Abstract
Cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies target the underlying cause of cystic fibrosis (CF), and are generally well-tolerated; however, real-world studies indicate the frequency of discontinuation and adverse events (AEs) may be higher than what was observed in clinical trials. The objectives of this systematic review were to summarize real-world AEs reported for market-available CFTR modulators (i.e., ivacaftor (IVA), lumacaftor/ivacaftor (LUM/IVA), tezacaftor/ivacaftor (TEZ/IVA), and elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA)), and to identify ways in which the pharmacist on CF healthcare teams may contribute to mitigating and managing these AEs. The MEDLINE, EMBASE, CINAHL, and Web of Science Core Collection online databases were searched from 2012 to 1 Aug 2020. Full manuscripts or conference abstracts of observational studies, case series, and case reports were eligible for inclusion. The included full manuscripts and conference abstracts comprised of 54 observational studies, 5 case series, and 9 case reports. The types of AEs reported generally aligned with what have been observed in clinical trials. LUM/IVA was associated with a higher frequency of respiratory-related AE and discontinuation in real-world studies. A signal for mental health and neurocognitive AEs was identified with all 4 CFTR modulators. A systematic approach to monitoring for AEs in people with CF on CFTR modulators in the real-world setting is necessary to help better understand potential AEs, as well as patient characteristics that may be associated with higher risk of certain AEs. Pharmacists play a key role in the safe initiation and monitoring of people with CF on CFTR modulator therapies.
Keywords:
CFTR modulator; ivacaftor; lumacaftor; tezacaftor; elexacaftor; real-world; adverse events; safety 1. Introduction
Cystic fibrosis (CF) is an autosomal recessive disorder affecting the CF transmembrane conductance regulator (CFTR) gene, resulting in alteration of CFTR protein synthesis, processing, or function. The CFTR protein is a channel that transports chloride and bicarbonate across cell membranes, regulating fluid and electrolyte balance in several organs throughout the body; dysfunctional CFTR protein results in viscous mucus and secretions [1]. In the lungs, this viscous mucus causes impaired mucociliary clearance as well as chronic infection and inflammation, which, over time, leads to irreversible airway damage and progressive lung function decline [1]. The pancreas, gastrointestinal tract, and biliary ducts are among the extrapulmonary organs impacted by CF, resulting in complications such as nutrient malabsorption, bowel obstruction and hepatobiliary disease, respectively [1]. Optimal management of CF requires a complex, multimodal medication regimen that may take upwards of 2 h to administer on a daily basis [2]. Pharmacists have therefore been identified as integral members of multidisciplinary teams providing care to people with CF, with essential roles such as: optimizing the effectiveness of medication regimens and tailoring to each individuals’ needs; providing medication education and counselling; promoting adherence to therapies; as well as monitoring and managing drug-related adverse events (AE) and interactions [3,4,5,6].
Prior to the development of CFTR modulator therapies, available treatments for CF only targeted symptom management, whereas CFTR modulators target the underlying cause [1,7]. CFTR modulators are small molecule therapies, with four single or combination therapies currently available on the market: ivacaftor (IVA; Kalydeco®), lumacaftor/ivacaftor (LUM/IVA; Orkambi®), tezacaftor/ivacaftor (TEZ/IVA; Symdeko® or Symkevi®), and elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA; Trikafta® or Kaftrio®) [8,9,10,11,12,13]. IVA was the first CFTR modulator brought to market, approved by the Food and Drug Administration (FDA) in 2012; it is a CFTR “potentiator” that increases the amount of time the CFTR protein channel remains open, targeting CFTR mutations that impact channel gating and conductance (e.g., G551D, R117H) [8]. The remaining three CFTR modulator therapies combine IVA with one or two CFTR “correctors”. “Correctors” primarily target the most common CFTR mutation, F508del, improving CFTR protein conformation and subsequent processing and trafficking to the cell surface [9,10,11,12,13]. Results from randomized, placebo-controlled clinical trials support the efficacy of CFTR modulator therapies in the treatment of people with CF: IVA in patients with at least one G551D or R117H mutation, non-G551D gating mutation, or residual function mutation [14,15,16,17,18]; LUM/IVA in patients who are F508del homozygous [19,20]; TEZ/IVA in patients who are F508del homozygous or heterozygous with a residual function mutation [18,21]; and ELX/TEZ/IVA in patients who are F508del homozygous or heterozygous with a minimal function mutation [22,23].
In the aforementioned clinical trials, CFTR modulators were generally well-tolerated, with the exception of LUM/IVA having higher rates of respiratory-related AE [7,19,20]. However, observational studies with real-world CFTR modulator safety data have raised flags for higher rates of discontinuation as well as AE that were rarely observed or not described in the clinical trial setting. The objective of this systematic review was to summarize real-world AEs reported for CFTR modulator therapies, as well as to identify ways in which the pharmacist on CF healthcare teams may contribute to mitigating and managing these AEs.
2. Methods
2.1. Search Strategy
The MEDLINE, EMBASE, CINAHL, and Web of Science Core Collection online databases were systematically searched from 2012 to 1 August 2020 to identify literature related to the primary research question. The search terms and Boolean operators utilized in MEDLINE and EMBASE are outlined in Appendix A as an example. References of relevant literature were searched manually to identify additional studies not identified in the electronic search, and grey literature was explored via conference abstracts identified in the search. The systematic review protocol was prospectively registered with PROSPERO (CRD42020188189).
2.2. Selection of Studies
Observational studies, case series, and case reports were eligible for inclusion in the review if study participants had a diagnosis of CF, received at least one dose of a market-available CFTR modulator (i.e., IVA (Kalydeco®), LUM/IVA (Orkambi®), TEZ/IVA (Symdeko® or Symkevi®), or ELX/TEZ/IVA (Trikafta® or Kaftrio®) [8,9,10,11,12,13]) in the real-world setting, and reported AEs that occurred while participants were receiving the CFTR modulator. Conference abstracts that met the above criteria were also included. Studies were excluded if it was a clinical trial or study utilizing clinical trial data, was not available in the English language, if none of the AE were specified, or if it was reported that no AEs occurred in the study participants. Two authors (R.D. and V.S.) independently conducted a search and reviewed potentially relevant citations to determine whether the pre-defined criteria for inclusion were satisfied; any inconsistencies in studies selected for inclusion were resolved by discussion and consensus.
2.3. Data Extraction and Management
Data from each included study were extracted and tabulated. Data extracted from full manuscripts included: first author; year of publication; study design; study location; study population age group, CFTR genotype, and baseline percent predicted of the forced expiratory volume in the first second (ppFEV1); CFTR modulator(s) received by study participants; follow-up time of study participants; type and frequency of AEs; CFTR modulator interruption, discontinuation, and/or dose modification secondary to AEs; and strategies to address or mitigate reported AEs (if described). Data extracted from conference abstracts was the same as for full manuscripts, with the exception of study location and follow-up time, as these were infrequently specified. Data were first extracted by the primary reviewer (R.D.) and checked by the secondary reviewer (V.S.); disagreements or inconsistencies regarding extracted data were resolved by discussion and consensus.
2.4. Quality Assessment
All full manuscripts included in the review were independently assessed for quality by the primary and secondary reviewer (R.D. and V.S.) using a National Institutes of Health, National Heart, Lung, and Blood Institute Quality Assessment Tool appropriate for the study design [24]. The quality of the manuscripts was assessed from the perspective of addressing safety events (i.e., a study may have been methodologically sound for assessment of effectiveness or other outcomes unrelated to safety but may have received a lower rating for this systematic review due to seemingly not having a robust approach to assessing safety outcomes). An overall rating of ‘Good’, ‘Fair’, or ‘Poor’ was assigned to each manuscript after discussion and consensus based on the assessment tool criteria and study-specific factors that may affect the quality and internal validity. Case reports were not systematically assessed for quality due to absence of a standardized quality assessment tool, and conference abstracts were not formally assessed due to inherent lack of detail necessary to appropriately assess the quality of study methodology.
2.5. Data Analysis
Descriptive analyses were performed on data extracted from the included studies.
3. Results
3.1. Description of Studies
Of the 2333 articles identified in the search or through other sources, 278 were screened for eligibility. Of the 278 screened, 31 studies with full manuscripts and 37 conference abstracts met the inclusion criteria, and 210 articles were excluded (Figure 1). Of the 31 full manuscripts, 20 were observational studies, 5 were case series, and 6 were case reports. Of the 37 conference abstracts, 34 were observational studies and 3 were case reports. Characteristics of the full manuscripts and conference abstracts describing observational cohort studies are summarized in Table 1 and Table 2, respectively. Two observational cohort studies included control subjects as comparison for non-safety outcomes related to LUM/IVA therapy [25,26], but only data regarding the participants on LUM/IVA was extracted and included in the descriptive analysis of this review. One case series was based on survey results presented in aggregate [27]; therefore, it was included in Table 1. Characteristics of case series and case reports are otherwise summarized in Table 3.
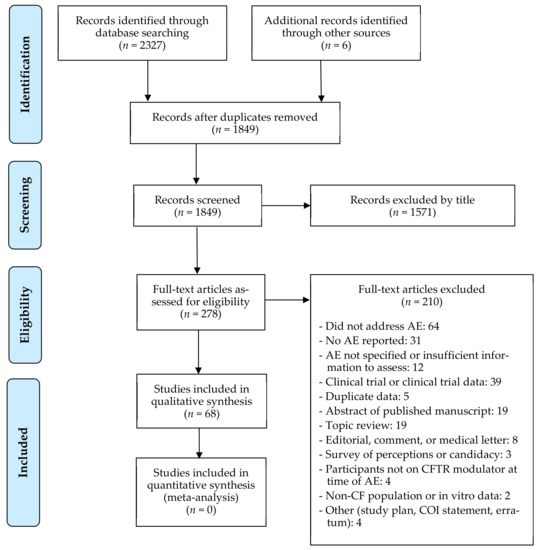
Figure 1.
PRISMA flow diagram of study selection 1. 1 From: Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097, doi:10.1371/journal.pmed1000097.

Table 1.
Summary of characteristics and results of cohort or survey studies with full manuscript.

Table 2.
Summary of characteristics and results of cohort or survey studies in abstract form.

Table 3.
Summary of characteristics and results of case series and case reports.
The majority of the included studies and case series/reports regarded LUM/IVA, totaling 47 (69%) of the 68 included. IVA, TEZ/IVA, and ELX/TEZ/IVA were the subject of 11 (16%), 7 (10%), and 4 (6%) of the included manuscripts and abstracts, respectively (one study discussed both LUM/IVA and TEZ/IVA, so was included in the totals for both modulators [28]). Eleven (16%) of the included studies and case series/reports included only pediatrics (age < 18 years old), 33 (49%) included only adults, 16 (24%) included both adult and pediatric patients, and the age groups included was not specified in 8 (12%). In the observational studies, the number of subjects included ranged from 4 to 845 (median: 28). Fourteen (26%) of the observational studies focused on patients with severe airflow obstruction, defined as ppFEV1 < 40%; in 11 of these studies, patients accessed the CFTR modulator via a compassionate access program (also referred to as a “managed access”, “early access’, “expanded access”, or “named patient” program) through the manufacturer [25,26,29,30,31,32,33,34,35,36,37,38,39,40]. Twenty (65%) of the full manuscripts were deemed to have safety as a primary focus or outcome [27,28,29,30,32,33,41,42,43,44,45,46,47,48,49,50,51,52,53,54].
3.2. Quality Assessment
The 20 observational studies and 5 case series with full manuscripts were assessed for quality from the standpoint of AE follow-up and assessment. Ten of the observational studies were rated as ‘Good’ quality, eight were rated as ‘Fair’, and two were rated as ‘Poor’ (Appendix B). Only one of the case series was rated as ‘Good’ quality, while one was rated as ‘Fair’ and three were rated as ‘Poor’ (Appendix C). The two leading factors contributing to lower quality ratings were unclear and/or inconsistent methods for follow-up and assessment of AEs, and lack of a reliable, validated tool or method to accurately assess and confirm a given AE.
3.3. AE-Related Outcomes
The AE reported for IVA, LUM/IVA, TEZ/IVA, and ELX/TEZ/IVA are summarized in Table 1, Table 2 and Table 3.
3.3.1. Ivacaftor (IVA)
In the studies evaluating IVA, a large proportion of the reported AEs were respiratory-related. In two observational studies that reported serious respiratory-related AEs such as pulmonary exacerbation or respiratory infection, hemoptysis, and acute respiratory failure, these respiratory AEs did not prompt discontinuation and were attributable to patients’ underlying disease [29,55]. Another study reported increased bronchial and nasal secretions after initiating IVA in a patient with low lung function (baseline ppFEV1 13.9%) prompting a severe pulmonary exacerbation, and IVA ultimately had to be discontinued due to difficulty clearing “liquefied” secretions despite decreases in dose [30]. Otherwise, respiratory AE reportedly resolved over time without changes to IVA therapy [30,46].
Relatively common were transaminitis and other hepatic AEs [29,30,55,56,58,64]. In one observational study, transaminitis and a diagnosis of liver cirrhosis resulted in IVA discontinuation in one individual each, and hepatitis warranted interruption of therapy [58]. One case report indicated a decrease in IVA dose was necessary for normalization of transaminases [46]. Hepatic AEs were not otherwise reported to require changes in IVA therapy.
Abdominal pain was reported in a number of studies, though the overall frequency was often not specified [29,30,58]. In some cases, abdominal pain was severe enough to warrant interruption or discontinuation of therapy [29,58]. Nausea or vomiting, intestinal dysmotility, and gastroenteritis were other gastrointestinal-related AEs reported, of which vomiting was the only one to prompt interruption of therapy [29,58].
