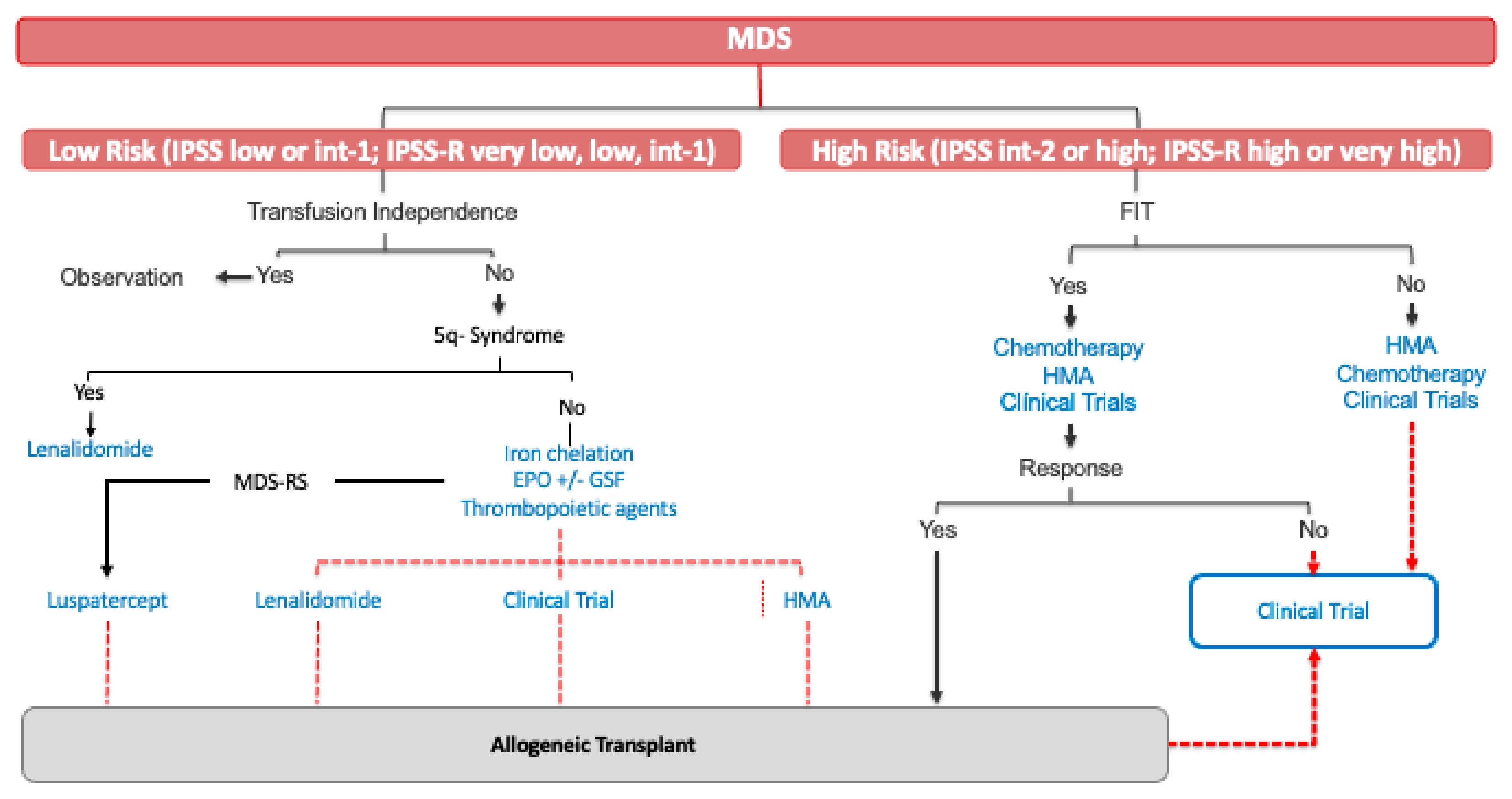
:1. Introduction
2. Lower-Risk MDS (LR-MDS)
2.1. Newly Diagnosed Patients with LR-MDS
2.1.1. Erythroid Stimulating Agents
2.1.2. Thrombopoietic Agents
2.1.3. Lenalidomide
2.1.4. Iron Chelation
2.1.5. Immunosuppressive Therapy
2.2. Relapsed/Refractory LR-MDS
2.2.1. Luspatercept
2.2.2. Hypomethylating Agents
2.2.3. Allogenic Stem Cell Transplantation
2.3. Emerging Strategies for Management LR-MDS
2.3.1. Roxadustat
2.3.2. Imetelstat
3. High-Risk MDS (HR-MDS)
3.1. Newly Diagnosed HR-MDS
3.1.1. Hypomethylating Agents
3.1.2. AML-Like Chemotherapy
3.1.3. Allogenic Stem Cell Transplantation
3.2. Future Therapies for HR-MDS: HMA-Based Combination Therapies
3.2.1. Azacitidine + Pevonedistat
3.2.2. Azacitidine + Magrolimab
3.2.3. Azacitidine + Eprenetapopt
3.2.4. Azacitidine + Venetoclax
3.3. Hypomethylating Agent Failure in HR-MDS
3.3.1. Rigosertib
3.3.2. Immune Checkpoint Inhibitors
3.3.3. Venetoclax
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McKenna, R.W.; Kyle, R.A.; Kuehl, W.M.; Harris, N.L.; Coupland, R.W.; Fend, F. Plasma cell neoplasms. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Eds.; World Health Organization: Lyon, France, 2017. [Google Scholar]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.; Cox, C.; Lebeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Nazha, A.; Narkhede, M.; Radivoyevitch, T.; Seastone, D.J.; Patel, B.J.; Gerds, A.T.; Mukherjee, S.; Kalaycio, M.; Advani, A.; Przychodzen, B.; et al. Incorporation of molecular data into the Revised International Prognostic Scoring System in treated patients with myelodysplastic syndromes. Leukemia 2016, 30, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Moyo, V.; Lefebvre, P.; Duh, M.S.; Yektashenas, B.; Mundle, S. Erythropoiesis-stimulating agents in the treatment of anemia in myelodysplastic syndromes: A meta-analysis. Ann. Hematol. 2008, 87, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Hellström-Lindberg, E.; Gulbrandsen, N.; Lindberg, G.; Ahlgren, T.; Dahl, I.M.S.; Dybedal, I.; Grimfors, G.; Hesse-Sundin, E.; Hjorth, M.; Kanter-Lewensohn, L.; et al. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: Significant effects on quality of life. Br. J. Haematol. 2003, 120, 1037–1046. [Google Scholar] [CrossRef]
