Comprehensive Perspective for Lung Cancer Characterisation Based on AI Solutions Using CT Images
Abstract
1. Introduction
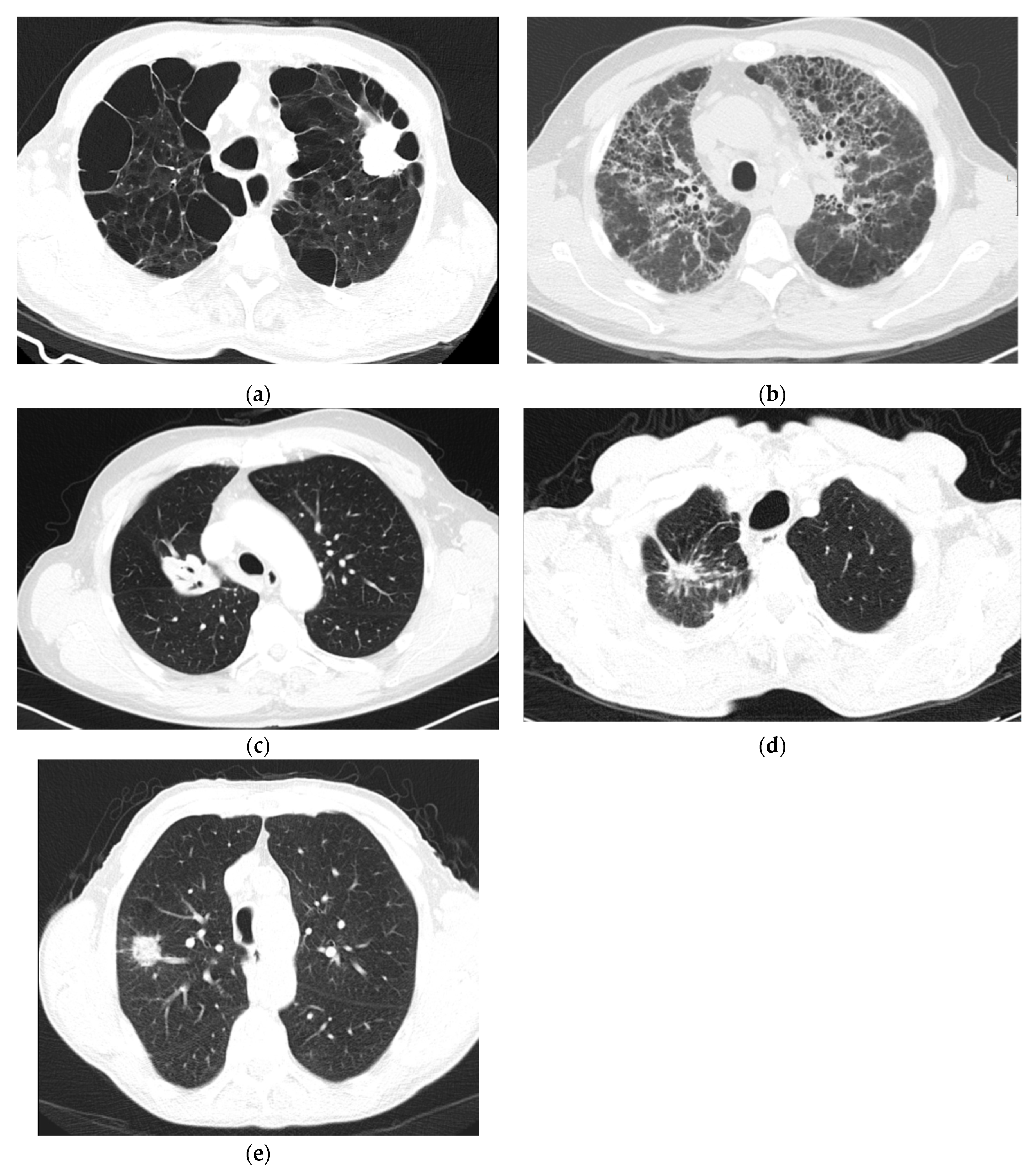
2. Pathophysiologic Features
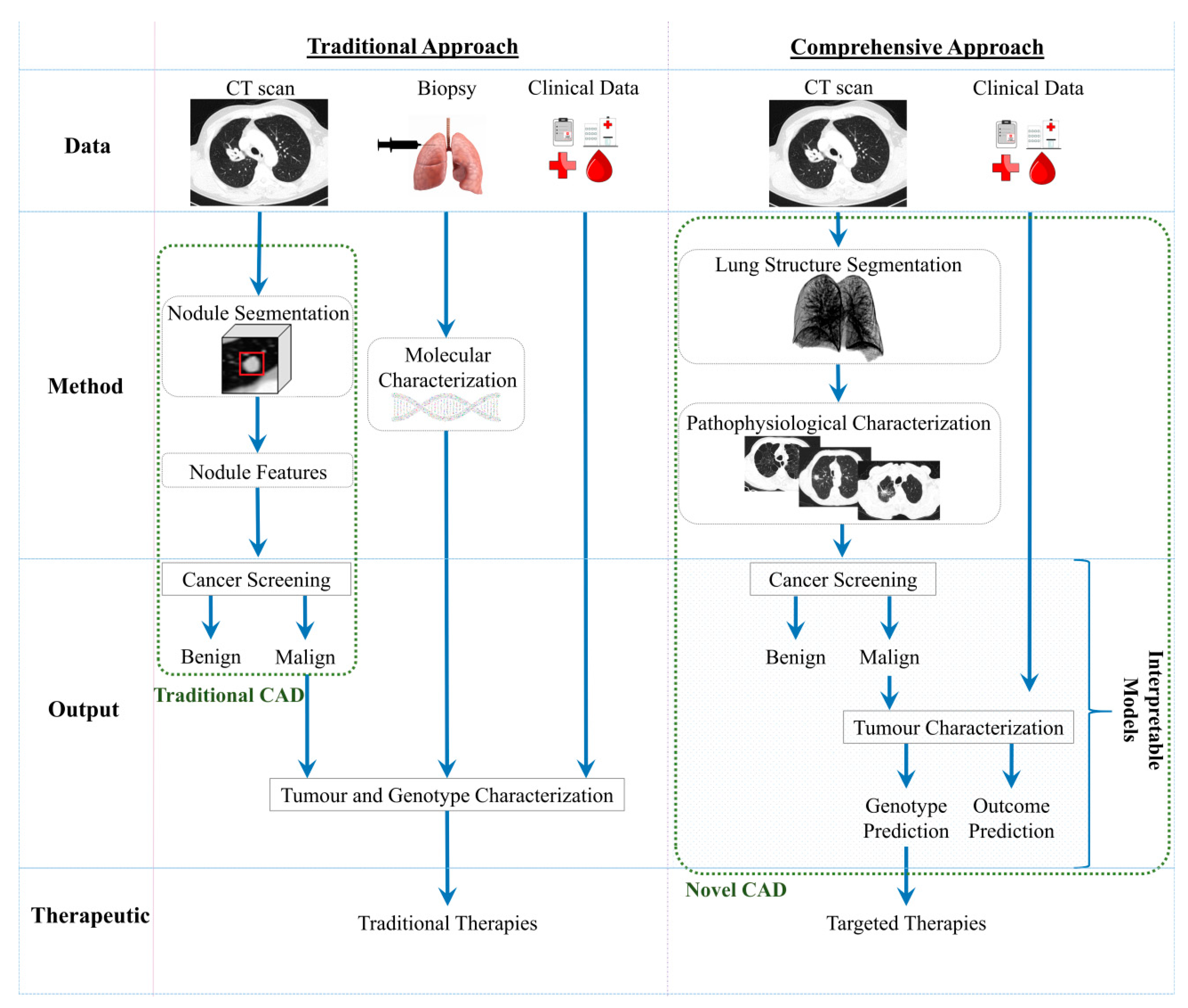
3. Comprehensive Perspective for the Next Generation of CADs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lin, L.; Yan, L.; Liu, Y.; Yuan, F.; Li, H.; Ni, J. Incidence and death in 29 cancer groups in 2017 and trend analysis from 1990 to 2017 from the Global Burden of Disease Study. J. Hematol. Oncol. 2019, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Yang, X.; Huang, Y.; Zhao, M.; Li, M.; Ma, K.; Yin, J.; Zhan, C.; Wang, Q. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag. Res. 2019, 11, 943–953. [Google Scholar] [CrossRef]
- Shigematsu, H.; Lin, L.; Takahashi, T.; Nomura, M.; Suzuki, M.; Wistuba, I.I.; Fong, K.M.; Lee, H.; Toyooka, S.; Shimizu, N.; et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl. Cancer Inst. 2005, 97, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-G.; Zhang, S.-M.; Ding, X.-X.; He, B.; Zhang, H.-Q. Driver genes in non-small cell lung cancer: Characteristics, detection methods, and targeted therapies. Oncotarget 2017, 8, 57680–57692. [Google Scholar] [CrossRef] [PubMed]
- Nakra, T.; Mehta, A.; Bal, A.; Nambirajan, A.; Mishra, D.; Midha, D.; Gupta, N.; Arora, N.; Gupta, P.; Gupta, P.; et al. Epidermal growth factor receptor mutation status in pulmonary adenocarcinoma: Multi-institutional data discussion at national conference of “Lung Cancer Management in Indian context”. Curr. Probl. Cancer 2020, 44, 100561. [Google Scholar] [CrossRef]
- Tas, F.; Ciftci, R.; Kilic, L.; Karabulut, S. Age is a prognostic factor affecting survival in lung cancer patients. Oncol. Lett. 2013, 6, 1507–1513. [Google Scholar] [CrossRef]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef]
- Paez, J.G.; Jänne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J.; et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef]
- Pao, W.; Miller, V.; Zakowski, M.; Doherty, J.; Politi, K.; Sarkaria, I.; Singh, B.; Heelan, R.; Rusch, V.; Fulton, L.; et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. USA 2004, 101, 13306–13311. [Google Scholar] [CrossRef]
- Vallath, S.; Hynds, R.E.; Succony, L.; Janes, S.M.; Giangreco, A. Targeting EGFR signalling in chronic lung disease: Therapeutic challenges and opportunities. Eur. Respir. J. 2014, 44, 513–522. [Google Scholar] [CrossRef]
- Zhang, C.; Leighl, N.B.; Wu, Y.-L.; Zhong, W.-Z. Emerging therapies for non-small cell lung cancer. J. Hematol. Oncol. 2019, 12, 1–24. [Google Scholar] [CrossRef]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.-L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Holleman, M.S.; Al, M.J.; Zaim, R.; Groen, H.J.M.; Uyl-de Groot, C.A. Cost-effectiveness analysis of the first-line EGFR-TKIs in patients with non-small cell lung cancer harbouring EGFR mutations. Eur. J. Health Econ. 2020, 21, 153–164. [Google Scholar] [CrossRef]