Headache and rash were also common, but only prompted IVA interruption in one study [29,30,55,57,58,62,63,65]. Less common but notable AEs included cataracts, depression, dizziness, and tinnitus; the latter two prompted discontinuation of IVA in one patient, while depression warranted interruption of therapy [29,55,57,58,65]. IVA was also implicated in causing recurrent morbilliform drug eruption (Type IV drug hypersensitivity) to trials of LUM/IVA and TEZ/IVA, with successful IVA desensitization thereafter [28].
3.3.2. Lumacaftor/Ivacaftor (LUM/IVA)
The majority of AEs reported with LUM/IVA were respiratory-related. Chest tightness, dyspnea, increased sputum, and declines in ppFEV1 were among the most common respiratory AEs and tended to occur within the first few days after initiation, even as quickly as 3–4 h after the first dose [25,26,27,31,32,33,34,35,37,38,41,42,43,44,45,60,61,66,67,68,69,70,71,72,73,74,75,76,77,78,83,84,85,90]. Bronchodilators were beneficial in mitigating symptoms of chest tightness, wheeze, and increased work of breathing in some individuals [31,41,66,67,75]. Improvement in or resolution of respiratory AEs generally occurred over the 1–4 weeks following initiation, but symptoms and/or ppFEV1 below baseline could persist beyond this time [26,31,32,34,42,43,73]. In studies assessing ppFEV1 changes post-initial dose of LUM/IVA, a mean absolute decline in ppFEV1 around 10% within 4 h was common [41,43,67], with declines of up to 20% at 4 and 24 h reported even when bronchodilators were administered pre-dose [43]. One study reported a ppFEV1 drop 39% below baseline in an individual; the timing of repeat spirometry after LUM/IVA initiation was not specified [44]. Declines in ppFEV1 were not always accompanied by respiratory symptoms, and bronchodilators did not consistently improve acute drops in ppFEV1 [32,41,43,66,67,84]. ppFEV1 could take upwards of three months after initiation to recover to baseline values [34,43]. Increased cough was common, sometimes resulting in discontinuation [33,42,44,45,69,73,76,85], while chest pain was infrequently reported [37,45,72,85]. Two cases of hemoptysis and one case of pneumothorax reportedly caused discontinuation of LUM/IVA in one study; whether the hemoptysis and pneumothorax were attributable to underlying disease was not specified [42].
Intolerable respiratory AE could be overcome in some patients by reducing the dose of LUM/IVA [66,70]; however, discontinuation due to respiratory AEs remained common. Chest tightness and/or dyspnea were the most common respiratory AEs to prompt discontinuation, with reported frequencies between from 5 to 31%. In one case report, halving the LUM/IVA dose mitigated severe chest tightness, dyspnea, and exertional hypoxemia that onset after initiation, but therapy was ultimately discontinued with rapid recovery of symptoms and ppFEV1 thereafter [90]. A cohort study also reported resolution of respiratory AE upon LUM/IVA discontinuation [35]. In observational studies that described retrials after LUM/IVA was discontinued, there were mixed outcomes among patients. In one study, 32 of the 90 patients who discontinued LUM/IVA at least once due to respiratory AE were retrialed—16 were able to successfully restart therapy, and 16 had to discontinue indefinitely [42]. Three studies also described LUM/IVA retrial following discontinuation due AE: in the first, of 25 patients who discontinued, 9 were retrialed, and 6 had recurrence of the same AE [72]; in the second, 4 of 8 patients who discontinued were retrialed at full- or half-dose and experienced AE recurrence [76]; and in the third, 6 of 8 patients who discontinued were retrialed and able to restart therapy, but 4 required half-dose in order to do so [78]. The three studies were reported in abstracts and did not specify whether it was only patients with respiratory AE that were retrialed, but respiratory AE accounted for a large proportion of the AEs prompting discontinuation.
A number of factors and patient characteristics were explored as potential risk factors for respiratory AE and/or discontinuation due to AE with LUM/IVA. Having a lower baseline ppFEV1 was generally associated with greater risk of respiratory AE or discontinuation [26,42,73,75,77], but differences were not always statistically significant [33,35,44,61], and one study found the contrary [43]. One study, using multivariable logistic regression, found for every 10% decrease in baseline ppFEV1, there was a greater likelihood of LUM/IVA discontinuation overall and due to respiratory AE—odds ratio (OR) 1.13 (95%CI, 1.02–1.25; p = 0.02) and OR 1.32 (95%CI, 1.14–1.51; p = 0.0001), respectively [42]. Older age and greater use of IV antibiotics in the past year had conflicting results for whether they were significant risk factors for LUM/IVA discontinuation [42,44,61]. The association with reactive airway disease and/or atopy was discussed in three studies. Reversible airway obstruction ≥12%, but not personal or familial history of atopy, was significantly associated with a greater drop in ppFEV1 when evaluated in one study [41], and higher frequency of discontinuation or drop in ppFEV1 was found in patients with asthma or asthma/atopy, respectively [67,77]; whether statistical significance was reached in the latter two studies was not specified. Interestingly, higher rates of discontinuation overall were associated with female sex in one study (adjusted OR 3.12 (95%CI, 1.04–9.38; p = 0.04) [44]), but this was not observed in another evaluating this relationship [61]. Discontinuation due to respiratory AE was associated with diabetes (OR 1.71 (95%CI, 1.03–2.85; p = 0.04)) and low body mass index (BMI) (OR 1.11 (95%CI, 1.00–1.23; p = 0.03) per 1 kg/m2 decrease) [42].
Similar to IVA, hepatic AE were relatively common with LUM/IVA. Transaminitis and other liver function test (LFT) elevations were seemingly mild and/or transient without need for intervention for the most part, but sometimes warranted discontinuation [34,42,45,59,60,61,72,76,78,81]. One study specified that transaminases were over 6 times the upper limit of normal when discontinued [42]. A case of isolated alkaline phosphatase elevation was also reported, taking several months for the alkaline phosphatase to normalize after discontinuation of LUM/IVA [91].
Similarly common with LUM/IVA were gastrointestinal AEs, of which abdominal pain, nausea and/or vomiting, and diarrhea were the most frequently reported and resulted in discontinuation in two studies each [26,33,34,42,44,45,61,69,71,73,78,79,85]. Uncommon gastrointestinal AEs were dysphagia and decreased appetite; the one case of dysphagia resulted in discontinuation [44,61].
Rash and hypersensitivity reactions were notable with LUM/IVA. In several observational studies reporting rash, LUM/IVA was seemingly continued without intervention, but prompted discontinuation in a small number of patients [26,33,35,42,44,73,76,78]. More serious reactions in the observational studies were rash with facial swelling, allergic reaction with chest tightness, and suspected Stevens–Johnson syndrome (SJS), reported in one individual each [61,75]. The LUM component of LUM/IVA was implicated via in vitro testing to have triggered a T-cell-mediated reaction that caused a progressive severe, pruritic rash and facial swelling in one case report [50]. Another case report described morbilliform drug eruption recurring on three separate trials of LUM/IVA, but IVA was deemed the likely culprit in the reactions [28]. In both case reports, the hypersensitivity reactions resolved quickly following discontinuation [28,50].
An important signal was noted for the impact of LUM/IVA on mental health. One large prospective cohort study reported four individuals (0.5% of the study cohort) discontinued LUM/IVA due to depression [42], and one individual in each of two smaller retrospective cohorts reported anxiety as the cause of discontinuation (4 and 5% of the respective cohorts) [45,75]. One case series described five adolescent females, comprising 24% of the adolescent females who initiated LUM/IVA at the reporting center, with new or worsening depression as early as two weeks following LUM/IVA initiation [47]. Two of the patients had suicidal ideation (one each with baseline and new-onset depression), while three of the patients had an attempted suicide requiring hospitalization (one with baseline and two with new-onset depression) [47]. In all but one patient, mood improved after discontinuing LUM/IVA [47]. One of the patients with new-onset depression and suicidal ideation also had worsening of baseline anxiety, which, along with menstrual irregularity, was the ultimate cause of LUM/IVA discontinuation [47]. A second case series described four individuals with worsening of their baseline psychiatric diagnoses, including depression, anxiety, bipolar disorder, and/or substance use disorder, as early as one month following LUM/IVA initiation [48]. In three individuals, worsening mental health triggered a clinical decline and pulmonary exacerbation, likely attributable to medication non-adherence [48]. One patient discontinued LUM/IVA due to worsening depression and anxiety, and required adjustment of psychotropic therapy to return to baseline [48]. The other two patients remained on LUM/IVA; for one patient, the mood returned to baseline only after adjustment of psychotropic medications and the other patient did not return to baseline levels of anxiety despite appropriate treatment interventions [48]. The final patient discontinued LUM/IVA due to non-adherence [48].
Two uncommon but consequential AEs were elevations in blood pressure and creatine kinase (CK). In 1 cohort of 22 patients, 5 (23%) had elevations in blood pressure, of whom 4 discontinued [45]. Two of the patients had symptomatic hypertension: one presented with headache and required two antihypertensives to manage her blood pressure before LUM/IVA was stopped, and the second had a hypertensive emergency that onset within 12 h of his first LUM/IVA dose (notably, he was initiated at one-quarter dose due to a drug–drug interaction with posaconazole) [45]. One of the patients with asymptomatic hypertension remained above his baseline blood pressure even after LUM/IVA discontinuation [45]. In a cohort of 30 patients admitted to hospital for LUM/IVA initiation, 1 (3%) discontinued due to hypertension; the onset of the hypertension was seemingly quick, as it occurred before the patient was discharged from hospital [37]. An elevation in CK was reported in a large cohort study wherein 20 (2%) patients had a CK greater than five times the upper limit of normal, and five (0.6%) had to discontinue LUM/IVA due to a CK greater than 10 times the upper limit of normal accompanied by myalgia [42]. One case report also describes an adult male who experienced rhabdomyolysis with a CK upwards of 17,000 U/L, attributed to a drug–drug interaction between LUM/IVA and acyclovir; despite normalization of the CK following discontinuation of both LUM/IVA and acyclovir, lower limb myalgia persisted [92].
Headache and fatigue were reported relatively often overall but were not a common cause of LUM/IVA discontinuation [25,26,33,34,42,45,69,73,76]. Menstrual irregularities and menorrhagia were reported infrequently but did result in discontinuation of LUM/IVA—the former in one patient who also discontinued due to worsening anxiety [47], and the latter in three individuals (0.4%) in a large cohort [42]. Uncommon AEs of note were cataracts, which prompted discontinuation in one individual [60]; one case each of bradycardia and tachycardia, the latter of which prompted discontinuation [42,45]; and a case of acute myelocytic leukemia (AML) post-partum [27]. The authors of the case series reporting AML deemed it likely an unfortunate coincidence, unrelated to LUM/IVA [27].
3.3.3. Tezacaftor/Ivacaftor (TEZ/IVA)
Limited data were available regarding real-world AEs with TEZ/IVA. Notable were neuropsychiatric AEs. In a cohort of 44 adults, 5 (11%) experienced neuropsychiatric AEs: out of body experience and visual hallucination; depersonalization and “brain fog”; severe migraine; and sleep pattern disturbance, which occurred in 2 individuals [88]. The two individuals with sleep pattern disturbance remained on TEZ/IVA, but the remaining three individuals changed back to LUM/IVA and had resolution of symptoms thereafter [88]. Another cohort study reported discontinuation due to unspecified mental health changes; the frequency was not reported [86].
A case study described morbilliform drug eruption that recurred on retrial of TEZ/IVA and resolved upon discontinuation; however, IVA was deemed the likely cause of the reaction, and after a stepwise desensitization to IVA the patient was able to resume TEZ/IVA [28]. One other cohort reported rash in two (9%) individuals that did not require discontinuation [39]. Liver enzyme abnormalities or elevations resulted in TEZ/IVA discontinuation in two cohort studies [86,87], while hair loss and fatigue, and blurred vision were the cause of TEZ/IVA discontinuation in one individual each [39,89]. Acholic stools, persistent nausea and vomiting, changes in blood glucose, and new-onset hemoptysis were also reported to require discontinuation of TEZ/IVA, but the frequency was not specified [86].
One case report describes a delayed suspected drug–drug interaction between TEZ/IVA and azithromycin [51]. In an adult female with no history of cardiac arrhythmia and who had been on long-term therapy with azithromycin prior to TEZ/IVA initiation, an electrocardiogram (ECG) at 36 weeks of concomitant therapy revealed first-degree heart block (PR interval of 334 ms); ECGs at baseline and 4, 12, and 24 weeks after initiating TEZ/IVA were normal [51]. The first-degree heart block resolved by four weeks after discontinuation of TEZ/IVA, and therapy was retrialed eight months later. Baseline and follow-up ECGs at 4, 8, 12, and 24 weeks were normal, but an ECG at 39 weeks revealed recurrence of the first-degree heart block (PR interval of 215 ms) [51]. Azithromycin was discontinued and the PR interval normalized by four weeks thereafter [51].
3.3.4. Elexacaftor/Tezacaftor/Ivacaftor (ELX/TEZ/IVA)
Given the relatively recent market approval in some countries, limited data were available regarding real-world AEs with ELX/TEZ/IVA. One case series reported on seven adults who experienced biliary colic following initiation of ELX/TEZ/IVA; all but one patient received a cholecystectomy [53]. Only one of the seven patients was CFTR modulator naïve prior to ELX/TEZ/IVA, and five of the patients had chronic cholecystitis and cholelithiasis [53]. In those receiving a cholecystectomy, ELX/TEZ/IVA remained unchanged in four patients and was briefly held perioperatively in two patients due to severe symptoms or postoperative complications; symptoms resolved in all six [53]. ELX/TEZ/IVA was held two weeks before and four weeks after the cholecystectomy in the fourth patient, and based on personal communication with the corresponding author, although symptoms of biliary colic resolved, ELX/TEZ/IVA was subsequently held due to transaminitis [53].