- Platzbecker, U.; Symeonidis, A.; Oliva, E.N.; Goede, J.S.; Delforge, M.; Mayer, J.; Slama, B.; Badre, S.; Gasal, E.; Mehta, B.; et al. A phase 3 randomized placebo-controlled trial of darbepoetin alfa in patients with anemia and lower-risk myelodysplastic syndromes. Leukemia 2017, 31, 1944–1950. [Google Scholar] [CrossRef] [Green Version]
- Fenaux, P.; Santini, V.; Spiriti, M.A.A.; Giagounidis, A.; Schlag, R.; Radinoff, A.; Gercheva-Kyuchukova, L.; Anagnostopoulos, A.; Oliva, E.N.; Symeonidis, A.; et al. A phase 3 randomized, placebo-controlled study assessing the efficacy and safety of epoetin-α in anemic patients with low-risk MDS. Leukemia 2018, 32, 2648–2658. [Google Scholar] [CrossRef] [Green Version]
- Affentranger, L.; Bohlius, J.; Hallal, M.; Bonadies, N. Efficacy of granulocyte colony stimulating factor in combination with erythropoiesis stimulating agents for treatment of anemia in patients with lower risk myelodysplastic syndromes: A systematic review. Crit. Rev. Oncol. 2019, 136, 37–47. [Google Scholar] [CrossRef]
- Jädersten, M.; Montgomery, S.M.; Dybedal, I.; Porwit-MacDonald, A.; Hellström-Lindberg, E. Long-term outcome of treatment of anemia in MDS with erythropoietin and G-CSF. Blood 2005, 106, 803–811. [Google Scholar] [CrossRef]
- Park, S.; Grabar, S.; Kelaidi, C.; Beyne-Rauzy, O.; Picard, F.; Bardet, V.; Coiteux, V.; Leroux, G.; Lepelley, P.; Daniel, M.-T.; et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: The GFM experience. Blood 2008, 111, 574–582. [Google Scholar] [CrossRef]
- Alfonso, A.; Alonso, E.; Alonso, S. Guías Españolas de SMD y LMMC.España. GESMD. 2020. Available online: https://www.gesmd.es/guias-smd-y-lmmc/ (accessed on 30 April 2021).