- Xu, M.J.; Johnson, D.E.; Grandis, J.R. EGFR-targeted therapies in the post-genomic era. Cancer Metastasis Rev. 2017, 36, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liang, S.-Q.; Schmid, R.A.; Peng, R.-W. New horizons in KRAS-mutant lung cancer: Dawn after darkness. Front. Oncol. 2019, 9, 953. [Google Scholar] [CrossRef] [PubMed]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef]
- Adderley, H.; Blackhall, F.H.; Lindsay, C.R. KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. EBioMedicine 2019, 41, 711–716. [Google Scholar] [CrossRef]
- Mullard, A. Cracking KRAS. Nat. Rev. Drug Discov. 2019, 18, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Dine, J.; Gordon, R.; Shames, Y.; Kasler, M.K.; Barton-Burke, M. Immune checkpoint inhibitors: An innovation in immunotherapy for the treatment and management of patients with cancer. Asia-Pac. J. Oncol. Nurs. 2017, 4, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Immune checkpoint therapy and the search for predictive biomarkers. Cancer J. 2016, 22, 68–72. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Backman, M.; La Fleur, L.; Kurppa, P.; Djureinovic, D.; Elfving, H.; Brunnström, H.; Mattsson, J.S.M.; Pontén, V.; Eltahir, M.; Mangsbo, S.; et al. Characterization of patterns of immune cell infiltration in NSCLC. J. Thorac. Oncol. 2020. [Google Scholar] [CrossRef]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Tuzi, A.; Bolzacchini, E.; Suter, M.B.; Giaquinto, A.; Passaro, A.; Gobba, S.; Vallini, I.; Pinotti, G. Biopsy and re-biopsy in lung cancer: The oncologist requests and the role of endobronchial ultrasounds transbronchial needle aspiration. J. Thorac. Dis. 2017, 9, S405–S409. [Google Scholar] [CrossRef][Green Version]
- Wu, C.C.; Maher, M.M.; Shepard, J.-A.O. Complications of CT-guided percutaneous needle biopsy of the chest: Prevention and management. Am. J. Roentgenol. 2011, 196, W678–W682. [Google Scholar] [CrossRef]
- Chaudhry, A.; Gul, M.; Chaudhry, A. Utility of computed tomography lung cancer screening and the management of computed tomography screen-detected findings. J. Thorac. Dis. 2018, 10, 1352–1355. [Google Scholar] [CrossRef]
- The National Lung Screening Trial Research Team; Church, T.R.; Black, W.C.; Aberle, D.R.; Berg, C.D.; Clingan, K.L.; Duan, F.; Fagerstrom, R.M.; Gareen, I.L.; Gierada, D.S.; et al. Results of initial low-dose computed tomographic screening for lung cancer. N. Engl. J. Med. 2013, 368, 1980–1991. [Google Scholar] [CrossRef]