A second case series described testicular pain that onset shortly after ELX/TEZ/IVA initiation in seven males [52]. For five patients, ELX/TEZ/IVA continued uninterrupted—three had no interventions and two required over-the-counter analgesics to manage pain [52]. One patient required a brief interruption in therapy and restarted at full-dose, while the final patient required a dose reduction and titration back to full-dose in addition to over-the-counter analgesics and antibiotics for epididymoorchitis and scrotal wall cellulitis [52]. All patients had resolution of the testicular symptoms, and at least three patients also experienced abdominal bloating or constipation [52]. Transaminitis was reported in four (36%) patients in a cohort study, but intervention was not required [40].
ELX/TEZ/IVA was also associated with mental health-related AE, as described in a case report of a 19-year-old female [54]. In the described case, the patient experienced worsening of baseline depression and sleep paralysis with hypnopompic hallucinations, and new passive suicidal ideation following initiation of ELX/TEZ/IVA [54]. The latter resolved upon discontinuation, but initiation of an antidepressant was necessary to improve mood [54]. Over the course of several months, the ELX/TEZ/IVA dose was titrated, and dosage times changed in response to worsening mood, suicidality, anxiety, and sleep paralysis. Although interpersonal conflicts may have affected symptoms, the reported symptoms were seemingly worst at the full-dose of ELX/TEZ/IVA and improved with dose reductions [54]. The dose eventually settled on two ELX/TEZ/IVA tabs once daily in the morning, with depression and anxiety symptoms above baseline and recurrent episodes of sleep paralysis with hypnopompic hallucinations [54].
3.3.5. Described Strategies to Address or Mitigate Reported Adverse Events
Protocols and strategies utilized in studies and/or suggestions that arose as a result of observed study outcomes are summarized in Table 4. To minimize respiratory-related AE and risk of discontinuation with LUM/IVA, two studies implemented an initiation protocol [31,43], while another study informally made efforts to optimize inhaled therapies in patients with ppFEV1 ≤ 40% [44]. A fourth study established a desensitization protocol for IVA and transition to TEZ/IVA following recurrent morbilliform drug eruption [28]. The majority of suggestions that resulted from study observations pertained to LUM/IVA, primarily in relation to respiratory AE [25,26,32,33,41,49,61,75], as well as new or worsening depression and anxiety [47,48], hypertension [45], and the potential drug–drug interaction with PPIs [79]. Three studies provided suggestions for ELX/TEZ/IVA based on direct observations—one each for testicular pain, biliary colic, and worsening mental health—and a fourth based indirectly on observed delayed-onset first-degree heart block with concomitant TEZ/IVA and azithromycin [51,52,53,54]. In addition to the latter study, suggestions for TEZ/IVA were provided based on observed neurocognitive AEs [88]. Suggestions for IVA regarded initiation in patients with severe lung disease and monitoring of pediatric patients for cataract formation [30,57].

Table 4.
Study protocols, strategies, or suggested management and monitoring for reported adverse events.
4. Discussion
4.1. Putting Real-World Adverse Events into Context
The primary objective of this systematic review was to evaluate the real-world AE profile of currently-marketed CFTR modulator therapies in the treatment of people with CF, with a secondary objective of evaluating reported strategies to address or mitigate reported AEs.
When interpreting the real-world AE findings in the context of clinical trial data, it is prudent to keep in mind that study populations in clinical trials are fundamentally different from real-world populations due to reasons such as strict inclusion/exclusion criteria and participants being inherently more motivated to continue with the assigned therapy [93]. In clinical trials evaluating CFTR modulators, study participants were clinically stable and those with severe or minimal lung disease (i.e., ppFEV1 < 40% and > 90%, respectively) were typically excluded or grossly underrepresented [94,95]. Another important consideration is the differences between observational studies and clinical trials in AE evaluation and reporting. Clinical trials have more rigorous monitoring for AEs, increasing the likelihood of identifying AEs, and AEs are reported even if not attributed to the intervention; this is demonstrated in 3 clinical trials evaluating CFTR modulators, wherein an absolute difference in the frequency of AE overall and AE deemed related to trial drug ranged from 12 to 53% within the treatment arms [18,22,96]. That said, patients and study investigators may also be less inclined to report AEs or attribute an AE to study drug to avoid the patient being removed from the trial, especially if the patient has experienced a dramatic clinical benefit. Any relative comparisons made hereinafter are general and should be interpreted with the aforementioned considerations in mind.
For IVA and LUM/IVA, the majority of reported AE were reflective of what may be expected based on the findings in clinical trials; the same comparison could not be made for TEZ/IVA and ELX/TEZ/IVA due to the limited published real-world data available. Of the four CFTR modulators, LUM/IVA was seemingly associated with a higher frequency of AE, respiratory-related AE in particular. While this observation may be due to real-world studies predominantly reporting on experience with LUM/IVA, it reflects what has also been observed in the clinical trial setting [7,94,95]. Dyspnea and chest tightness with LUM/IVA appear to have occurred more often in real-world studies, with higher frequencies overall and of discontinuation than reported in clinical trials. This is likely due to the larger proportion of patients with severe lung disease in real-world studies. In fact, when Burgel et al. re-examined the data from their prospective cohort study [42], they found that in patients who discontinued LUM/IVA, respiratory AE were the cause in 74% of patients with a baseline ppFEV1 < 40%, compared to 42% and 29% in patients with a baseline ppFEV1 of 40–90% and ≥ 90%, respectively [97]. However, even patients with a baseline ppFEV1 ≥ 40% had overall LUM/IVA discontinuation rates upwards of threefold higher than in the clinical trial setting [42]. Moreover, in the TRAFFIC/TRANSPORT trials, while the frequency of dyspnea was higher in the pooled LUM/IVA group compared to placebo overall, the frequency in study participants with a baseline ppFEV1 < 40% was approximately twice that of those with a baseline ppFEV1 ≥ 40% [98]. It should be noted that although there is greater reporting of respiratory-related AEs in patients with more advanced lung disease on LUM/IVA, asymptomatic acute drops in ppFEV1 were also common in pediatric patients with milder lung disease [41,43].
The mechanism behind the respiratory-related AE reported for LUM/IVA has not been fully elucidated but is unlikely explained by CFTR modulation alone as it has not been described with other more effective CFTR modulators. Furthermore, this effect is likely specific to LUM as it was not described with IVA monotherapy. Bronchoconstriction is a likely contributor, with the mitigation of respiratory AE observed with bronchodilators in real-world studies; this was also demonstrated in an open-label study in healthy subjects, wherein administration of a short-acting bronchodilator reversed the drop in ppFEV1 observed at 4 h post-LUM/IVA dose, and long-acting bronchodilator administered 12 h pre-dose diminished the drop in ppFEV1 [99]. The incidence of respiratory-related AE with LUM/IVA is seemingly highest around initiation of therapy, justifying suggestions to initiate at lower doses, especially in those with lower lung function. Despite efforts to optimize inhaled therapy and initiate LUM/IVA at lower doses for at least a week in patients with ppFEV1 ≤ 40%, Jennings et al. [44] still observed higher frequencies of respiratory-related AE in this subgroup; however, it is possible the frequency would have been higher without these precautions, which was the case in an open-label study of LUM/IVA by Taylor-Cousar et al. [100]. In this study involving patients with advanced CF lung disease, treating physicians were permitted to initiate patients at half-dose for up to 15 days and titrate thereafter to full-dose; those initiated at half-dose had a lower incidence and duration of respiratory-related AE, and unlike those initiated at full-dose, respiratory-related AE did not necessitate discontinuation or dose modification of LUM/IVA in those initiated at half-dose [100]. Moreover, Murer et al. [31] attributed the relatively low frequency of discontinuation due to respiratory-related AE in their single-center study of patients with severe CF-related lung disease to their step-wise LUM/IVA initiation protocol. Burgel et al. [42] reported higher discontinuation rates in those who initiated LUM/IVA at a reduced dose instead of full dose (25% vs. 17.3%, respectively), but this difference was not statistically significant and the majority of patients initiating at lower doses were due to potential drug interactions, not necessarily precautions due to low lung function. Further, in the case that lower initiation doses were utilized in patients deemed higher risk for respiratory-related AE, the higher discontinuation rate could be a reflection of confounding by indication.
When considering the factors found to be associated with increased risk for AE or discontinuation of LUM/IVA, increased risk of respiratory AE and discontinuation due to AE in patients with lower lung function is expected from what has been observed in the literature. Accordingly, so is more frequent need for IV antibiotics, which may be a reflection of more advanced lung disease, as well as CF-related diabetes and low BMI, as both are correlated and associated with worse pulmonary function [101,102]. Older age as a risk factor may also be a reflection of lower lung function by nature of CF being a progressive disease over time, but the basis of female sex as a risk factor is unclear, and the authors too could not rationalize why this was observed [44]. With bronchoconstriction as a likely contributing mechanism in LUM/IVA respiratory-related AE, reversible airway obstruction ≥12% as a risk factor is also justifiable, and although the studies evaluating asthma with or without atopy did not report on statistical significance, the sample sizes were likely too small to detect one [67,77].
Rash was not uncommon, reported in real-world studies for each of IVA, LUM/IVA, and TEZ/IVA, with few individuals requiring interruption or discontinuation of therapy for rash or allergic reactions. Similar was seen in clinical trials, with cases of rash being reported for all four CFTR modulators, and serious rash or discontinuation due to rash being reported for ELX/TEZ/IVA [22,23,103] and LUM/IVA [19,104]. Anecdotally at our site, one case of suspected anaphylaxis occurred following the first dose of TEZ/IVA; no CFTR modulators have ever been retrialed in this patient. Given no additional information regarding the suspected case of SJS was provided [61], it is not possible to evaluate whether it was potentially attributable to LUM/IVA; however, with the report of the severe delayed CD4+ lymphocyte-mediated reaction to LUM/IVA [50], the possibility for SJS and other severe Type-IV (i.e., delayed) hypersensitivity reactions to CFTR modulators is not inconceivable. Unlike the described morbilliform drug reaction with successful desensitization to IVA [28], it would be inappropriate to attempt desensitization to these more serious delayed hypersensitivity reactions [105]. In a phase 3 clinical trial of ELX/TEZ/IVA, the incidence of rash was highest in females, particularly those on hormonal contraceptives [23]; the basis of the increased incidence of rash in either of these subgroups is unclear.
An important signal of AE identified in the real-world studies that were uncommon or not reported in the clinical trials was related to mental health and neurocognitive or neuropsychiatric events. Though evidence-based interventions were also implemented in several cases, discontinuation or dose adjustment of the CFTR modulator seemingly resulted in symptom improvement for some individuals [47,48,54]. In PERSIST, the open-label extension study of the STRIVE and ENVISION trials, one study participant discontinued IVA due to depression and one suicide occurred; the latter was deemed unlikely related to IVA [106]. Serious AE reported in one individual each were affective disorder, depression, major depression, suicidal ideation, and suicidal depression, but whether or not these were attributable to IVA was not stated [106]. Though not published in the original manuscripts, McKinzie et al. [47] reported that a number of cases of depression, depressed mood, or anxiety were reported in the TRAFFIC/TRANSPORT trials and PROGRESS extension study for LUM/IVA; for two participants in the TRAFFIC/TRANSPORT trials the symptoms were reportedly considered related to study drug, but this was not specified for the PROGRESS study. Though causation cannot be attributed to CFTR modulators it is prudent to consider the potential for these medications to affect mental health outcomes, as depression has been associated with accelerated lung function decline in adolescents and adults with CF [107] as well as increased five-year mortality in adults with CF, particularly in those with severe depression [108]. Indeed, in three of the reported cases there was significant lung function decline following worsening mental health with LUM/IVA, potentially due to the negative impact of mood on adherence to maintenance therapies [48]. The mechanism by which CFTR modulators may impact mental health is not well-understood; induction of cytochrome P450 (CYP) enzymes by LUM and resultant decreased exposure to substrate psychotropic medications is one potential contributor [9,109], but this does not account for the other CFTR modulators which do not share this drug–drug interaction or individuals who do not have a known history of mental health concerns and/or who are not on a psychotropic medication.
A potential mechanism by which ELX/TEV/IVA (or other CFTR modulators) may trigger biliary colic was hypothesized by Safirstein et al. [53]; restoring CFTR function in the biliary epithelium will cause changes in the fluidity and acidity of bile fluids, which may result in mobilization of existing gallstones and precipitation of biliary colic. This hypothesis has merit, given the underlying pathophysiology of CF-related hepatobiliary disease [110]. That restoration of CFTR function may also dislodge mucus blockages from the testes and/or vas deferens was posed by Rotolo et al. [52] as a potential the mechanism behind testicular pain in males following initiation of ELX/TEZ/IVA. One serious AE that was not mentioned in the included real-world studies but warrants discussion is distal intestinal obstruction syndrome (DIOS). DIOS has been reported in clinical trials for all four CFTR modulators, albeit at a low frequency [15,18,19,96,103,106,111,112,113,114]. Although DIOS may be secondary to the underlying CF itself, there is a plausible mechanism for how DIOS may be of concern with the CFTR modulators, particularly at the time of initiation. Chronic constipation is not uncommon in CF, with viscous intestinal mucus and gastrointestinal dysmotility resulting in undigested food adhering to the intestinal walls [115]. Theoretically then, with initiation of a highly effective CFTR modulator and resultant hydration of viscous intestinal mucus, fecal matter may detach from the luminal wall and begin to move along the bowels simultaneously, increasing the potential for DIOS. Abdominal pain is a symptom of DIOS and can be for constipation as well [115]; therefore, abdominal pain reported in real-world studies, particularly cases that were severe and/or warranted interruption or discontinuation of therapy, may have been in relation to sudden increased fecal transit. More information, such as timing of onset, location and quality of abdominal pain, findings on physical assessment and imaging, would be necessary to evaluate this possibility.