- Fenaux, P.; Muus, P.; Kantarjian, H.; Lyons, R.M.; Larson, R.A.; Sekeres, M.A.; Becker, P.S.; Orejudos, A.; Franklin, J. Romiplostim monotherapy in thrombocytopenic patients with myelodysplastic syndromes: Long-term safety and efficacy. Br. J. Haematol. 2017, 178, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Oliva, E.N.; Alati, C.; Santini, V.; Poloni, A.; Molteni, A.; Niscola, P.; Salvi, F.; Sanpaolo, G.; Balleari, E.; Germing, U.; et al. Eltrombopag versus placebo for low-risk myelodysplastic syndromes with thrombocytopenia (EQoL-MDS): Phase 1 results of a single-blind, randomised, controlled, phase 2 superiority trial. Lancet Haematol. 2017, 4, e127–e136. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Chien, K.S.; Montalban-Bravo, G. Myelodysplastic syndromes: 2021 update on diagnosis, risk stratification and management. Am. J. Hematol. 2020, 95, 1399–1420. [Google Scholar] [CrossRef] [PubMed]
- List, A.; Kurtin, S.; Roe, D.J.; Buresh, A.; Mahadevan, D.; Fuchs, D.; Rimsza, L.; Heaton, R.; Knight, R.; Zeldis, J.B. Efficacy of Lenalidomide in Myelodysplastic Syndromes. N. Engl. J. Med. 2005, 352, 549–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- List, A.; Dewald, G.; Bennett, J.; Giagounidis, A.; Raza, A.; Feldman, E.; Powell, B.; Greenberg, P.; Thomas, D.; Stone, R.; et al. Lenalidomide in the Myelodysplastic Syndrome with Chromosome 5q Deletion. N. Engl. J. Med. 2006, 355, 1456–1465. [Google Scholar] [CrossRef] [Green Version]
- Dutt, S.; Narla, A.; Lin, K.; Mullally, A.; Abayasekara, N.; Megerdichian, C.; Wilson, F.H.; Currie, T.; Khanna-Gupta, A.; Berliner, N.; et al. Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood 2011, 117, 2567–2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fumagalli, S.; Di Cara, A.; Neb-Gulati, A.; Natt, F.; Schwemberger, S.; Hall, J.; Babcock, G.F.; Bernardi, R.; Pandolfi, P.P.; Thomas, G.J. Absence of nucleolar disruption after impairment of 40S ribosome biogenesis reveals an rpL11-translation-dependent mechanism of p53 induction. Nat. Cell Biol. 2009, 11, 501–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krönke, J.; Fink, E.C.; Hollenbach, P.W.; Macbeth, K.J.; Hurst, S.N.; Udeshi, N.D.; Chamberlain, P.P.; Mani, D.R.; Man, H.W.; Gandhi, A.K.; et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature 2015, 523, 183–188. [Google Scholar] [CrossRef]
- Fenaux, P.; Giagounidis, A.; Selleslag, D.; Beyne-Rauzy, O.; Mufti, G.; Mittelman, M.; Muus, P.; Boekhorst, P.T.; Sanz, G.; Del Cañizo, C.; et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood 2011, 118, 3765–3776. [Google Scholar] [CrossRef]
- Jaedersten, M.; Saft, L.; Smith, A.; Kulasekararaj, A.; Pomplun, S.; Goehring, G.; Hedlund, A.; Hast, R.; Schlegelberger, B.; Porwit, A.; et al. TP53 Mutations in Low-Risk Myelodysplastic Syndromes With del(5q) Predict Disease Progression. J. Clin. Oncol. 2011, 29, 1971–1979. [Google Scholar] [CrossRef]
- Mallo, M.; del Rey, M.; Ibáñez, M.; Calasanz, M.J.; Arenillas, L.; Larráyoz, M.J.; Pedro, C.; Jerez, A.; Maciejewski, J.; Costa, D.; et al. Response to lenalidomide in myelodysplastic syndromes with del(5q): Influence of cytogenetics and mutations. Br. J. Haematol. 2013, 162, 74–86. [Google Scholar] [CrossRef]
- Sallman, D.A.; Komrokji, R.; Vaupel, C.; Cluzeau, T.; Geyer, S.M.; McGraw, K.L.; Al Ali, N.H.; Lancet, J.; McGinniss, M.J.; Nahas, S.; et al. Impact of TP53 mutation variant allele frequency on phenotype and outcomes in myelodysplastic syndromes. Leukemia 2016, 30, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.; Kosmider, O.; Chevret, S.; Delaunay, J.; Stamatoullas, A.; Rose, C.D.; Beynerauzy, O.; Banos, A.; Guercibresler, A.; Wickenhauser, S.; et al. Lenalidomide with or without erythropoietin in transfusion-dependent erythropoiesis-stimulating agent-refractory lower-risk MDS without 5q deletion. Leukemia 2016, 30, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Giri, S.; Deveaux, M.; Ballas, S.K.; Duong, V.H. Systematic review and meta-analysis of the effect of iron chelation therapy on overall survival and disease progression in patients with lower-risk myelodysplastic syndromes. Ann. Hematol. 2018, 98, 339–350. [Google Scholar] [CrossRef]