- Al Mohammad, B.; Brennan, P.; Mello-Thoms, C. A review of lung cancer screening and the role of computer-aided detection. Clin. Radiol. 2017, 72, 433–442. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; Van Der Aalst, C.M.; De Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- El-Baz, A.; Beache, G.M.; Gimel’Farb, G.; Suzuki, K.; Okada, K.; Elnakib, A.; Soliman, A.; Abdollahi, B. Computer-aided diagnosis systems for lung cancer: Challenges and methodologies. Int. J. Biomed. Imaging 2013, 2013, 942353. [Google Scholar] [CrossRef] [PubMed]
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G.; et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 2019, 25, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, C.; Zhang, H.; Song, J.; Wu, L. Radiomics for the prediction of EGFR mutation subtypes in non-small cell lung cancer. Med. Phys. 2019, 46, 4545–4552. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kim, J.; Balagurunathan, Y.; Li, Q.; Garcia, A.L.; Stringfield, O.; Ye, Z.; Gillies, R.J. Radiomic features are associated with EGFR mutation status in lung adenocarcinomas. Clin. Lung Cancer 2016, 17, 441–448.e6. [Google Scholar] [CrossRef]
- Wang, X.; Kong, C.; Xu, W.; Yang, S.; Shi, D.; Zhang, J.; Du, M.; Wang, S.; Bai, Y.; Zhang, T.; et al. Decoding tumor mutation burden and driver mutations in early stage lung adenocarcinoma using CT-based radiomics signature. Thorac. Cancer 2019, 10, 1904–1912. [Google Scholar] [CrossRef]
- Jia, T.-Y.; Xiong, J.-F.; Li, X.-Y.; Yu, W.; Xu, Z.-Y.; Cai, X.-W.; Ma, J.-C.; Ren, Y.-C.; Larsson, R.; Zhang, J.; et al. Identifying EGFR mutations in lung adenocarcinoma by noninvasive imaging using radiomics features and random forest modeling. Eur. Radiol. 2019, 29, 4742–4750. [Google Scholar] [CrossRef]
- Tu, W.; Sun, G.; Fan, L.; Wang, Y.; Xia, Y.; Guan, Y.; Li, Q.; Zhang, D.; Liu, S.; Li, Z. Radiomics signature: A potential and incremental predictor for EGFR mutation status in NSCLC patients, comparison with CT morphology. Lung Cancer 2019, 132, 28–35. [Google Scholar] [CrossRef]
- Gevaert, O.; Echegaray, S.; Khuong, A.; Hoang, C.D.; Shrager, J.B.; Jensen, K.C.; Berry, G.J.; Guo, H.H.; Lau, C.; Plevritis, S.K.; et al. Predictive radiogenomics modeling of EGFR mutation status in lung cancer. Sci. Rep. 2017, 7, 41674. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, G.; Pereira, T.; Dias, C.; Freitas, C.; Hespanhol, V.; Costa, J.L.; Cunha, A.; Oliveira, H.P. Identifying relationships between imaging phenotypes and lung cancer-related mutation status: EGFR and KRAS. Sci. Rep. 2020, 10, 3625. [Google Scholar] [CrossRef]
- Shah, P.L.; Herth, F.J.; Van Geffen, W.H.; Deslee, G.; Slebos, D.-J. Lung volume reduction for emphysema. Lancet Respir. Med. 2017, 5, 147–156. [Google Scholar] [CrossRef]
