Pancreatic Islet Purification from Large Mammals and Humans Using a COBE 2991 Cell Processor versus Large Plastic Bottles
Abstract
:1. Introduction
2. Continuous Density Gradient with a COBE 2991 Cell Processor
3. Purification Solutions
4. Controlled Density Gradient
5. Islet Purification Using Large Bottles
6. Modification of the Bottle Purification Method
7. Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| IFN-γ | interferon-γ |
| IL-1β | interleukin-1β |
| TNF-α | tumor necrosis factor-α |
| MK | trehalose-containing Kyoto solution with ulinastatin |
| IK | iodixanol + MK |
References
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [PubMed]
- Shapiro, A.M.; Ricordi, C.; Hering, B.J.; Auchincloss, H.; Lindblad, R.; Robertson, R.P.; Secchi, A.; Brendel, M.D.; Berney, T.; Brennan, D.C.; et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 2006, 355, 1318–1330. [Google Scholar] [PubMed] [Green Version]
- Froud, T.; Ricordi, C.; Baidal, D.A.; Hafiz, M.M.; Ponte, G.; Cure, P.; Pileggi, A.; Poggioli, R.; Ichii, H.; Khan, A.; et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am. J. Transplant. 2005, 5, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Hering, B.J.; Kandaswamy, R.; Ansite, J.D.; Eckman, P.M.; Nakano, M.; Sawada, T.; Matsumoto, I.; Ihm, S.H.; Zhang, H.J.; Parkey, J.; et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005, 293, 830–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, H.; Iwanaga, Y.; Okitsu, T.; Nagata, H.; Yonekawa, Y.; Matsumoto, S. Evaluation of islet transplantation from non-heart beating donors. Am. J. Transplant. 2006, 6, 2476–2482. [Google Scholar]
- Anazawa, T.; Matsumoto, S.; Yonekawa, Y.; Loganathan, G.; Wilhelm, J.J.; Soltani, S.M.; Papas, K.K.; Sutherland, D.E.; Hering, B.J.; Balamurugan, A.N. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation 2011, 91, 508–514. [Google Scholar] [CrossRef]
- Barbaro, B.; Salehi, P.; Wang, Y.; Qi, M.; Gangemi, A.; Kuechle, J.; Hansen, M.A.; Romagnoli, T.; Avila, J.; Benedetti, E.; et al. Improved human pancreatic islet purification with the refined UIC-UB density gradient. Transplantation 2007, 84, 1200–1203. [Google Scholar]
- Delaney, C.A.; Pavlovic, D.; Hoorens, A.; Pipeleers, D.G.; Eizirik, D.L. Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells. Endocrinology 1997, 138, 2610–2614. [Google Scholar] [CrossRef]
- Noguchi, H.; Matsushita, M.; Okitsu, T.; Moriwaki, A.; Tomizawa, K.; Kang, S.; Li, S.T.; Kobayashi, N.; Matsumoto, S.; Tanaka, K.; et al. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat. Med. 2004, 10, 305–309. [Google Scholar]
- Noguchi, H.; Naziruddin, B.; Jackson, A.; Shimoda, M.; Fujita, Y.; Chujo, D.; Takita, M.; Peng, H.; Sugimoto, K.; Itoh, T.; et al. Comparison of ulinastatin, gabexate mesilate, and nafamostat mesilate in preservation solution for islet isolation. Cell Transplant. 2012, 21, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, H.; Naziruddin, B.; Jackson, A.; Shimoda, M.; Ikemoto, T.; Fujita, Y.; Chujo, D.; Takita, M.; Peng, H.; Sugimoto, K.; et al. Fresh islets are more effective for islet transplantation than cultured islets. Cell Transplant. 2012, 21, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, H. Regulation of c-Jun NH2-Terminal Kinase for Islet Transplantation. J. Clin. Med. 2019, 8, 1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y.; Ebi, N.; Hamada, E.; Tamaki, Y.; Kuwae, K.; Kobayashi, N.; Saitoh, I.; Watanabe, M. Modified cell-permeable JNK inhibitors efficiently prevents islet apoptosis and improves the outcome of islet transplantation. Sci. Rep. 2018, 8, 11082. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y.; Ebi, N.; Hamada, E.; Tamaki, Y.; Kuwae, K.; Kitamura, S.; Kobayashi, N.; Saitoh, I.; et al. A Novel Preservation Solution Containing a JNK Inhibitory Peptide Efficiently Improves Islet Yield for Porcine Islet Isolation. Transplantation 2019, 103, 344–352. [Google Scholar] [CrossRef]