- Angelucci, E.; Li, J.; Greenberg, P.; Wu, D.; Hou, M.; Figueroa, E.H.; Rodriguez, M.G.; Dong, X.; Ghosh, J.; Izquierdo, M.; et al. Iron chelation in transfusion-dependent patients with low- To intermediate-1-risk myelodysplastic syndromes: A randomized trial. Ann. Intern. Med. 2020, 172, 513–522. [Google Scholar] [CrossRef]
- Ganangomez, I.; Wei, Y.; Starczynowski, D.T.; Colla, S.; Yang, H.; Cabrero-Calvo, M.; Bohannan, Z.; Verma, A.; Steidl, U.; Garciamanero, G. Deregulation of innate immune and inflammatory signaling in myelodysplastic syndromes. Leukemia 2015, 29, 1458–1469. [Google Scholar] [CrossRef]
- Parikh, A.R.; Olnes, M.J.; Barrett, A.J. Immunomodulatory Treatment of Myelodysplastic Syndromes: Antithymocyte Globulin, Cyclosporine, and Alemtuzumab. Semin. Hematol. 2012, 49, 304–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passweg, J.R.; Giagounidis, A.A.N.; Simcock, M.; Aul, C.; Dobbelstein, C.; Stadler, M.; Ossenkoppele, G.; Hofmann, W.K.; Schilling, K.; Tichelli, A.; et al. Immunosuppressive therapy for patients with myelodysplastic syndrome: A prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care—SAKK 33/99. J. Clin. Oncol. 2011, 29, 303–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, M.; Bewersdorf, J.P.; Giri, S.; Wang, R.; Zeidan, A.M. Use of immunosuppressive therapy for management of myelodysplastic syndromes: A systematic review and meta-analysis. Haematologica 2019, 105, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sloand, E.M.; Olnes, M.J.; Shenoy, A.; Weinstein, B.; Boss, C.; Loeliger, K.; Wu, C.O.; More, K.; Barrett, A.J.; Scheinberg, P.; et al. Alemtuzumab Treatment of Intermediate-1 Myelodysplasia Patients Is Associated With Sustained Improvement in Blood Counts and Cytogenetic Remissions. J. Clin. Oncol. 2010, 28, 5166–5173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bataller, A.; Montalban-Bravo, G.; Soltysiak, K.A.; Garcia-Manero, G. The role of TGFβ in hematopoiesis and myeloid disorders. Leukemia 2019, 33, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Platzbecker, U.; Mufti, G.J.; Garcia-Manero, G.; Buckstein, R.; Santini, V.; Díez-Campelo, M.; Finelli, C.; Cazzola, M.; Ilhan, O.; et al. Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 382, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Germing, U.; Götze, K.S.; Kiewe, P.; Mayer, K.; Chromik, J.; Radsak, M.; Wolff, T.; Zhang, X.; Laadem, A.; et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): A multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol. 2017, 18, 1338–1347. [Google Scholar] [CrossRef]
- Jabbour, E.J.; Garcia-Manero, G.; Strati, P.; Mishra, A.; Al Ali, N.H.; Padron, E.; Lancet, J.; Kadia, T.; Daver, N.; O’Brien, S.; et al. Outcome of patients with low-risk and intermediate-1-risk myelodysplastic syndrome after hypomethylating agent failure: A report on behalf of the MDS Clinical Research Consortium. Cancer 2015, 121, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; Almeida, A.; Giagounidis, A.; Platzbecker, U.; Garcia, R.; Voso, M.T.; Larsen, S.R.; Valcarcel, D.; Silverman, L.R.; Skikne, B.; et al. Design and rationale of the QUAZAR lower-risk MDS (AZA-MDS-003) trial: A randomized phase 3 study of CC-486 (oral azacitidine) plus best supportive care vs placebo plus best supportive care in patients with IPSS lower-risk myelodysplastic syndromes and po. BMC Hematol. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komrokji, R.; Swern, A.S.; Grinblatt, D.; Lyons, R.M.; Tobiasson, M.; Silverman, L.R.; Sayar, H.; Vij, R.; Fliss, A.; Tu, N.; et al. Azacitidine in Lower-Risk Myelodysplastic Syndromes: A Meta-Analysis of Data from Prospective Studies. Oncologist 2017, 23, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Manero, G.; Jabbour, E.; Borthakur, G.; Faderl, S.; Estrov, Z.; Yang, H.; Maddipoti, S.; Godley, L.A.; Gabrail, N.; Berdeja, J.G.; et al. Randomized Open-Label Phase II Study of Decitabine in Patients with Low- or Intermediate-Risk Myelodysplastic Syndromes. J. Clin. Oncol. 2013, 31, 2548–2553. [Google Scholar] [CrossRef]