- Friedman, P.J. Imaging Studies in Emphysema. Proc. Am. Thorac. Soc. 2008, 5, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.O.; Weissfeld, J.L.; Balkan, A.; Schragin, J.G.; Fuhrman, C.R.; Fisher, S.N.; Wilson, J.; Leader, J.K.; Siegfried, J.M.; Shapiro, S.D.; et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am. J. Respir. Crit. Care Med. 2008, 178, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Jeon, K.N.; Lee, S.J.; Kim, H.C.; Ha, J.Y.; Park, S.E.; Baek, H.J.; Choi, B.H.; Cho, S.B.; Moon, J.I. Severity of pulmonary emphysema and lung cancer. Medicine 2016, 95, e5494. [Google Scholar] [CrossRef] [PubMed]
- Hohberger, L.A.; Schroeder, D.R.; Bartholmai, B.J.; Yang, P.; Wendt, C.H.; Bitterman, P.B.; Larsson, O.; Limper, A.H. Correlation of regional emphysema and lung cancer: A lung tissue research consortium-based study. J. Thorac. Oncol. 2014, 9, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Wille, M.M.W.; Thomsen, L.H.; Petersen, J.; De Bruijne, M.; Dirksen, A.; Pedersen, J.H.; Shaker, S.B. Visual assessment of early emphysema and interstitial abnormalities on CT is useful in lung cancer risk analysis. Eur. Radiol. 2016, 26, 487–494. [Google Scholar] [CrossRef]
- Nishio, M.; Kubo, T.; Togashi, K. Estimation of lung cancer risk using homology-based emphysema quantification in patients with lung nodules. PLoS ONE 2019, 14, e0210720. [Google Scholar] [CrossRef]
- King, T.E.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- Gotway, M.B.; Freemer, M.M.; E King, T., Jr. Challenges in pulmonary fibrosis. 1: Use of high resolution CT scanning of the lung for the evaluation of patients with idiopathic interstitial pneumonias. Thorax 2007, 62, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Karampitsakos, T.; Tzilas, V.; Tringidou, R.; Steiropoulos, P.; Aidinis, V.; Papiris, S.A.; Bouros, D.; Tzouvelekis, A. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther. 2017, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef]
- Raju, S.; Ghosh, S.; Mehta, A.C. Chest CT signs in pulmonary disease: A pictorial review. Chest 2017, 151, 1356–1374. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Xing, Y.; Jiang, S.; Chen, G.; Mao, H.; Labh, K.; Jia, X.; Sun, X. Morphological factors differentiating between early lung adenocarcinomas appearing as pure ground-glass nodules measuring ≤10 mm on thin-section computed tomography. Cancer Imaging 2014, 14, 33. [Google Scholar] [CrossRef]
- Qiang, J.; Zhou, K.; Lu, G.; Wang, Q.; Ye, X.; Xu, S.; Tan, L. The relationship between solitary pulmonary nodules and bronchi: Multi-slice CT–pathological correlation. Clin. Radiol. 2004, 59, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Snoeckx, A.; Reyntiens, P.; Desbuquoit, D.; Spinhoven, M.J.; Van Schil, P.E.; Van Meerbeeck, J.P.; Parizel, P.M. Evaluation of the solitary pulmonary nodule: Size matters, but do not ignore the power of morphology. Insights Imaging 2018, 9, 73–86. [Google Scholar] [CrossRef]