Although CFTR is expressed in cervical epithelium and plays a role in hydration of cervical mucus throughout the menstrual cycle [116], this does not explain the menstrual abnormalities reported with LUM/IVA in real-world studies. In the phase 3 clinical trial setting, menstrual abnormalities reportedly occurred more frequently in female participants treated with LUM/IVA compared to placebo, with an even higher frequency in the subgroup of participants taking hormonal contraceptives [9]. This observation is very likely due to the induction of CYP3A and UDP-glucuronosyltransferase (UGT) enzymes by LUM, increasing the metabolism of and decreasing exposure to particular hormones in some hormonal contraceptive formulations [9]; this induction is not observed with other CFTR modulators, nor is the increased frequency of menstrual abnormalities. It was not specified whether the females in the real-world studies who experienced menstrual abnormalities were taking hormonal contraceptives, but this drug–drug interaction is a possible explanation.
Elevations in blood pressure were reported in the phase 3 clinical trials for LUM/IVA. At the end of the 96-week open-label extension of the TRAFFIC/TRANSPORT trials, study participants who continued on LUM/IVA 400 mg/250 mg every 12 h after or who rolled over to this regimen from placebo had mean absolute increases from baseline systolic blood pressure (SBP) of 5.9 mmHg and 5.1 mmHg, respectively, and mean absolute increases from baseline diastolic blood pressure (DBP) of 4.4 mmHg and 4.1 mmHg, respectively [113]. Moreover, one serious AE related to hypertension was reported in each of the TRAFFIC/TRANSPORT and PROGRESS trials [113]. A recent case report describes a six-year-old boy with CF whose intermittently-elevated blood pressure became persistently elevated (> 99th percentile for his age) after just two doses of LUM/IVA [117]. Upon further investigation, posaconazole, initiated about two weeks prior to the first documented incidence of hypertension, was deemed the likely culprit through inhibition of 11β-hydroxylase and resultant accumulation of mineralocorticoid precursors [117]. Interestingly, the individual in the real-world study who experienced a hypertensive emergency 12 h after his first LUM/IVA dose had been on posaconazole for five months prior, and, subjectively, his baseline blood pressure before LUM/IVA was relatively high (157/85 mmHg) [45]. Therefore, posaconazole and LUM independently may contribute to elevations in blood pressure, with more rapid elevations when used in combination. Elevations in blood pressure were also observed in the phase 3 study of ELX/TEZ/IVA, with mean absolute changes from baseline SBP of 3.1 mmHg and −0.1 mmHg in the ELX/TEZ/IVA and placebo groups at 24 weeks, respectively, and mean absolute changes from baseline DBP of 1.9 mmHg and 0.3 mmHg, respectively [23]. It has been hypothesized that people with CF have lower blood pressure when compared to age- and sex-matched controls due to salt wasting [118]; with increased CFTR function and diminished salt wasting, it may then be expected for elevations in blood pressure to occur with these agents. That said, the elevations in blood pressure are not seemingly a class effect and a greater increase in blood pressure was not observed with highly-effective CFTR modulators, as would have been expected. Single cases each of tachycardia and bradycardia were reported, but only the latter is reflective of other available information. Statistically significant decreases in heart rate were reportedly observed with LUM/IVA over placebo in the clinical trial setting, with a greater proportion of patients in the LUM/IVA group having heart rates < 50 beats per minute (bpm) [9]. Whether these changes were clinically significant cannot be determined from the information available, but the single case of bradycardia reported was asymptomatic [45]. In one study, people with CF were found to have a higher baseline heart rate when compared to healthy controls [119]; therefore, correction of CFTR function may theoretically reduce heart rate. However, again, this heart rate-lowering effect is seemingly unique to LUM/IVA.
CK elevations have been reported in clinical trials for all four CFTR modulators, with some cases being serious enough to warrant interruption or discontinuation [18,19,21,103,114]. The clinical significance of the CK elevations observed in both the clinical trials and real-world studies is unclear. In a large population-based study, 5.3% of individuals were found to have CK levels above the upper limit of normal established by age and sex; of those who obtained a “control” CK after three days of refraining from specific activities that may elevate CK (e.g., alcohol use, physical activity, and muscle training), 70% had normalization of CK values [120]. In four cases where the initial CK measures ranged from 5660 U/L to 15,941 U/L, the individuals had participated in significant physical activity and all had normalization of CK in their subsequent “control” test [120]. One clinical trial specified the CK elevations observed in both the placebo and ELX/TEZ/IVA groups were associated with exercise [23]; the frequency of CK elevations were 10% and 5% in the ELX/TEZ/IVA and placebo groups, respectively, perhaps reflecting that those receiving active therapy had greater capacity to be physically active. CFTR protein is expressed in peripheral muscular tissue and has been studied as a potential contributor to CF-related muscle abnormalities such as atrophy and weakness [121]; whether this too may explain some of the observed CK elevations with CFTR modulators is unclear.
An important consideration to keep in mind is the potential for erroneous attribution of AEs to medication; assignment of causality is subjective, and it is not uncommon for clinicians to inadvertently ascribe an AE to a medication in error, as is readily observed in placebo-controlled trials [122]. Moreover, while rare and unexpected AE may occur, a number of the AE reported in one or few individuals receiving CFTR modulators in the real-world studies did not have a clear pharmacokinetic or pharmacodynamic basis. Examples include dysphagia, pericarditis, worsening restless leg syndrome, swollen ear, tinnitus, secondary adrenal cortical insufficiency, and AML. Similar can be said for the single case of delayed onset first-degree heart block observed with concomitant TEZ/IVA and azithromycin reported [51]. Phase 3 clinical trials were significantly shorter than the upwards of eight months that it took for this drug interaction to be observed; however, available preliminary data for the open-label phase 3 rollover study for TEZ/IVA indicate this drug–drug interaction was not detected within the 100 weeks of total follow-up time [114]. Additionally important is the potential for reported AE to be secondary to CF itself, such as pulmonary exacerbation, hemoptysis, pneumothorax, and respiratory failure, especially in patients with lower levels of lung function.
4.2. Role of the CF Pharmacist in the Safe Prescribing of CFTR Modulators
As described, there are a number of potential AEs to be aware of with CFTR modulators. Although the safe initiation and monitoring of patients on CFTR modulators is a shared responsibility within a multidisciplinary CF team, pharmacists play an integral role. The following are highlights of pharmacists’ role in this, with additional suggestions and insights from the findings of this review.
4.2.1. Selection of a CFTR Modulator
Depending on an individual’s CFTR genotype, there may be more than one CFTR modulator that he or she is eligible for. Efficacy outcomes aside, pharmacists can provide guidance into how the AE and drug–drug interaction profile of each CFTR modulator differs and may impact selection based on patient-specific factors. For example, LUM/IVA is not a favorable alternative in general for individuals who are homozygous for the F508del mutation with severe lung disease (i.e., ppFEV1 < 40%) and/or reactive airway disease due to the high frequency of respiratory-related AE and associated discontinuation. Moreover, due to the induction of CYP and UGT enzymes, LUM is prone to a myriad of drug–drug interactions [9,109]; pharmacists can help navigate which drug–drug interactions are clinically significant and warrant avoidance in favor of an alternative CFTR modulator when possible. Selection may be limited, however, when CFTR modulators are not accessible (e.g., due to lack of regulatory approval, or exclusion from formularies of eligible public or private healthcare benefits).
4.2.2. Patient Counseling and Education
Before patients are initiated on CFTR modulators, pharmacists can provide the necessary counseling and education. Patients who are informed about potential AEs in advance may be more willing to trial a new medication [123,124], especially if strategies to address potential AEs are provided [123]. Anecdotally, this has been true at our site: some patients have expressed hesitancy and concern about potential AEs secondary to CFTR modulators, but once monitoring and strategies to address potential AE were discussed, they were more open to a trial. Similarly, some patients at our site were reluctant to obtain baseline and follow-up assessments, such as blood work or imaging, but were amenable once educated about the rationale (i.e., to ensure appropriate monitoring of both the safety and effectiveness of the medication).
It goes without saying that patients should be advised to inform their CF healthcare providers if new or changing symptoms arise, even if presenting as an expected AE, as it can be difficult to distinguish between something minor or self-limiting and something requiring timely attention. An example of this is increased respiratory secretions in the short-term following CFTR modulator initiation, often referred to as “the purge”. Though expected, increased respiratory secretions may also be manifest of a pulmonary exacerbation; the differentiation may be even more difficult with LUM/IVA due to additional respiratory AEs such as dyspnea, chest tightness, and declines in lung function.
Further salient is education regarding the potential for withdrawal syndrome upon discontinuation of CFTR modulators. There are several reports of patients experiencing clinical decline and/or pulmonary exacerbations upon discontinuation of a CFTR modulator, even requiring IV antibiotics in the community or hospital setting in some cases [27,125,126,127,128,129,130,131]. Therefore, patients should be informed to notify the CF healthcare providers if considering discontinuation of a CFTR modulator so that a plan to do so safely and with close monitoring can be established, and also to ensure refills are requested in advance to avoid lapses in therapy.
4.2.3. Initiation and Monitoring Plan
Establishing patients’ baseline for parameters such as spirometry, blood work, vital signs, weight, and BMI, as well as clinical status for CF-related comorbidities is essential in monitoring for a number of the described AE following initiation of CFTR modulators. Pharmacists can also contribute to safe initiation and follow-up of CFTR modulators beyond these routine parameters.
Respiratory AE: The respiratory AE with LUM/IVA are well-established, but in cases where better-tolerated alternatives are not accessible, pharmacists can help establish an initiation plan to mitigate these AE. Insight and recommendations gleaned from the real-world studies are summarized in Table 4; although the majority of suggestions pertain to patients with severe lung disease, they may still benefit patients with higher lung function. Prior to LUM/IVA initiation, pharmacists can optimize patients’ pre-existing inhaled corticosteroid and bronchodilator therapies as well as establish a titration schedule. The titration schedule utilized by Murer et al. [31] was the most conservative, starting with just one tablet (one-quarter dose) and increasing to the target dose of two tablets twice daily over several days to weeks, depending on whether patients experienced AEs. Given that CFTR modulator therapy is intended to be long-term and LUM/IVA-related respiratory AE were predominantly reported in the initial weeks of therapy, a “start low, go slow” approach is reasonable. Further advisable is having patients in a closely-monitored clinic or inpatient setting at least for their first dose, with bronchodilators readily available. At our site, pre-treatment with a course of oral or IV antibiotics has also been a strategy to optimize lung health and minimize the potential for confounding in the assessment of a patient who does experience significant respiratory AE upon initiation of LUM/IVA. Walayat et al. [49] suggest patients may benefit from discontinuing hypertonic saline and continuing dornase alfa while on LUM/IVA to avoid patients “drowning” in liquefied secretions; although, a justifiable suggestion based on their case report, this may not be the experience of all individuals started on LUM/IVA or alternative CFTR modulators, and the risks versus benefits of discontinuing hypertonic saline (or dornase alfa) must be weighed against demonstrated benefit in pulmonary outcomes [132,133,134]. Whether it is safe to stop hypertonic saline or dornase alfa in patients on ELX/TEZ/IVA is currently under investigation [135], but unlike LUM/IVA, ELX/TEZ/IVA is considered a highly effective CFTR modulator and the study results would not be generalizable. Given that respiratory AEs have not been a notable concern with IVA, TEZ/IVA, and ELX/TEZ/IVA, there may be more comfort with initiating therapy at full-dose and in the community setting. That said, based on experience at our site and as described by Hebestreit et al. [30], when initiating patients with severe lung disease on highly effective CFTR modulators (i.e., IVA or ELX/TEZ/IVA) it is reasonable to consider an antibiotic “tune-up” and/or admission for close monitoring during the initial “purge”. We also recommend initiating ELX/TEZ/IVA at one orange tablet in the morning and one blue tablet in the evening for the first week in patients with severe lung disease to minimize the intensity of “the purge”, even if transitioning from LUM/IVA or TEZ/IVA.
DIOS Risk: Another potential reason our site would recommend a reduced dose of ELX/TEZ/IVA for the first week is the aforementioned concern for potential DIOS and other gastrointestinal-related AE, with the goal again being to reduce the intensity of effects at initiation. For the same reason, our site has included a bowel preparation (or abdominal X-ray to rule-out fecal loading) in the initiation protocol for ELX/TEZ/IVA; though not guided by published literature, it was felt the benefit of this intervention outweighed the risk. The pharmacist now educates patients regarding these gastrointestinal precautions and ensures completion of the preparation.
Drug–Drug Interactions with LUM: As previously mentioned, LUM is prone to drug–drug interactions due to induction of CYP and UGT enzymes; examples of medications impacted by this include azole antifungals, select psychotropic and antiepileptic medications (e.g., citalopram, sertraline, carbamazepine, and phenytoin), hormonal contraceptives, select immunosuppressants (e.g., tacrolimus and cyclosporine), and proton pump inhibitors [9,109]. How and whether to address a given interaction is not always straightforward. For individuals established on interacting medications prior to LUM/IVA initiation, depending on the medication and indication, close monitoring and adjusting the dose if necessary to maintain clinical stability may be a reasonable approach in some cases, whereas therapeutic drug monitoring (TDM) and close monitoring of drug levels before and after LUM/IVA initiation may be imperative in others. Although the pharmacist on the CF healthcare team may not be involved in the management of the medication(s) potentially impacted by LUM/IVA, he or she can liaise with the healthcare provider(s) responsible so that a plan may be implemented proactively.