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y.; Saitoh, I.; Watanabe, M. Novel cell-permeable p38-MAPK inhibitor efficiently prevents porcine islet apoptosis and improves islet graft function. Am. J. Transplant. 2020, 20, 1296–1308. [Google Scholar] [CrossRef]
- Eckhard, M.; Brandhorst, D.; Brandhorst, H.; Brendel, M.D.; Bretzel, R.G. Optimization in osmolality and range of density of a continuous ficoll-sodium-diatrizoate gradient for isopycnic purification of isolated human islets. Transplant. Proc. 2004, 36, 2849–2854. [Google Scholar] [CrossRef]
- Noguchi, H.; Naziruddin, B.; Shimoda, M.; Fujita, Y.; Chujo, D.; Takita, M.; Peng, H.; Sugimoto, K.; Itoh, T.; Kobayashi, N.; et al. Evaluation of osmolality of density gradient for human islet purification. Cell Transplant. 2012, 21, 493–500. [Google Scholar] [CrossRef]
- Shimoda, M.; Noguchi, H.; Fujita, Y.; Takita, M.; Ikemoto, T.; Chujo, D.; Naziruddin, B.; Levy, M.F.; Kobayashi, N.; Grayburn, P.A.; et al. Islet purification method using large bottles effectively achieves high islet yield from pig pancreas. Cell Transplant. 2012, 21, 501–508. [Google Scholar] [CrossRef]
- Miyagi-Shiohira, C.; Kobayashi, N.; Saitoh, I.; Watanabe, M.; Noguchi, Y.; Matsushita, M.; Noguchi, H. The Evaluation of Islet Purification Methods That Use Large Bottles to Create a Continuous Density Gradient. Cell Med. 2016, 9, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Miyagi-Shiohira, C.; Kobayashi, N.; Saitoh, I.; Watanabe, M.; Noguchi, Y.; Matsushita, M.; Noguchi, H. Comparison of Purification Solutions with Different Osmolality for Porcine Islet Purification. Cell Med. 2016, 9, 53–59. [Google Scholar] [CrossRef]
- Ebi, N.; Miyagi-Shiohira, C.; Hamada, E.; Tamaki, Y.; Masamoto, M.; Makishi, E.; Nakashima, Y.; Kobayashi, N.; Saitoh, I.; Watanabe, M.; et al. Evaluation of Islet Purification Methods for Making a Continuous Density Gradient and Loading Tissue. Cell Med. 2018, 10, 2155179017733090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyagi-Shiohira, C.; Nakashima, Y.; Ebi, N.; Hamada, E.; Tamaki, Y.; Kuwae, K.; Kobayashi, N.; Saitoh, I.; Watanabe, M.; Kinjo, T.; et al. Comparison of Tissue Loading Before and After the Creation of a Continuous Density Gradient in Porcine Islet Purification. Cell Med. 2018, 10, 2155179018781343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoda, M.; Itoh, T.; Iwahashi, S.; Takita, M.; Sugimoto, K.; Kanak, M.A.; Chujo, D.; Naziruddin, B.; Levy, M.F.; Grayburn, P.A.; et al. An effective purification method using large bottles for human pancreatic islet isolation. Islets 2012, 4, 398–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwae, K.; Miyagi-Shiohira, C.; Hamada, E.; Tamaki, Y.; Nishime, K.; Sakai, M.; Yonaha, T.; Makishi, E.; Saitoh, I.; Watanabe, M.; et al. Excellent Islet Yields after 18-h Porcine Pancreas Preservation by Ductal Injection, Pancreas Preservation with MK Solution, Bottle Purification, and Islet Purification Using Iodixanol with UW Solution and Iodixanol with MK Solution. J. Clin. Med. 2019, 8, 1561. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, H.; Ikemoto, T.; Naziruddin, B.; Jackson, A.; Shimoda, M.; Fujita, Y.; Chujo, D.; Takita, M.; Kobayashi, N.; Onaca, N.; et al. Iodixanol-controlled density gradient during islet purification improves recovery rate in human islet isolation. Transplantation 2009, 87, 1629–1635. [Google Scholar] [CrossRef]