- Chu, Z.-G.; Zhang, Y.; Li, W.-J.; Li, Q.; Zheng, Y.-N.; Lv, F.-J. Primary solid lung cancerous nodules with different sizes: Computed tomography features and their variations. BMC Cancer 2019, 19. [Google Scholar] [CrossRef]
- Rizzo, S.; Raimondi, S.; De Jong, E.E.C.; Van Elmpt, W.; De Piano, F.; Petrella, F.; Bagnardi, V.; Jochems, A.; Bellomi, M.; Dingemans, A.M.; et al. Genomics of non-small cell lung cancer (NSCLC): Association between CT-based imaging features and EGFR and K-RAS mutations in 122 patients—An external validation. Eur. J. Radiol. 2019, 110, 148–155. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, J.; Qu, F.; Liu, S.; Wang, H.; Balagurunathan, Y.; Ye, Z.; Gillies, R.J. CT features associated with epidermal growth factor receptor mutation status in patients with lung adenocarcinoma. Radiology 2016, 280, 271–280. [Google Scholar] [CrossRef]
- Hu, H.; Wang, Q.; Tang, H.; Xiong, L.; Lin, Q. Multi-slice computed tomography characteristics of solitary pulmonary ground-glass nodules: Differences between malignant and benign. Thorac. Cancer 2016, 7, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, H.; Geng, C.; Dai, Y.; Ji, J. Advances in intelligent diagnosis methods for pulmonary ground-glass opacity nodules. Biomed. Eng. Online 2018, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, F.; Raja, G.; Frangi, A.F. Computer-aided detection of lung nodules: A review. J. Med. Imaging 2019, 6, 02090. [Google Scholar] [CrossRef]
- Holzinger, A.; Biemann, C.; Pattichis, C.S.; Kell, D.B. What do we need to build explainable AI systems for the medical domain? arXiv 2017, arXiv:1712.09923. [Google Scholar]
- Carvalho, D.V.; Pereira, E.M.; Cardoso, J.S. Machine Learning Interpretability: A Survey on Methods and Metrics. Electronics 2019, 8, 832. [Google Scholar] [CrossRef]
- Kawagishi, M.; Kubo, T.; Sakamoto, R.; Yakami, M.; Fujimoto, K.; Aoyama, G.; Emoto, Y.; Sekiguchi, H.; Sakai, K.; Iizuka, Y.; et al. Automatic inference model construction for computer-aided diagnosis of lung nodule: Explanation adequacy, inference accuracy, and experts’ knowledge. PLoS ONE 2018, 13, e0207661. [Google Scholar] [CrossRef]
- Akhtar, N.; Bansal, J.G. Risk factors of Lung Cancer in nonsmoker. Curr. Probl. Cancer 2017, 41, 328–339. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the college of American pathologists, the international association for the study of lung cancer, and the association for molecular pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef]
- Videtic, G.M.M.; Donington, J.; Giuliani, M.; Heinzerling, J.; Karas, T.Z.; Kelsey, C.R.; Lally, B.E.; Latzka, K.; Lo, S.S.-M.; Moghanaki, D.; et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pr. Radiat. Oncol. 2017, 7, 295–301. [Google Scholar] [CrossRef]