Rash and Hypersensitivity: If a patient experiences a rash or other hypersensitivity reaction secondary to a CFTR modulator, pharmacists can work with the healthcare team to appropriately manage based on the severity and confirmed or suspected diagnosis. Anecdotally there are a myriad of approaches to managing patients who experience rash or hypersensitivity reactions, such as holding therapy until resolved then rechallenging (if appropriate), use of one or more medications (e.g., first- or second-generation antihistamines, topical corticosteroids, and systemic corticosteroids) until resolution, or, as described by Patterson et al. [28], developing and implementing a desensitization protocol. In females taking both ELX/TEZ/IVA and hormonal contraceptives, there are recommendations to consider interruption of both therapies should rash occur, and to consider stepwise resumption as tolerated following rash resolution [12]. Though these recommendations are founded on a clinical trial observation [23], the basis of the association is not clear or confirmed. Pharmacists may help evaluate the risks versus benefits of interrupting or discontinuing hormonal contraceptives in females who experience rash while on ELX/TEZ/IVA, and to identify suitable contraceptive alternatives, as necessary.
4.3. Limitations
The results of this review must be interpreted in the context of its limitations. Foremost, none of the studies had a comparator; however, even if there were a placebo or active comparator, it is possible, and not uncommon, for AEs to be misattributed to medications inadvertently [122]. It is possible that reported AEs were secondary to concomitant medications, comorbid conditions, the underlying CF itself, or other unrelated causes. Furthermore, validated methods to evaluate AEs were not consistently utilized. For example, in the case reports and case series describing changes in mental health and neuropsychiatric AEs following CFTR modulator initiation, in only one of the described cases was use of a validated tool (the GAD-7) reported [47,48,54]. This has the potential to overestimate AEs by misclassifying subjectively-reported symptoms, or underestimate AEs, as patients who do not self-report symptoms and are otherwise not evaluated regarding specific symptoms may go undetected. Similarly, safety was not a primary outcome for over one third of the studies available as full manuscripts; without systematic evaluation, it is possible AEs went undetected.
In addition to differences in the methodology for AE assessment, there was significant heterogeneity in both the size and characteristics of the patient populations across the included studies. As observed in this review and in the clinical trial setting, certain patient characteristics may be associated with a higher risk of particular AEs, and with smaller sample sizes, each individual AE results in a larger incremental change in the reported overall AE frequency. Therefore, the reported frequencies are not generalizable and must be interpreted in the context of the individual studies.
Although a robust search strategy was utilized in this systematic review, it is possible that relevant literature was missed. The majority of included studies were in the form of conference abstracts, which inherently do not include sufficient detail to assess the study quality or methodology for evaluating AEs. However, it was felt that exclusion of conference abstracts would risk missing potential signals for AEs that were not otherwise reported in the literature. Finally, a number of studies did not specify or clearly describe all of the AEs reported and/or did not specify the frequency of each AE within the study population, limiting the ability to more accurately characterize the CFTR modulator AE profiles.
5. Conclusions
As a growing number of people with CF gain access to CFTR modulators, it is prudent for healthcare providers to be aware of potential AEs as well as approaches for prevention and/or management to optimize patient safety. While the studies included in this review add value to the growing collective knowledge of potential AEs associated with CFTR modulators, this review also highlights the need for a systematic, comprehensive approach to monitoring for AEs in patients on these medications. It is also imperative that CF centers continue to share and publish their real-world experiences with patients on CFTR modulators to help better understand the potential short- and long-term AEs with these medications as well as patient characteristics that may be associated with higher risk of certain AEs. With this knowledge and their unique expertise within the CF healthcare team, pharmacists can play a key role in the safe initiation of people with CF on CFTR modulator therapy, as well as monitoring for and managing AEs that may arise thereafter.
Author Contributions
Conceptualization, R.V.E.D., V.C.S., and B.S.Q.; methodology, R.V.E.D., V.C.S., and B.S.Q.; writing—original draft preparation, R.V.E.D.; writing—review and editing, R.V.E.D., V.C.S., and B.S.Q.; supervision, B.S.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest
Appendix A. Search Terms and Boolean Operators Used in Medline and Embase Electronic Databases
MEDLINE:
- exp Cystic Fibrosis Transmembrane Conductance Regulator/or CFTR modulator*.mp.
- ivacaftor.mp.
- lumacaftor.mp.
- tezacaftor.mp.
- elexacaftor.mp.
- kalydeco.mp.
- orkambi.mp.
- symdeko.mp.
- trikafta.mp.
- VX-770.mp.
- VX-445.mp.
- VX-809.mp.
- VX-661.mp.
- adverse event*.mp.
- side effect*.mp. or exp Treatment Outcome/
- phase IV.mp.
- phase 4.mp.
- adverse reaction*.mp.
- toxicity.mp.
- toxicities.mp.
- exp Product Surveillance, Postmarketing/or pharmacovigilance.mp. or exp Pharmacovigilance/or exp “Drug-Related Side Effects and Adverse Reactions”/or exp Adverse Drug Reaction Reporting Systems/
- post-marketing.mp.
- tolerability.mp.
- exp Drug Tolerance/or tolerance.mp.
- harm*.mp
- complication*.mp.
- drug safety.mp.
- drug hypersensitivity.mp. or exp Drug Hypersensitivity/
- 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
- 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13
- 29 and 30
EMBASE:
- exp cystic fibrosis transmembrane conductance regulator/or CFTR modulator *.mp.
- exp ivacaftor plus tezacaftor/or exp ivacaftor/or exp ivacaftor plus lumacaftor/or exp elexacaftor plus ivacaftor plus tezacaftor/or ivacaftor.mp.
- tezacaftor.mp. or exp tezacaftor/
- exp lumacaftor/or lumacaftor.mp.
- exp elexacaftor/or elexacaftor.mp.
- kalydeco.mp.
- orkambi.mp.
- symdeko.mp.
- symkevi.mp.
- trikafta.mp.
- VX-770.mp.
- VX-445.mp.
- VX-809.mp.
- VX-661.mp.
- 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
- exp adverse event/or adverse event*.mp. or exp adverse drug reaction/
- exp side effect/or side effect*.mp.
- adverse reaction*.mp. or exp drug hypersensitivity/
- toxicity/or toxicity.mp. or exp drug toxicity/
- toxicities.mp.
- post-marketing.mp. or exp drug surveillance program/or exp postmarketing surveillance/
- exp drug safety/or exp pharmacovigilance/or pharmacovigilance.mp.
- phase 4.mp.
- exp phase 4 clinical trial/or phase IV.mp.
- drug tolerance.mp. or exp drug tolerance/
- drug hypersensitivity.mp.
- complication*.mp.
- exp patient harm/or harm*.mp.
- 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
- 15 and 29
Appendix B

Table A1.
Summary of methodological ratings of included observational studies a,b.
Table A1.
Summary of methodological ratings of included observational studies a,b.
| Criteria | Burgel et al. 2020 [42] | Diab-Cáceres et al. 2018 [34] | Dryden et al. 2018 [57] | Finnegan et al. 2020 [43] | Gomez-Pastrana et al. 2019 [56] | Hebestreit et al. 2013 [30] | Hubert et al. 2017 [33] |
| 1. Study objective clearly stated | Y | Y | Y | Y | Y | Y | Y |
| 2. Study population clearly defined | Y | Y | N | Y | Y | Y | Y |
| 3. Participation ≥ 50% of eligible persons | Y | CD | CD | Y | Y | Y | Y |
| 4. Study subjects from same or similar population, inclusion/exclusion criteria pre-specified and uniformly applied | Y | Y | Y | Y | Y | Y | Y |
| 5. Sample size justification, power description, or variance and effect estimates provided | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 6. Exposure of interest measured prior to outcome assessed | Y | Y | Y | Y | Y | Y | Y |
| 7. Timeframe sufficient to observe association between exposure/outcome | Y | Y | Y | Y | Y | Y | Y |
| 8. Study examined different levels of exposure as related to outcome | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 9. Exposure measures clearly defined, valid, reliable, implemented consistently | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 10. Exposure assessed more than once | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 11. Outcome measures clearly defined, valid, reliable, implemented consistently | Y | Y | N | Y | CD | CD | Y |
| 12. Outcome assessors blinded to exposure status of study subjects | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 13. Loss to follow-up ≤ 20% | Y | Y | Y | Y | Y | Y | Y |
| 14. Key potential confounders measured and adjusted statistically for impact on relationship between exposure/outcome | Y | N | N | Y | N | N | N |
| Final rating | GOOD | GOOD | FAIR | GOOD | FAIR | FAIR | GOOD |
| Criteria | Hubert et al. 2018 [58] | Jennings et al. 2017 [44] | Kopp et al. 2018 [59] | Labaste et al. 2017 [41] | Loukou et al. 2020 [60] | Murer et al. 2018 [31] | Pohl et al. 2018 [61] |
| 1. Study objective clearly stated | Y | Y | Y | Y | Y | Y | Y |
| 2. Study population clearly defined | Y | Y | Y | Y | Y | Y | Y |
| 3. Participation ≥ 50% of eligible persons | CD | Y | CD | CD | Y | Y | Y |
| 4. Study subjects from same or similar population, inclusion/exclusion criteria pre-specified and uniformly applied | Y | Y | CD | CD | Y | Y | Y |
| 5. Sample size justification, power description, or variance and effect estimates provided | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 6. Exposure of interest measured prior to outcome assessed | Y | Y | Y | Y | Y | Y | Y |
| 7. Timeframe sufficient to observe association between exposure/outcome | Y | Y | Y | Y | Y | Y | Y |
| 8. Study examined different levels of exposure as related to outcome | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 9. Exposure measures clearly defined, valid, reliable, implemented consistently | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 10. Exposure assessed more than once | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 11. Outcome measures clearly defined, valid, reliable, implemented consistently | CD | CD | N | Y | CD | Y | CD |
| 12. Outcome assessors blinded to exposure status of study subjects | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 13. Loss to follow-up ≤ 20% | Y | Y | Y | Y | Y | Y | Y |
| 14. Key potential confounders measured and adjusted statistically for impact on relationship between exposure/outcome | N | Y | N | Y | N | N | Y |
| Final rating | FAIR | GOOD | POOR | GOOD | FAIR | GOOD | GOOD |
| Criteria | Popowicz et al. 2017 [32] | Sergeev et al. 2019 [45] | Stalling et al. 2018 [55] | Taylor-Cousar et al. 2016 [29] | Tong et al. 2019 [26] | Wark et al. 2019 [25] | |
| 1. Study objective clearly stated | Y | Y | Y | Y | Y | Y | |
| 2. Study population clearly defined | Y | Y | Y | Y | Y | Y | |
| 3. Participation ≥ 50% of eligible persons | Y | Y | N | CD | Y | Y | |
| 4. Study subjects from same or similar population, inclusion/exclusion criteria pre-specified and uniformly applied | Y | Y | Y | Y | Y | Y | |
| 5. Sample size justification, power description, or variance and effect estimates provided | N/A | N/A | N/A | N/A | N/A | N/A | |
| 6. Exposure of interest measured prior to outcome assessed | Y | Y | Y | Y | Y | Y | |
| 7. Timeframe sufficient to observe association between exposure/outcome | Y | Y | Y | Y | Y | Y | |
| 8. Study examined different levels of exposure as related to outcome | N/A | N/A | N/A | N/A | N/A | N/A | |
| 9. Exposure measures clearly defined, valid, reliable, implemented consistently | N/A | N/A | N/A | N/A | N/A | N/A | |
| 10. Exposure assessed more than once | N/A | N/A | N/A | N/A | N/A | N/A | |
| 11. Outcome measures clearly defined, valid, reliable, implemented consistently | Y | N | N | CD | CD | N | |
| 12. Outcome assessors blinded to exposure status of study subjects | N/A | N/A | N/A | N/A | N/A | N/A | |
| 13. Loss to follow-up ≤ 20% | Y | Y | Y | Y | Y | Y | |
| 14. Key potential confounders measured and adjusted statistically for impact on relationship between exposure/outcome | N | N | N | N | Y | N | |
| Final rating | GOOD | FAIR | POOR | FAIR | GOOD | FAIR |
a Studies were rated against the 14 criteria of the Quality Assessment for Observational Cohort and Cross-Sectional Studies from the National Institutes of Health, National Heart, Lung, and Blood Institute [24] from the standpoint of AE assessment. b Only observational studies with a full manuscript were assessed for quality. AE, adverse event; CD, cannot determine; N, no; N/A, not applicable; Y, yes.
Appendix C

Table A2.
Summary of methodological ratings of included case series a,b.
Table A2.
Summary of methodological ratings of included case series a,b.
| Criteria | McKinzie et al., 2017 [47] | Nash et al., 2020 [27] | Rotolo et al., 2020 [52] | Safirstein et al., 2020 [53] | Talwalkar et al., 2017 [48] |
|---|---|---|---|---|---|
| 1. Study objective clearly stated | Y | Y | N | Y | Y |
| 2. Study population clearly defined, using case definition | N | N | N | N | N |
| 3. Cases consecutive | NR | NR | NR | NR | NR |
| 4. Subjects comparable | CD | CD | CD | N | N |
| 5. Intervention clearly described | Y | Y | Y | Y | Y |
| 6. Outcome measures clearly defined, valid, reliable, implemented consistently | N | N | N | Y | N |
| 7. Adequate length of follow-up | Y | Y | Y | Y | CD |
| 8. Statistical methods well-described | N/A | N/A | N/A | N/A | N |
| 9. Results well-described | Y | N | Y | Y | Y |
| Final rating | Poor | Poor | Fair | Good | Poor |
a Studies were rated against the 9 criteria of the Quality Assessment for Case Series Studies from the National Institutes of Health, National Heart, Lung, and Blood Institute [24] from the standpoint of AE assessment; b Only case series with a full manuscript were assessed for quality. AE, adverse event; CD, cannot determine; N, no; N/A, not applicable; NR, not reported; Y, yes.