- Jabbour, E.; Short, N.J.; Montalban-Bravo, G.; Huang, X.; Bueso-Ramos, C.; Qiao, W.; Yang, H.; Zhao, C.; Kadia, T.; Borthakur, G.; et al. Randomized phase 2 study of low-dose decitabine vs low-dose azacitidine in lower-risk MDS and MDS/MPN. Blood 2017, 130, 1514–1522. [Google Scholar] [CrossRef] [Green Version]
- Cutler, C.S.; Lee, S.J.; Greenberg, P.; Deeg, H.J.; Pérez, W.S.; Anasetti, C.; Bolwell, B.J.; Cairo, M.S.; Gale, R.P.; Klein, J.P.; et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: Delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 2004, 104, 579–585. [Google Scholar] [CrossRef]
- Saygin, C.; Carraway, H.E. Current and emerging strategies for management of myelodysplastic syndromes. Blood Rev. 2020, 100791. [Google Scholar] [CrossRef]
- Steensma, D.P.; Platzbecker, U.; Van Eygen, K.; Raza, A.; Santini, V.; Germing, U.; Font, P.; Samarina, I.; Díez-Campelo, M.; Thepot, S.; et al. Imetelstat Treatment Leads to Durable Transfusion Independence (TI) in RBC Transfusion-Dependent (TD), Non-Del(5q) Lower Risk MDS Relapsed/Refractory to Erythropoiesis-Stimulating Agent (ESA) Who Are Lenalidomide (LEN) and HMA Naive. Blood 2018, 132, 463. [Google Scholar] [CrossRef]
- Fenaux, P.; Ades, L. Review of azacitidine trials in Intermediate-2-and High-risk myelodysplastic syndromes. Leuk. Res. 2009, 33, S7–S11. [Google Scholar] [CrossRef]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized Controlled Trial of Azacitidine in Patients With the Myelodysplastic Syndrome: A Study of the Cancer and Leukemia Group B. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Itzykson, R.; Thépot, S.; Quesnel, B.; Dreyfus, F.; Beyne-Rauzy, O.; Turlure, P.; Vey, N.; Recher, C.; Dartigeas, C.; Legros, L.; et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood 2011, 117, 403–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantarjlan, H.; Issa, J.P.J.; Rosenfeld, C.S.; Bennett, J.M.; Albitar, M.; DiPersio, J.; Klimek, V.; Slack, J.; De Castro, C.; Ravandi, F.; et al. Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study. Cancer 2006, 106, 1794–1803. [Google Scholar] [CrossRef]
- Kantarjian, H.; Oki, Y.; Garcia-Manero, G.; Huang, X.; O’Brien, S.; Cortes, J.; Faderl, S.; Bueso-Ramos, C.; Ravandi, F.; Estrov, Z.; et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 2006, 109, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P.; Baer, M.R.; Slack, J.L.; Buckstein, R.; Godley, L.A.; Garcia-Manero, G.; Albitar, M.; Larsen, J.S.; Arora, S.; Cullen, M.T.; et al. Multicenter Study of Decitabine Administered Daily for 5 Days Every 4 Weeks to Adults With Myelodysplastic Syndromes: The Alternative Dosing for Outpatient Treatment (ADOPT) Trial. J. Clin. Oncol. 2009, 27, 3842–3848. [Google Scholar] [CrossRef] [PubMed]
- Savona, M.R.; Odenike, O.; Amrein, P.C.; Steensma, D.P.; De Zern, A.E.; Michaelis, L.C.; Faderl, S.; Harb, W.; Kantarjian, H.; Lowder, J.; et al. An oral fixed-dose combination of decitabine and cedazuridine in myelodysplastic syndromes: A multicentre, open-label, dose-escalation, phase 1 study. Lancet Haematol. 2019, 6, e194–e203. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; McCloskey, J.; Griffiths, E.A.; Yee, K.W.; Zeidan, A.M.; Al-Kali, A.; Dao, K.-H.; Deeg, H.J.; Patel, P.A.; Sabloff, M.; et al. Pharmacokinetic Exposure Equivalence and Preliminary Efficacy and Safety from a Randomized Cross over Phase 3 Study (ASCERTAIN study) of an Oral Hypomethylating Agent ASTX727 (cedazuridine/decitabine) Compared to IV Decitabine. Blood 2019, 134, 846. [Google Scholar] [CrossRef]
- Sekeres, M.A.; Watts, J.; Radinoff, A.; Sangerman, M.A.; Cerrano, M.; Lopez, P.F.; Zeidner, J.F.; Campelo, M.D.; Graux, C.; Liesveld, J.; et al. Randomized phase 2 trial of pevonedistat plus azacitidine versus azacitidine for higher-risk MDS/CMML or low-blast AML. Leukemia 2021. [Google Scholar] [CrossRef] [PubMed]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D.; Van Rooijen, N.; Weissman, I.L. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef] [Green Version]