- Najafabadi, M.M.; Villanustre, F.; Khoshgoftaar, T.M.; Seliya, N.; Wald, R.; Muharemagic, E. Deep learning applications and challenges in big data analytics. J. Big Data 2015, 2. [Google Scholar] [CrossRef]
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; et al. ImageNet large scale visual recognition challenge. Int. J. Comput. Vis. 2015, 115, 211–252. [Google Scholar] [CrossRef]
- McNitt-Gray, M.F.; Armato, S.G.; Meyer, C.R.; Reeves, A.P.; McLennan, G.; Pais, R.C.; Freymann, J.; Brown, M.S.; Engelmann, R.M.; Bland, P.H.; et al. The Lung Image Database Consortium (LIDC) data collection process for nodule detection and annotation. Acad. Radiol. 2007, 14, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
| Traditional Approach | Comprehensive Approach | |
|---|---|---|
| Pros | -Clinically validated | -Non-invasive assessment, faster and with lower costs; -Safety repeated; -Leverage the personalised medicine; -Interpretable models; -Comprehensive perspective |
| Cons | -Invasive and with clinical implications; -Restriction for the repetitions of the procedure; -AI based solutions with residual help in the diagnosis | -In development; -Requirement large datasets to train the predictive models |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, T.; Freitas, C.; Costa, J.L.; Morgado, J.; Silva, F.; Negrão, E.; de Lima, B.F.; da Silva, M.C.; Madureira, A.J.; Ramos, I.; et al. Comprehensive Perspective for Lung Cancer Characterisation Based on AI Solutions Using CT Images. J. Clin. Med. 2021, 10, 118. https://doi.org/10.3390/jcm10010118
Pereira T, Freitas C, Costa JL, Morgado J, Silva F, Negrão E, de Lima BF, da Silva MC, Madureira AJ, Ramos I, et al. Comprehensive Perspective for Lung Cancer Characterisation Based on AI Solutions Using CT Images. Journal of Clinical Medicine. 2021; 10(1):118. https://doi.org/10.3390/jcm10010118
Chicago/Turabian StylePereira, Tania, Cláudia Freitas, José Luis Costa, Joana Morgado, Francisco Silva, Eduardo Negrão, Beatriz Flor de Lima, Miguel Correia da Silva, António J. Madureira, Isabel Ramos, and et al. 2021. "Comprehensive Perspective for Lung Cancer Characterisation Based on AI Solutions Using CT Images" Journal of Clinical Medicine 10, no. 1: 118. https://doi.org/10.3390/jcm10010118
APA StylePereira, T., Freitas, C., Costa, J. L., Morgado, J., Silva, F., Negrão, E., de Lima, B. F., da Silva, M. C., Madureira, A. J., Ramos, I., Hespanhol, V., Cunha, A., & Oliveira, H. P. (2021). Comprehensive Perspective for Lung Cancer Characterisation Based on AI Solutions Using CT Images. Journal of Clinical Medicine, 10(1), 118. https://doi.org/10.3390/jcm10010118