References
- Elborn, J.S. Cystic Fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Sawicki, G.S.; Sellers, D.E.; Robinson, W.M. High treatment burden in adults with cystic fibrosis: Challenges to disease self-management. J. Cyst. Fibros. 2009, 8, 91–96. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation. Your CF Care Team. Available online: https://www.cff.org/Care/Your-CF-Care-Team/ (accessed on 12 October 2020).
- McIntosh, I.D. Health Human Resources Guidelines: Minimum Staffing Standards for CF Healthcare Teams. Cystic Fibrosis Canada. 2014. Available online: https://www.cysticfibrosis.ca/uploads/cf%20care/Health-Human-Resources-Guidelines.pdf (accessed on 12 October 2020).
- Conway, S.; Balfour-Lynn, I.M.; De Rijcke, K.; Drevinek, P.; Foweraker, J.; Havermans, T.; Heijerman, H.; Lannefors, L.; Lindblad, A.; Macek, M.; et al. European Cystic Fibrosis Society standards of care: Framework for the cystic fibrosis centre. J. Cyst. Fibros. 2014, 13, S3–S22. [Google Scholar] [CrossRef]
- Redfern, J.; Webb, A.K. Benefits of a dedicated cystic fibrosis pharmacist. J. R. Soc. Med. 2004, 97, 2–7. [Google Scholar] [PubMed]
- Habib, A.-R.R.; Kajbafzadeh, M.; Desai, S.; Yang, C.L.; Skolnik, K.; Quon, B.S. A systematic review of the clinical efficacy and safety of CFTR modulators in cystic fibrosis. Sci. Rep. 2019, 9, 7234:1–7234:9. [Google Scholar] [CrossRef]
- Vertex. Kalydeco® Product Monograph. 2019. Available online: https://pi.vrtx.com/files/Canadapm_kalydeco_en.pdf (accessed on 21 September 2020).
- Vertex. Orkambi® Product Monograph. 2019. Available online: https://pi.vrtx.com/files/Canadapm_orkambi_en.pdf (accessed on 21 September 2020).
- Vertex. Symdeko® Product Monograph. 2020. Available online: https://pi.vrtx.com/files/Canadapm_symdeko_en.pdf (accessed on 21 September 2020).
- European Medicines Agency. Symkevi. 2020. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/Symkevi (accessed on 21 September 2020).
- Vertex. Trikafta® Prescribing Information. 2020. Available online: https://pi.vrtx.com/files/uspi_elexacaftor_tezacaftor_ivacaftor.pdf (accessed on 21 September 2020).
- European Medicines Agency. Kaftrio. 2020. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/Kaftrio (accessed on 7 November 2020).
- Davies, J.C.; Wainwright, C.E.; Canny, G.J.; Chilvers, M.A.; Howenstine, M.S.; Munck, A.; Mainz, J.G.; Rodriguez, S.; Li, H.; Yen, K. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am. J. Respir. Crit. Care Med. 2013, 187, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, K.; Munck, A.; Walker, S.; Faro, A.; Hiatt, P.; Gilmartin, G.; Higgins, M. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J. Cyst. Fibros. 2014, 13, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, B.W.; Davies, J.; McElvaney, N.G.; Tullis, E.; Bell, S.C.; Drevinek, P.; Griese, M.; McKone, E.F.; Wainwright, C.E.; Konstan, M.W. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Eng. J. Med. 2011, 365, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.B.; Flume, P.A.; Elborn, J.S.; Cooke, J.; Rowe, S.M.; McColley, S.A.; Rubenstein, R.C.; Higgins, M. Efficacy and safety of ivacaftor treatment: Randomized trial in subjects with cystic fibrosis who have an R117H-CFTR mutation. Lancet Respir. Med. 2015, 3, 524–533. [Google Scholar] [CrossRef]
- Rowe, S.M.; Daines, C.; Ringshausen, F.C.; Kerem, E.; Wilson, J.; Tullis, E.; Nair, N.; Simard, C.; Han, L.; Ingenito, E.P.; et al. Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. N. Eng. J. Med. 2017, 377, 2024–2035. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N. Eng. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, F.; Hug, C.; Marigowda, G.; Tian, S.; Huang, X.; Stanojevic, S.; Milla, C.E.; Robinson, P.D.; Waltz, D.; Davies, J.C. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6–11 years with cystic fibrosis homozygous for F508del-CFTR: A randomized, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2017, 5, 557–567. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Munck, A.; McKone, E.F.; Van der Ent, C.K.; Moeller, A.; Simard, C.; Wang, L.T.; Ingenito, E.P.; McKee, C.; Lu, Y.; et al. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N. Eng. J. Med. 2017, 377, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Heijerman, H.G.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- Middleton, P.G.; Mall, M.A.; Drevinek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylour-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N. Eng. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- NIH National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 15 July 2020).
- Wark, P.A.; Cookson, K.; Thiruchelvam, T.; Brannan, J.; Dorahy, D.J. Lumacaftor/ivacaftor improves exercise tolerance in patients with cystic fibrosis and severe airflow obstruction. BMC Pulm. Med. 2019, 19, 106:1–106:8. [Google Scholar] [CrossRef]
- Tong, K.; Barker, D.; France, M.; Burr, L.; Greville, H.; Visser, S.; Middleton, P.; Wainwright, C.; Dorahy, D.; Wark, P. Lumacaftor/ivacaftor reduces exacerbations in adults homozygous for Phe508del mutation with severe lung disease. J. Cyst. Fibros. 2020, 19, 415–420. [Google Scholar] [CrossRef]
- Nash, E.F.; Middleton, P.G.; Taylor-Cousar, J.L. Outcomes of pregnancy in women with cystic fibrosis (CF) taking CFTR modulators—An international survey. J. Cyst. Fibros. 2020, 19, 521–526. [Google Scholar] [CrossRef]
- Patterson, A.; Autry, E.; Kuhn, R.; Murth, M. Ivacaftor drug desensitization. Pediatr. Pulmonol. 2019, 54, 672–674. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.; Niknian, M.; Gilmartin, G.; Pilewski, J.M. Effect of ivacaftor in patients with advanced cystic fibrosis and a G551D-CFTR mutation: Safety and efficacy in an expanded access program in the United States. J. Cyst. Fibros. 2016, 15, 116–122. [Google Scholar] [CrossRef]
- Hebestreit, H.; Sauer-Heilborn, A.; Fischer, R.; Kading, M.; Mainz, J.G. Effects of ivacaftor on severely ill patients with cystic fibrosis carrying a G551D mutation. J. Cyst. Fibros. 2013, 12, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Murer, C.; Huber, L.C.; Kurowski, T.; Hirt, A.; Robinson, C.; Bɒrgi, U.; Benden, C. First experience in Switzerland in Phe508del homozygous cystic fibrosis patients with end-stage pulmonary disease enrolled in a lumacaftor-ivacaftor therapy trial—Preliminary results. Swiss Med. Wkly. 2018, 148, w14593:1–w14593:5. [Google Scholar] [CrossRef]
- Popowicz, N.; Wood, J.; Tai, A.; Morey, S.; Mulrennan, S. Immediate effects of lumacaftor/ivacaftor administration on lung function in patients with severe cystic fibrosis lung disease. J. Cyst. Fibros. 2017, 16, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Hubert, D.; Chiron, R.; Camara, B.; Grenet, D.; Prévotat, A.; Bassinet, L.; Dominique, S.; Rault, G.; Macey, J.; Honoré, I.; et al. Real-life initiation of lumacaftor/ivacaftor combination in adults with cystic fibrosis homozygous for the Phe508del CFTR mutation and severe lung disease. J. Cyst. Fibros. 2017, 16, 388–391. [Google Scholar] [CrossRef]
- Diab-Cáceres, L.; Girón-Moreno, R.M.; Pastor-Sanz, M.T.; Quintana-Gallego, E.; Delgado-Pecellín, I.; Blanco-Aparicio, B.; Maiz, L.; García-Clemente, M.M.; Luna-Paredes, C.; Mondéjar-Lopez, P.; et al. Compassionate use of lumacaftor/ivacaftor in cystic fibrosis: Spanish experience. Arch. Bronconeumol. 2018, 54, 614–618. [Google Scholar] [CrossRef]
- Etherington, C.; Graham, T.; Spoletini, G.; Shaw, N.; Whitaker, P.; Clifton, I.; Peckham, D. Early experience of the Orkambi “managed Access Programme” in a regional adult UK CF centre. In Proceedings of the 40th European Cystic Fibrosis Conference, Seville, Spain, 7–10 June 2017; p. 56. [Google Scholar]
- Wareham, J.M.; Webb, K.A.; Jones, A.M.; Brennan, A.L.; Bright-Thomas, R.J.; Horsley, A.R.; Barry, P.J. Lumacaftor/ivacaftor is associated with high discontinuation rates in patients with baseline severe lung function but also benefits in those who tolerate therapy. In Proceedings of the British Thoracic Society Winter Meeting, London, UK, 6–8 December 2017; p. 247. [Google Scholar]
- Wadsworth, L.; Riley, D.; Johnson, S.; Barry, P. Physiotherapeutic objective and subjective evaluation of lumacaftor/ivacaftor initiation in patients with severe lung disease in a large adult CF centre. In Proceedings of the 33rd Annual North American Cystic Fibrosis Conference, Nashville, TN, USA, 31 October–2 November 2019; p. 468. [Google Scholar]
- Carter, S.C.; Kearns, S.; Grogan, B.; Hisert, K.B.; Cooke, G.; Singh, P.K.; Gallagher, C.G.; McKone, E.F. Effects of lumacaftor/ivacaftor in patients homozygous for F508del mutation with very advanced lung disease. In Proceedings of the 40th European Cystic Fibrosis Conference, Seville, Spain, 7–10 June 2017; p. 47. [Google Scholar]
- Robinson, R.; FitzMaurice, T.; Shaw, M.; Dawood, S.; Nazareth, D.; Walshaw, M. Symkevi: Real-world experience in an ill cohort at an adult cystic fibrosis centre. In Proceedings of the 43rd European Cystic Fibrosis Conference, Lyon, France, 3–6 June 2020; p. 219. [Google Scholar]
- Bowen, M.; Alemayehu, M.; Battle, E.; Brown, A.W. Promising results with elexacaftor/tezacaftor/ivacaftor use in cystic fibrosis patients with advanced lung disease: Beyond the clinical trial inclusion criteria. In Proceedings of the American Thoracic Society 2020 International Conference, Philadelphia, PA, USA, 15–20 May 2020; 1164. Available online: https://www.atsjournals.org/doi/book/10.1164/ajrccm-conference.2020.D12 (accessed on 1 August 2020).