- Sallman, D.A.; DeZern, A.E.; Garcia-Manero, G.; Steensma, D.P.; Roboz, G.J.; Sekeres, M.A.; Cluzeau, T.; Sweet, K.L.; McLemore, A.; McGraw, K.L.; et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J. Clin. Oncol. 2021, 39, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.S. Prospects for Venetoclax in Myelodysplastic Syndromes. Hematol. Clin. N. Am. 2020, 34, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Estey, E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J. Clin. Oncol. 2007, 25, 1908–1915. [Google Scholar] [CrossRef]
- Beran, M.; Shen, Y.; Kantarjian, H.; O’Brien, S.; Koller, C.A.; Giles, F.J.; Cortes, J.; Thomas, D.A.; Faderl, S.; Despa, S.; et al. High-dose chemotherapy in high-risk myelodysplastic syndrome: Covariate-adjusted comparison of five regimens. Cancer 2001, 92, 1999–2015. [Google Scholar] [CrossRef]
- Estey, E.H.; Thall, P.F.; Cortes, J.E.; Giles, F.J.; O’Brien, S.; Pierce, S.A.; Wang, X.; Kantarjian, H.M.; Beran, M. Comparison of idarubicin + ara-C–, fludarabine + ara-C–, and topotecan + ara-C–based regimens in treatment of newly diagnosed acute myeloid leukemia, refractory anemia with excess blasts in transformation, or refractory anemia with excess blasts. Blood 2001, 98, 3575–3583. [Google Scholar] [CrossRef] [Green Version]
- Kantarjian, H.; Beran, M.; Cortes, J.; O’Brien, S.; Giles, F.; Pierce, S.; Shan, J.; Plunkett, W.; Keating, M.; Estey, E.; et al. Long-term follow-up results of the combination of topotecan and cytarabine and other intensive chemotherapy regimens in myelodsplastic syndrome. Cancer 2006, 106, 1099–1109. [Google Scholar] [CrossRef]
- Venditti, A.; Tamburini, A.; Buccisano, F.; Scimò, M.T.; Del Poeta, G.; Maurillo, L.; Cox, M.C.; Abruzzese, E.; Tribalto, M.; Masi, M.; et al. A phase-II trial of all trans retinoic acid and low-dose cytosine arabinoside for the treatment of high-risk myelodysplastic syndromes. Ann. Hematol. 2000, 79, 138–142. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; O’Brien, S.; Huang, X.; Garcia-Manero, G.; Ravandi, F.; Cortes, J.; Shan, J.; Davisson, J.; Bueso-Ramos, C.E.; Issa, J.P.; et al. Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome: Comparison with historical experience. Cancer 2007, 109, 1133–1137. [Google Scholar] [CrossRef] [Green Version]
- Kadia, T.M.; Jain, P.; Ravandi, F.; Garcia-Manero, G.; Andreef, M.; Takahashi, K.; Borthakur, G.; Jabbour, E.; Konopleva, M.; Daver, N.G.; et al. TP53mutations in newly diagnosed acute myeloid leukemia: Clinicomolecular characteristics, response to therapy, and outcomes. Cancer 2016, 122, 3484–3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalban-Bravo, G.; Kanagal-Shamanna, R.; Sasaki, K.; Patel, K.; Ganan-Gomez, I.; Jabbour, E.; Kadia, T.; Ravandi, F.; Dinardo, C.; Borthakur, G.; et al. NPM1 mutations define a specific subgroup of MDS and MDS/MPN patients with favorable outcomes with intensive chemotherapy. Blood Adv. 2019, 3, 922–933. [Google Scholar] [CrossRef] [Green Version]
- Steensma, D.P. Myelodysplastic syndromes current treatment algorithm 2018. Blood Cancer J. 2018, 8, 47. [Google Scholar] [CrossRef]
- Chang, C.K.; Storer, B.E.; Scott, B.L.; Bryant, E.M.; Shulman, H.M.; Flowers, M.E.; Sandmaier, B.M.; Witherspoon, R.P.; Nash, R.A.; Sanders, J.E.; et al. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: Similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic d. Blood 2007, 110, 1379–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Festuccia, M.; Baker, K.; Gooley, T.A.; Sandmaier, B.M.; Deeg, H.J.; Scott, B.L. Hematopoietic Cell Transplantation in Myelodysplastic Syndromes after Treatment with Hypomethylating Agents. Biol. Blood Marrow Transplant. 2017, 23, 1509–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koreth, J.; Pidala, J.; Perez, W.S.; Deeg, H.J.; Garcia-Manero, G.; Malcovati, L.; Cazzola, M.; Park, S.; Itzykson, R.; Ades, L.; et al. Role of Reduced-Intensity Conditioning Allogeneic Hematopoietic Stem-Cell Transplantation in Older Patients With De Novo Myelodysplastic Syndromes: An International Collaborative Decision Analysis. J. Clin. Oncol. 2013, 31, 2662–2670. [Google Scholar] [CrossRef] [PubMed]