- Labaste, A.; Ohlmann, C.; Mainguy, C.; Jubin, V.; Perceval, M.; Coutier, L.; Reix, P. Real-life acute lung function changes after lumacaftor/ivacaftor first administration in pediatric patients with cystic fibrosis. J. Cyst. Fibros. 2017, 16, 709–712. [Google Scholar] [CrossRef]
- Burgel, P.R.; Munck, A.; Durieu, I.; Chiron, R.; Mely, L.; Prevotat, A.; Murris-Espin, M.; Porzio, M.; Abely, M.; Reix, P.; et al. Real-life safety and effectiveness of lumacaftor-ivacaftor in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2020, 201, 188–197. [Google Scholar] [CrossRef]
- Finnegan, R.; O’Grady, E.; Smyth, A.; Ryan, S.; Williamson, M. Evidence of small airways disease and the immediate effects of lumacaftor/ivacaftor in children with cystic fibrosis. Ir. Med. J. 2020, 113, 70:1–70:7. [Google Scholar]
- Jennings, M.T.; Dezube, R.; Paranjape, S.; West, N.E.; Hong, G.; Braun, A.; Grant, J.; Merlo, C.A.; Lechtzin, N. An observational study of outcomes and tolerances in patients with cystic fibrosis initiated on lumacaftor/ivacaftor. Ann. Am. Thorac. Soc. 2017, 14, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, V.; Desai, S.; Flores, E.; Kerr, J.; Su, V.; Wilcox, P.; Quon, B.S. Safety and effectiveness of lumacaftor-ivacaftor in adults with cystic fibrosis: A single-centre Canadian experience. Can. J. Respir. Crit. Care Sleep Med. 2019, 4, 174–179. [Google Scholar] [CrossRef]
- Welsner, M.; Strasburg, S.; Taube, C.; Sivagurunathan, S. Use of ivacaftor in late diagnosed cystic fibrosis monozygotic twins heterozygous for F508del and R117H-7T: A case report. BMC Pulm. Med. 2019, 19, 76:1–76:6. [Google Scholar] [CrossRef] [PubMed]
- McKinzie, C.J.; Goralski, J.L.; Noah, T.L.; Retsch-Bogart, G.Z.; Prieur, M.B. Worsening anxiety and depression after initiation of lumacaftor/ivacaftor combination therapy in adolescent females with cystic fibrosis. J. Cyst. Fibros. 2017, 16, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Talwalkar, J.S.; Koff, J.L.; Lee, H.B.; Britto, C.J.; Mulenos, A.M.; Georgiopoulos, A.M. Cystic fibrosis transmembrane regulator modulators: Implications for the management of depression and anxiety in cystic fibrosis. Psychosomatics 2017, 58, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Walayat, S.; Hussain, N.; Patel, J.; Hussain, F.; Patel, P.; Dhillon, S.; Aulakh, B.; Chittivelu, S. Drug-induced dyspnea versus cystic fibrosis exacerbation: A diagnostic dilemma. Int. Med. Case Rep. J. 2017, 10, 243–246. [Google Scholar] [CrossRef][Green Version]
- Roehmel, J.F.; Ogese, M.O.; Rohrbach, A.; Mall, M.A.; Naisbitt, D.J. Drug allergy to CFTR modulator therapy associated with lumacaftor-specific CD4+ T lymphocytes. J. Allergy Clin. Immunol. 2020. [Google Scholar] [CrossRef]
- Song, Y.; Palacios, A.C.; Thiagalingam, A.; Middleton, P.G. Azithromycin and tezacaftor/ivacaftor is associated with first-degree heart block in an adult with cystic fibrosis. J. Cyst. Fibros. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, S.M.; Duehlmeyer, S.; Slack, S.M.; Jacobs, H.R.; Heckman, B. Testicular pain following initiation of elexacaftor/tezacaftor/ivacaftor in males with cystic fibrosis. J. Cyst. Fibros. 2020, 19, e39–e41. [Google Scholar] [CrossRef] [PubMed]
- Safirstein, J.; Grant, J.J.; Clausen, E.; Savant, D.; Dezube, R.; Hong, G. Biliary disease and cholecystectomy after initiation of elexacaftor/ivacaftor/tezacaftor in adults with cystic fibrosis. J. Cyst. Fibros. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tindell, W.; Su, A.; Oros, S.M.; Rayapati, A.O.; Rakesh, G. Trikafta and psychopathology in cystic fibrosis: A case report. Psychosomatics 2020, 61, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Stalling, V.A.; Sainath, N.; Oberle, M.; Bertolaso, C.; Schall, J.I. Energy balance and mechanisms of weight gain with ivacaftor treatment of cystic fibrosis gating mutations. J. Pediatr. 2018, 201, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pastrana, D.; Nwokoro, C.; McLean, M.; Brown, S.; Christiansen, N.; Pao, C.S. Real-world effectiveness of ivacaftor in children with cystic fibrosis and the G551D mutation. An. Pediatr. (Barc.) 2019, 90, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Dryden, C.; Wilkinson, J.; Young, D.; Brooker, J.R. The impact of 12 months treatment with ivacaftor on Scottish paediatric patients with cystic fibrosis with the G115D mutation: A review. Arch. Dis. Child. 2018, 103, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Hubert, D.; Dehillotte, C.; Munck, A.; David, V.; Baek, J.; Mely, L.; Dominique, S.; Ramel, S.; Danner-Boucher, I.; Lefeuvre, S.; et al. Retrospective observational study of French patients with cystic fibrosis and a Gly551Asp-CFTR mutation after 1 and 2 years of treatment with ivacaftor in a real-world setting. J. Cyst. Fibros. 2018, 17, 89–95. [Google Scholar] [CrossRef]
- Kopp, B.T.; McCulloch, S.; Shrestha, C.L.; Zhang, S.; Sarzynski, L.; Woodley, F.W.; Hayes, D., Jr. Metabolomic responses to lumacaftor/ivacaftor in cystic fibrosis. Pediatr. Pulmonol. 2018, 53, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Loukou, I.; Moustaki, M.; Plyta, M.; Douros, K. Longitudinal changes in lung function following initiation of lumacaftor/ivacaftor combination. J. Cyst. Fibros. 2020, 19, 534–539. [Google Scholar] [CrossRef]
- Pohl, K.; Nichols, D.P.; Taylor-Cousar, J.L.; Saavedra, M.T.; Strand, M.J.; Nick, J.A.; Bratcher, P.E. Corticosteroid use and increased CXCR2 levels on leukocytes are associated with lumacaftor/ivacaftor discontinuation in cystic fibrosis patients homozygous for the F508del CFTR mutation. PLoS ONE 2018, 13, e0209026:1–e0209026:19. [Google Scholar] [CrossRef]
- De Gregorio, F.; Tosco, A.; Casale, A.; Sepe, A.; Cimbalo, C.; Catzola, A.; Castaldo, A.; Slavadori, L.; Raia, V. Efficacy and safety of Kalydeco oral granules: The experience of our centre. In Proceedings of the 23rd Italian Congress of Cystic Fibrosis and the 13th National Congress of Cystic Fibrosis Italian Society, Naples, Italy, 22–25 November 2017; p. 29. [Google Scholar]
- Robson, E.A.; Feltbower, R.; Lee, T. Real world ivacaftor efficacy in children: Five years on…. In Proceedings of the 42nd European Cystic Fibrosis Conference, Liverpool, UK, 5–8 June 2019; p. 252. [Google Scholar]
- Al-Rashdi, Z.; Al-Busaidi, N. The effect of ivacaftor on adult cystic fibrosis patients at the Royal Hospital in Oman. In Proceedings of the 42nd European Cystic Fibrosis Conference, Liverpool, UK, 5–8 June 2019; p. 256. [Google Scholar]
- Spencer-Clegg, E.; Sapina-Vivo, R.; Cullen, D.; Lloyd, E.; Walshaw, M.J. Ivacaftor in the real world-early experience in a large adult CF centre. In Proceedings of the 27th Annual North American Cystic Fibrosis Conference, Salt Lake City, UT, USA, 17–19 October 2013; p. 228. [Google Scholar]
- Flanagan, M.; Donovan, D.; Murphy, C.; Keating, E.; Jennings, R.; Shanahan, P.; Crowley, J.; Cronin, K.; Mullane, D.; Ni Chroinin, M. Orkambi: The Cork pediatric experience Cork University Hospital. In Proceedings of the Irish Thoracic Society Annual Scientific Meeting, Limerick, Ireland, 10–11 November 2017. [Google Scholar]
- McKinney, M.L.; Noizelier, S.; Tellier, I.; Marcottee, J.; Berube, D.; Tse, S. Change in FEV1 and bronchodilator response after first dose of lumacaftor/ivacaftor. In Proceedings of the 31st Annual North American Cystic Fibrosis Conference, Indianapolis, IN, USA, 2–4 November 2017; p. 314. [Google Scholar]
- Chiron, R.; Hubert, D.; Reix, P.; Collas-Aubert, V.; Tian, S.; Kiefer, P.; Kinnman, N. Lumacaftor/ivacaftor (LUM/IVA) treatment in patients (pts) with cystic fibrosis (CF) aged ≥12 years homozygous for F508del-CFTR: Description of a French Temporary Authorization for Use (ATU) cohort. In Proceedings of the 40th European Cystic Fibrosis Conference, Seville, Spain, 7–10 June 2017; p. 49. [Google Scholar]
- Brokaar, E.; Van Leeuwen, M.; Leegwater, E.; Ploeger, M.; Van der Meer, R. Adverse drug reactions and discontinuation rate during the first year on Orkambi—The earliest results of the STORM study. In Proceedings of the 42nd European Cystic Fibrosis Conference, Liverpool, UK, 5–8 June 2019; p. 260. [Google Scholar]
- Anstead, M.; Tupayachi, G.; Murphy, D.; Autry, E.; Bulkley, V.; Kuhn, R. Lumacaftor/ivacaftor: Real world experience in a CF center. In Proceedings of the 30th Annual North American Cystic Fibrosis Conference, Orlando, FL, USA, 27–29 October 2016; p. 290. [Google Scholar]
- Taylor, T.; Brown, R.F.; Tolle, J. Lumacaftor-ivacaftor tolerance in cystic fibrosis patients homozygous for Phe508del CFTR: A single-center observational study. In Proceedings of the 30th Annual North American Cystic Fibrosis Conference, Orlando, FL, USA, 27–29 October 2016; p. 264. [Google Scholar]
- Ahmad, D.; Zanni, R.; Marmolegos, G.; Allwein, L.; Kitch, D.; Brosius, H.; Isenberg, M.; Patel, M.; Mills, N.; Stephen, M.J. Side effect profile of lumacaftor/ivacaftor. In Proceedings of the 31st Annual North American Cystic Fibrosis Conference, Indianapolis, IN, USA, 2–4 November 2017; p. 310. [Google Scholar]
- Hubert, D.; Kanaan, R.; Honoré, I.; Martin, C.; Burgel, P. Reasons for not starting or discontinuing lumacaftor/ivacaftor: A single center observational study. In Proceedings of the 31st Annual North American Cystic Fibrosis Conference, Indianapolis, IN, USA, 2–4 November 2017; p. 224. [Google Scholar]
- Walker, S.D.; Henry, B.; Sueblinvong, V.; Hunt, W.R. Experience of a large academic CF center with lumcaftor/ivacaftor. In Proceedings of the 30th Annual North American Cystic Fibrosis Conference, Orlando, FL, USA, 27–29 October 2016; p. 291. [Google Scholar]
- Wang, J.; Lester, M.K.; Benitez, D.; Rao, A.; Beringer, P. Use of in-clinic challenge dosing to assess safety and tolerability of lumcaftor/ivacaftor (L/I). In Proceedings of the 30th Annual North American Cystic Fibrosis Conference, Orlando, FL, USA, 27–29 October 2016; p. 448. [Google Scholar]
- Indihar, M.V.; Trapnell, J.P.; Burns, L.A.; Meyers, M.; Major, T.; Montag, K. Early experience initiating Orkambi therapy in adult cystic fibrosis patients. In Proceedings of the 30th Annual North American Cystic Fibrosis Conference, Orlando, FL, USA, 27–29 October 2016; p. 286. [Google Scholar]
- Albon, D.; Cagnina, E. Patient factors contributing to Orkambi discontinuation in adult cystic fibrosis, single center experience. In Proceedings of the 31st Annual North American Cystic Fibrosis Conference, Indianapolis, IN, USA, 2–4 November 2017; p. 305. [Google Scholar]
- Ahmad, D.; Patel, M.; Mills, N.; Stephen, M.J. Real world experience of lumacaftor-ivacaftor at an urban cystic fibrosis center. In Proceedings of the 30th Annual North American Cystic Fibrosis Conference, Orlando, FL, USA, 27–29 October 2016; p. 32. [Google Scholar]
- Roth, K.; Novak, K.J. Retrospective review of proton pump inhibitor use in cystic fibrosis patients initiating lumacaftor-ivacaftor (Orkambi) treatment. In Proceedings of the 31st Annual North American Cystic Fibrosis Conference, Indianapolis, IN, USA, 2–4 November 2017; p. 624. [Google Scholar]
- Wang, J.; Rao, A.; Beringer, P. Efficacy of lumacaftor/ivacaftor in patients with mild lung disease. In Proceedings of the 31st Annual North American Cystic Fibrosis Conference, Indianapolis, IN, USA, 2–4 November 2017; p. 40. [Google Scholar]
- Paluck, F.; Linnane, B. Liver tests in F508del homozygous cystic fibrosis patients on Orkambi. In Proceedings of the 9th Eruopaediatrics Congress of Royal College of Paediatrics and Child Health, Dublin, Ireland, 13–15 June 2019; p. 274. [Google Scholar]
- Melotti, P.; Tridello, G.; Pintani, E.; Meneghelli, I.; Volpi, S.; Spinelli, E.; D’Orazio, C. Effects of ivacaftor/lumacaftor in cystic fibrosis patients homozygous for F508del CFTR mutation. In Proceedings of the 24th Congress of the Italian Society of Cystic Fibrosis and the 14th National Congress of Cystic Fibrosis Italian Society, Salerno, Italy, 8–10 November 2018; p. 251. [Google Scholar]
- Bourgani, E.; Stagaki, E.; Gioka, M.; Nakou, A.; Antonogiannaki, E.-M.; Padapopoulos, D.; Diamantea, F. Two years’ experience of lumacaftor/ivacaftor treatment at an adult cystic fibrosis centre in Athens, Greece. In Proceedings of the 42nd European Cystic Fibrosis Conference, Liverpool, UK, 5–8 June 2019; p. 267. [Google Scholar]
- Bonizzoni, G.D.M.; Bellocchia, M.; Bena, C.; Clivati, E.; Biglia, C.; Demichelis, S.; Bandelli, G.; Grande, A.; Traversa, F.; Albera, C.; et al. Real life experience of lumacaftor/ivacaftor in adult Phe508del homozygous cystic fibrosis patients. In Proceedings of the European Respiratory Society International Congress, Paris, France, 15–19 September 2018; p. 1322. [Google Scholar]
- Ong, T.; Cassidy, J.; McNamara, S.; Davis, C.; Gibson, R.; Lion, K. Standardized clinical assessment of CFTR modulator use and safety monitoring: A descriptive quality improvement project. In Proceedings of the 30th Annual North American Cystic Fibrosis Conference, Orlando, FL, USA, 27–29 October 2016; p. 554. [Google Scholar]
- Pan, A.; Autry, E.; McKinzie, C.J. Real world multicenter experience of pediatric cystic fibrosis patients started on tezacaftor/ivacaftor + ivacaftor. In Proceedings of the 33rd Annual North American Cystic Fibrosis Conference, Nashville, TN, USA, 31 October–2 November 2019; p. 477. [Google Scholar]
- Robinson, N.J.; McMullan, C.; Berwick, A.; Robertson, J.; McIntosh, L.; McCabe, D.; Hardisty, G.R.; Gray, R.D. CFTR modulators in the real world: An observational study of patient response to tezacaftor/ivacaftor therapy. In Proceedings of the 43rd European Cystic Fibrosis Conference, Lyon, France, 3–6 June 2020; p. 218. [Google Scholar]
- Perez, A.; Jue, I.; Dawson, D.; Kleinhenz, M. Neurocognitive side effects thwart transition to tezacaftor/ivacaftor CFTR modulator therapy in patients with F508del homozygous cystic fibrosis. In Proceedings of the American Thoracic Society International Conference, Dallas, TX, USA, 17–22 May 2019. [Google Scholar]
- Ridge, P.C.; Campbell, C.D.; O’Mahony, M. Real-world outcomes of adult patients transitioning from Orkambi to Symkevi. In Proceedings of the 43rd European Cystic Fibrosis Conference, Lyon, France, 3–6 June 2020; p. 217. [Google Scholar]
- Richards, C.J.; Sicilian, L.; Neuringer, I. Decline and recovery of lung function with the initiation and cessation of lumacaftor-ivacaftor. In Proceedings of the American Thoracic Society 2017 International Conference, Washington, DC, USA, 19–24 May 2017. [Google Scholar]
- Howsare, M.M.; El-Kersh, K. Isolated extreme elevation of alkaline phosphatase associated with lumacaftor-ivacaftor therapy: First report. In Proceedings of the American Thoracic Society 2017 International Conference, Washington, DC, USA, 19–24 May 2017. [Google Scholar]
- Joyau, C.; Veyrac, G.; Haloun, A.; Jolliet, P. Severe rhabdomyolysis in a patient treated with lumacaftor/ivacaftor association and aciclovir. In Proceedings of the Annual Meeting of French Society of Pharmacology and Therapeutics, and INSERM Clinical Research Centers, CIC Meeting, Rouen, France, 19–21 April 2017. [Google Scholar]
- SØrensen, H.T.; Lash, T.L.; Rothman, K.J. Beyond randomized controlled trials: A critical comparison of trials with nonrandomized studies. Hepatology 2006, 44, 1075–1082. [Google Scholar] [CrossRef]
- Shteinberg, M.; Taylor-Cousar, J.L. Impact of CFTR modulator use on outcomes in people with severe cystic fibrosis lung disease. Eur. Respir. Rev. 2020, 29, 190112:1–190112:8. [Google Scholar] [CrossRef]
- Gramegna, A.; Contarini, M.; Aliberti, S.; Casciaro, R.; Blasi, F.; Castellani, C. From ivacaftor to triple combination: A systematic review of efficacy and safety of CFTR modulators in people with cystic fibrosis. Int. J. Mol. Sci. 2020, 21, 5882. [Google Scholar] [CrossRef]
- Schwarz, C.; Sivagurunathan, S.; Epaud, R.; Klingsberg, R.C.; Fischer, R.; Rowe, S.M.; Audhya, P.K.; Ahluwalia, N.; You, X.; Ferro, T.J.; et al. Tezacaftor/ivacaftor in people with cystic fibrosis who stopped lumacaftor/ivacaftor due to respiratory adverse events. J. Cyst. Fibros. 2020. [Google Scholar] [CrossRef]
- Burgel, P.-R.; Durieu, I.; Chiron, R.; Mely, L.; Prevotat, A.; Murris-Espin, M.; Porzio, M.; Abely, M.; Reix, P.; Marguet, C.; et al. Clinical response to lumacaftor-ivacaftor in patients with cystic fibrosis according to baseline lung function. J. Cyst. Fibros. 2020. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S.; Ramsey, B.W.; Boyle, M.P.; Konstan, M.W.; Huang, X.; Marigowda, G.; Waltz, D.; Wainwright, C.E. Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: A pooled analysis. Lancet Respir. Med. 2016, 4, 617–626. [Google Scholar] [CrossRef]
- Marigowda, G.; Liu, F.; Waltz, D. Effect of bronchodilators in healthy individuals receiving lumacaftor/ivacaftor combination therapy. J. Cyst. Fibros. 2017, 16, 246–249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taylor-Cousar, J.L.; Jain, M.; Barto, T.L.; Haddad, T.; Atkinson, J.; Tian, S.; Tang, R.; Marigowda, G.; Waltz, D.; Pilewski, J. Lumacaftor/ivacaftor in patients with cystic fibrosis and advanced lung disease homozygous for F508del-CFTR. J. Cyst. Fibros. 2018, 17, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Granados, A.; Chan, C.L.; Ode, K.L.; Moheet, A.; Moran, A.; Holl, R. Cystic fibrosis related diabetes: Pathophysiology, screening and diagnosis. J. Cyst. Fibros. 2019, 18, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Culhane, S.; George, C.; Pearo, B.; Spoede, E. Malnutrition in cystic fibrosis: A review. Nutr. Clin. Pract. 2013, 28, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Keating, D.; Marigowda, G.; Burr, L.; Daines, C.; Mall, M.M.; McKone, E.F.; Ramsey, B.W.; Rowe, S.M.; Sass, L.A.; Tullis, E.; et al. VX-445-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N. Eng. J. Med. 2018, 379, 1612–1620. [Google Scholar] [CrossRef]
- Milla, C.E.; Ratjen, F.; Marigowda, G.; Liu, F.; Waltz, D.; Rosenfeld, M. Lumacaftor/ivacaftor in patients aged 6–11 years with cystic fibrosis and homozygous for F508del-CFTR. Am. J. Respir. Crit. Care Med. 2017, 195, 912–920. [Google Scholar] [CrossRef]
- Scherer, K.; Brockow, K.; Aberer, W.; Gooi, J.H.C.; Demoly, P.; Romano, A.; Schnyder, B.; Whitaker, P.; Cernadas, J.S.R.; Bircher, A.J. Desensitization in delayed drug hypersensitivity reactions—An EAACI position paper of the Drug Allergy Interest Group. Allergy 2013, 68, 844–852. [Google Scholar] [CrossRef]
- McKone, E.F.; Borowitz, D.; Drevinek, P.; Griese, M.; Konstan, M.W.; Wainwright, C.; Ratjen, F.; Sermet-Gaudelus, I.; Plant, B.; Munck, A.; et al. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: A phase 3, open-label extension study (PERSIST). Lancet Respir. Med. 2014, 2, 902–910. [Google Scholar] [CrossRef]
- Fidika, A.; Herle, M.; Goldbeck, L. Symptoms of depression impact the course of lung function in adolescents and adults with cystic fibrosis. BMC Pulm. Med. 2014, 14, 205:1–205:6. [Google Scholar] [CrossRef] [PubMed]
- Schechter, M.S.; Ostrenga, J.S.; Fink, A.K.; Barker, D.H.; Sawicki, G.S.; Quittner, A.L. Decreased survival in cystic fibrosis patients with a positive screen for depression. J. Cyst. Fibros. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.L.; Noah, T.L.; Henry, M.M. Therapeutic challenges posed by critical drug-drug interactions in cystic fibrosis. Pediatr. Pulmonol. 2016, 51, S61–S70. [Google Scholar] [CrossRef] [PubMed]
- Assis, D.N.; Debray, D. Gallbladder and bile duct disease in cystic fibrosis. J. Cyst. Fibros. 2017, 16, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Sheridan, H.; Bell, N.; Cunningham, S.; Davis, S.D.; Elborn, J.S.; Milla, C.E.; Starner, T.D.; Weiner, D.J.; Lee, P.-S.; et al. Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: A randomized controlled trial. Lancet Respir. Med. 2013, 1, 630–638. [Google Scholar] [CrossRef]
- Griese, M.; Costa, S.; Linnemann, R.W.; Mall, M.A.; McKone, E.F.; Polineni, D.; Quon, B.S.; Ringshausen, F.C.; Taylor-Cousar, J.L.; Withers, N.J.; et al. Safety and efficacy of elexacaftor/tezacaftor/ivacaftor for ≥24 weeks in people with CF and ≥1 F508del allele: Interim results of an open-label phase three clinical trial. Am. J. Respir. Crit. Care Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; McKone, E.F.; Moss, R.B.; Marigowda, G.; Tian, S.; Waltz, D.; Huang, X.; Lubarsky, B.; Rubin, J.; Millar, S.J.; et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): A phase 3, extension study. Lancet Respir. Med. 2017, 5, 107–118. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. A study to Evaluate the Safety and Efficacy of Long Term Treatment with VX-661 in Combination with Ivacaftor in Subjects with Cystic Fibrosis Who Have an F508del-CFTR Mutation. Identifier NCT02565914. October 2015. Available online: https://clinicaltrials.gov/ct2/show/results/NCT02565914?view=results (accessed on 12 October 2020).
- Assis, D.N.; Freedman, S.D. Gastrointestinal disorders in cystic fibrosis. Clin. Chest Med. 2016, 37, 109–118. [Google Scholar] [CrossRef]
- Edenborough, F.P. Women with cystic fibrosis and their potential for reproduction. Thorax 2001, 56, 649–655. [Google Scholar] [CrossRef]
- Agarwal, N.; Apperley, L.; Taylor, N.F.; Taylor, D.R.; Ghataore, L.; Rumsby, E.; Treslove, C.; Holt, R.; Thursfield, R.; Senniappan, S. Posaconazole-induced hypertension masquerading as congenital adrenal hyperplasia in a child with cystic fibrosis. Case Rep. Med. 2020, 8153012:1–8153012:5. [Google Scholar] [CrossRef]
- Lieberman, J.; Rodbard, S. Low blood pressure in young adults with cystic fibrosis: An effect of chronic salt loss in sweat? Ann. Int. Med. 1975, 82, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Bisch, A.L.; Wheatley, C.M.; Baker, S.E.; Peitzman, E.R.; Van Iterson, E.H.; Laguna, T.A.; Morgan, W.J.; Snyder, E.M. Cystic fibrosis transmembrane conductance regulator genotype, not circulating catecholamines, influences cardiovascular function in patients with cystic fibrosis. Clin. Med. Insights Circ. Respir. Pulm. Med. 2019, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lilleng, H.; Abeler, K.; Johnsen, S.H.; Stensland, E.; LØseth, S.; Jorde, R.; Figenschau, Y.; Lindal, S.; Wilsgaard, T.; Bekkelund, S.I. Variation of serum creatinine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The TromsØ Study. Neuromuscul. Disord. 2011, 21, 494–500. [Google Scholar] [CrossRef]
- Gruet, M.; Troosters, T.; Verges, S. Peripheral muscle abnormalities in cystic fibrosis: Etiology, clinical implications and response to therapeutic interventions. J. Cyst. Fibros. 2017, 16, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Le-Rademacher, J.; Hillman, S.L.; Meyers, J.; Loprinzi, C.L.; Limburg, P.J.; Mandrekar, S.J. Statistical controversies in clinical research: Value of adverse events relatedness to study treatment: Analyses of data from randomized double-blind placebo-controlled clinical trials. Ann. Oncol. 2017, 28, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Bitonti, M.; Patel, P.; Dickinson, R.; Knapp, P.; Blalock, S.J. The effect of counseling on willingness to use a hypothetical medication and perceptions of medication safety. Res. Soc. Adm. Pharm. 2018, 14, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Agyapong, V.I.O.; Nwankwo, V.; Bangaru, R.; Kirrane, R. Sources of patients’ knowledge of the adverse effects of psychotropic medication and the perceived influence of adverse effects on compliance among service users attending community mental health services. J. Clin. Psychopharmacol. 2009, 29, 565–570. [Google Scholar] [CrossRef]
- Keating, D.; Wilson, L.; Williams, E.; Kotsimbos, T.; Wilson, J. Ivacaftor withdrawal syndrome during a randomised placebo-controlled cross-over study. In Proceedings of the 42nd European Cystic Fibrosis Conference, Liverpool, UK, 5–8 June 2019; p. 259. [Google Scholar]
- McKone, E.; Connolly, A.; Gallagher, C. Impact of ivacaftor treatment disruption on clinical outcomes: A single centre study. In Proceedings of the 42nd European Cystic Fibrosis Conference, Liverpool, UK, 5–8 June 2019; p. 208. [Google Scholar]
- Trimble, A.T.; Donaldson, S.H. Ivacaftor withdrawal syndrome in cystic fibrosis patients with the G551D mutation. J. Cyst. Fibros. 2018, 17, e13–e16. [Google Scholar] [CrossRef]
- Vekaria, S.; Popowicz, N.; White, S.W.; Mulrennan, S. To be or not to be on CFTR modulators during pregnancy: Risks to be considered. J. Cyst. Fibros. 2020, 19, e7–e8. [Google Scholar] [CrossRef]
- Mainz, J.G.; Michl, R.K.; Beiersdorf, N.; Lorenz, M.; Schneider, U.; Groten, T.; Jaudszus, A. Successful pregnancy of a patient with cystic fibrosis genotype F508del/F508del and progressed pulmonary destruction on lumacaftor/ivacaftor. Klin. Padiatr. 2019, 231, 271–273. [Google Scholar] [CrossRef]
- Trimble, A.; McKinzie, C.; Terrell, M.; Stringer, E.; Esther, C.R. Measured fetal and neonatal exposure to lumacaftor and ivacaftor during pregnancy and while breastfeeding. J. Cyst. Fibros. 2018, 17, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Carpino, E.A.; Fowler, R.E.; Uluer, A.Z.; Sawicki, G.S. Acute clinical outcomes following participation in short-term CFTR modulator trials in adults with cystic fibrosis: A retrospective chart review. In Proceedings of the 32nd North American Cystic Fibrosis Conference, Denver, CO, USA, 18–20 October 2018; p. 300. [Google Scholar]
- Wark, P.; McDonald, V.M. Nebulized hypertonic saline for cystic fibrosis. Cochrane Database Syst. Rev. 2018, 9, CD001506:1–CD001506:123. [Google Scholar] [CrossRef]
- Yang, C.; Montgomery, M. Dornase alfa for cystic fibrosis. Cochrane Database Syst. Rev. 2018, 9, CD001127:1–CD001127:115. [Google Scholar] [CrossRef] [PubMed]
- Mogayzel, P.J.; Naureckas, E.T.; Robinson, K.A.; Mueller, G.; Hadjiliadis, D.; Hoag, J.B.; Lubsch, L.; Hazle, L.; Sabadosa, K.; Marshall, B. Cystic fibrosis pulmonary guidelines: Chronic medications for maintenance of lung health. Am. J. Respir. Crit. Care Med. 2013, 187, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.; Mayer-Hamblett, N.; Gifford, A. Impact of Discontinuing Chronic Therapies in People with Cystic Fibrosis on Highly Effective CFTR Modulator Therapy (SIMPLIFY). Identifier NCT04378153. 7 May 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04378153 (accessed on 12 October 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).