- Della Porta, M.G.; Gallì, A.; Bacigalupo, A.; Zibellini, S.; Bernardi, M.; Rizzo, E.; Allione, B.; Van Lint, M.T.; Pioltelli, P.; Marenco, P.; et al. Clinical Effects of Driver Somatic Mutations on the Outcomes of Patients With Myelodysplastic Syndromes Treated With Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2016, 34, 3627–3637. [Google Scholar] [CrossRef]
- Bejar, R.; Stevenson, K.E.; Caughey, B.; Lindsley, R.C.; Mar, B.G.; Stojanov, P.; Getz, G.; Steensma, D.P.; Ritz, J.; Soiffer, R.; et al. Somatic Mutations Predict Poor Outcome in Patients With Myelodysplastic Syndrome After Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2014, 32, 2691–2698. [Google Scholar] [CrossRef] [Green Version]
- Scott, B.L.; Pasquini, M.C.; Logan, B.R.; Wu, J.; Devine, S.M.; Porter, D.L.; Maziarz, R.T.; Warlick, E.D.; Fernandez, H.F.; Alyea, E.P.; et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J. Clin. Oncol. 2017, 35, 1154–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swords, R.T.; Coutre, S.; Maris, M.B.; Zeidner, J.F.; Foran, J.M.; Cruz, J.; Erba, H.P.; Berdeja, J.G.; Tam, W.; Vardhanabhuti, S.; et al. Pevonedistat, a first-in-class NEDD8-activating enzyme inhibitor, combined with azacitidine in patients with AML. Blood 2018, 131, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- Sallman, D.A.; Al Malki, M.; Asch, A.S.; Lee, D.J.; Kambhampati, S.; Donnellan, W.B.; Bradley, T.J.; Vyas, P.; Jeyakumar, D.; Marcucci, G.; et al. Tolerability and efficacy of the first-in-class anti-CD47 antibody magrolimab combined with azacitidine in MDS and AML patients: Phase Ib results. J. Clin. Oncol. 2020, 38, 7507. [Google Scholar] [CrossRef]
- Lambert, J.M.; Gorzov, P.; Veprintsev, D.B.; Söderqvist, M.; Segerbäck, D.; Bergman, J.; Fersht, A.R.; Hainaut, P.; Wiman, K.G.; Bykov, V.J. PRIMA-1 Reactivates Mutant p53 by Covalent Binding to the Core Domain. Cancer Cell 2009, 15, 376–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maslah, N.; Salomao, N.; Drevon, L.; Verger, E.; Partouche, N.; Ly, P.; Aubin, P.; Naoui, N.; Schlageter, M.-H.; Bally, C.; et al. Synergistic effects of PRIMA-1Met (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia. Haematologica 2019, 105, 1539–1551. [Google Scholar] [CrossRef] [Green Version]
- Cuzzubbo, S. Eprenetapopt Plus Azacitidine in TP53 -Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone des My elodysplasies (GFM) abstract. J. Clin. Oncol. 2021, 39, 1575–1583. [Google Scholar]
- Dinardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Montalban-Bravo, G.; Garcia-Manero, G.; Jabbour, E. Therapeutic choices after hypomethylating agent resistance for myelodysplastic syndromes. Curr. Opin. Hematol. 2018, 25, 146–153. [Google Scholar] [CrossRef]
- Clavio, M.; Crisà, E.; Miglino, M.; Guolo, F.; Ceccarelli, M.; Salvi, F.; Allione, B.; Ferrero, D.; Balleari, E.; Finelli, C.; et al. Overall survival of myelodysplastic syndrome patients after azacitidine discontinuation and applicability of the North American MDS Consortium scoring system in clinical practice (In press). Cancer 2021. [Google Scholar] [CrossRef] [PubMed]
- Björklund, A.T.; Carlsten, M.; Sohlberg, E.; Liu, L.L.; Clancy, T.; Karimi, M.; Cooley, S.; Miller, J.S.; Klimkowska, M.; Schaffer, M.; et al. Complete Remission with Reduction of High-Risk Clones following Haploidentical NK-Cell Therapy against MDS and AML. Clin. Cancer Res. 2018, 24, 1834–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harel, S.; Cherait, A.; Berthon, C.; Willekens, C.; Park, S.; Rigal, M.; Brechignac, S.; Thépot, S.; Quesnel, B.; Gardin, C.; et al. Outcome of patients with high risk Myelodysplastic Syndrome (MDS) and advanced Chronic Myelomonocytic Leukemia (CMML) treated with decitabine after azacitidine failure. Leuk. Res. 2015, 39, 501–504. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Fenaux, P.; Al-Kali, A.; Baer, M.R.; Sekeres, M.A.; Roboz, G.J.; Gaidano, G.; Scott, B.L.; Greenberg, P.; Platzbecker, U.; et al. Rigosertib versus best supportive care for patients with high-risk myelodysplastic syndromes after failure of hypomethylating drugs (ONTIME): A randomised, controlled, phase 3 trial. Lancet Oncol. 2016, 17, 496–508. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Sasaki, K.; Montalban-Bravo, G.; Daver, N.G.; Jabbour, E.J.; Alvarado, Y.; Dinardo, C.D.; Ravandi, F.; Borthakur, G.; Bose, P.; et al. A Phase II Study of Nivolumab or Ipilimumab with or without Azacitidine for Patients with Myelodysplastic Syndrome (MDS). Blood 2018, 132, 465. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Pollyea, D.A.; Garcia, J.S.; Brunner, A.; Roncolato, F.; Borate, U.; Odenike, O.; Bajel, A.R.; Watson, A.M.; Götze, K.; et al. A Phase 1b Study Evaluating the Safety and Efficacy of Venetoclax As Monotherapy or in Combination with Azacitidine for the Treatment of Relapsed/Refractory Myelodysplastic Syndrome. Blood 2019, 134, 565. [Google Scholar] [CrossRef]
| 0 | 0.5 | 1 | 1.5 | 2 | 3 | 4 | |
|---|---|---|---|---|---|---|---|
| Karyotype * | Very good | Good | Intermediate | Poor | Very poor | ||
| BM blasts (%) | 0–2 | 3–4.9 | 5–10 | >10 | |||
| Hemoglobin (g/dL) | ≥10 | 8–9.9 | <8 | ||||
| Platelets (×109/L) | ≥100 | 50–99 | <50 | ||||
| ANC (×109/L) | ≥0.8 | <0.8 |
| Autor (Reference) | Therapy | Phase | N | Outcomes | |
|---|---|---|---|---|---|
| Monotherapy | Silverman et al., 2002 [45] | AZA vs. BSC | III | 191 | ORR: AZA 60% vs. BSC 5% (p > 0.001) CR: AZA 7% vs. BSC 0% (p = 0.01) LFS: AZA 21 months vs. BSC 13 months (p = 0.007) |
| Fenaux et al., 2009 [46] | AZA vs. BSC | III | 358 | ORR: AZA 29% vs. BSC 12% (p = 0.0001) CR: AZA 17% vs. 8% (p = 0.015) OS: AZA 24.5 months vs. BSC 15 months (p = 0.0001) | |
| Kantarjian et al., 2006 [48] | DEC vs. BSC | III | 170 | ORR: DEC 17% vs. BSC 0% (p = 0.001) CR: DEC 9% vs. BSC 0% LFS: DEC 12.1 months vs. BSC 7.8 months (p = 0.16) OS: DEC 14.0 vs. BSC 14.9 (p = 0.636) | |
| Kantarjian et al., 2007 [49] | DEC (20 mg/m2 iv × 5 days vs. 20 mg/m2 sc × 5 days vs. 10 mg/m2 iv × 5 days) | II | 95 | ORR: 73% CR: 34% 18 month EFS: 51% 18 month OS: 56% | |
| Steensma et al., 2009 [50] | DEC | II | 99 | ORR: 32% CR: 17% OS: 19.4 months | |
| Combination therapies | Sekeres et al., 2021 [53] | AZA +/− pevonedistat | II | 120 | ORR: AZA + pevonedistat 79.3% vs. AZA 56.7% CR: AZA + pevonedistat 51.7% vs. AZA 26.7% DoR: AZA + pevonedistat 34.6 months vs. AZA 13.1 months EFS: AZA + pevonedistat 20.2 months vs. AZA 14.8 months (p = 0.045) OS: AZA + pevonedistat 23.9 months vs. AZA 19.1 months (p = 0.240) |
| Sallmann et al., 2020 [54] | AZA + magrolimab | I | 33 | ORR: 91% CR: 42% DoR: median not reached OS: median not reached | |
| Sallman et al., 2021 [55] | AZA + eprenetapopt | Ib/II | 40 (TP53 mut) | ORR: 73% CR: 50% DoR: 8.4 months OS: 10.4 months | |
| Garcia et al., 2020 [56] | AZA + venetoclax | Ib | 78 | ORR: 77% CR: 37% DoR: 12.9 moths OS: 27.5 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios-Berraquero, M.L.; Alfonso-Piérola, A. Current Therapy of the Patients with MDS: Walking towards Personalized Therapy. J. Clin. Med. 2021, 10, 2107. https://doi.org/10.3390/jcm10102107
Palacios-Berraquero ML, Alfonso-Piérola A. Current Therapy of the Patients with MDS: Walking towards Personalized Therapy. Journal of Clinical Medicine. 2021; 10(10):2107. https://doi.org/10.3390/jcm10102107
Chicago/Turabian StylePalacios-Berraquero, Maria Luisa, and Ana Alfonso-Piérola. 2021. "Current Therapy of the Patients with MDS: Walking towards Personalized Therapy" Journal of Clinical Medicine 10, no. 10: 2107. https://doi.org/10.3390/jcm10102107
APA StylePalacios-Berraquero, M. L., & Alfonso-Piérola, A. (2021). Current Therapy of the Patients with MDS: Walking towards Personalized Therapy. Journal of Clinical Medicine, 10(10), 2107. https://doi.org/10.3390/jcm10102107