- Ricordi, C.; Lacy, P.E.; Finke, E.H.; Olack, B.J.; Scharp, D.W. Automated method for isolation of human pancreatic islets. Diabetes 1988, 37, 413–420. [Google Scholar] [CrossRef]
- Ricordi, C.; Lacy, P.E.; Scharp, D.W. Automated islet isolation from human pancreas. Diabetes 1989, 38 (Suppl. 1), 140–142. [Google Scholar] [CrossRef]
- Brandhorst, H.; Brandhorst, D.; Brendel, M.D.; Hering, B.J.; Bretzel, R.G. Assessment of intracellular insulin content during all steps of human islet isolation procedure. Cell Transplant. 1998, 7, 489–495. [Google Scholar] [CrossRef]
- Lake, S.P.; Bassett, P.D.; Larkins, A.; Revell, J.; Walczak, K.; Chamberlain, J.; Rumford, G.M.; London, N.J.; Veitch, P.S.; Bell, P.R.; et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes 1989, 38, 143–145. [Google Scholar] [CrossRef]
- van Suylichem, P.T.; Wolters, G.H.; van Schilfgaarde, R. The efficacy of density gradients for islet purification: A comparison of seven density gradients. Transpl. Int. 1990, 3, 156–161. [Google Scholar] [CrossRef]
- Robertson, G.S.; Chadwick, D.R.; Contractor, H.; James, R.F.; Bell, P.R.; London, N.J. The use of continuous density gradients for the assessment of islet and exocrine tissue densities and islet purification. Acta Diabetol. 1993, 30, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.S.; Chadwick, D.R.; Contractor, H.; James, R.F.; London, N.J. The optimization of large-scale density gradient isolation of human islets. Acta Diabetol. 1993, 30, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ichii, H.; Wang, X.; Messinger, S.; Alvarez, A.; Fraker, C.; Khan, A.; Kuroda, Y.; Inverardi, L.; Goss, J.A.; Alejandro, R.; et al. Improved human islet isolation using nicotinamide. Am. J. Transplant. 2006, 6, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Kin, T.; Mirbolooki, M.; Salehi, P.; Tsukada, M.; O’Gorman, D.; Imes, S.; Ryan, E.A.; Shapiro, A.M.; Lakey, J.R. Islet isolation and transplantation outcomes of pancreas preserved with University of Wisconsin solution versus two-layer method using preoxygenated perfluorocarbon. Transplantation 2006, 82, 1286–1290. [Google Scholar] [CrossRef]
- Chadwick, D.R.; Robertson, G.S.; Rose, S.; Contractor, H.; James, R.F.; Bell, P.R.; London, N.J. Storage of porcine pancreatic digest prior to islet purification. The benefits of UW solution and the roles of its individual components. Transplantation 1993, 56, 288–293. [Google Scholar] [CrossRef]
- Hering, B.J. Repurification: Rescue rather than routine remedy. Am. J. Transplant. 2005, 5, 1–2. [Google Scholar] [CrossRef]
- London, N.J.; Swift, S.M.; Clayton, H.A. Isolation, culture and functional evaluation of islets of Langerhans. Diabetes Metab. 1998, 24, 200–207. [Google Scholar]
- Lake, S.P.; Anderson, J.; Chamberlain, J.; Gardner, S.J.; Bell, P.R.; James, R.F. Bovine serum albumin density gradient isolation of rat pancreatic islets. Transplantation 1987, 43, 805–808. [Google Scholar] [CrossRef]
- Williams, N.; Kraft, N.; Shortman, K. The separation of different cell classes from lymphoid organs. VI. The effect of osmolarity of gradient media on the density distribution of cells. Immunology 1972, 22, 885–899. [Google Scholar]
- Loos, J.A.; Roos, D. Ficoll-isopaque gradients for the determination of density distributions of human blood lymphocytes and other reticulo-endothelial cells. Exp. Cell Res. 1974, 86, 333–341. [Google Scholar] [CrossRef]
- Eckhardt, T.; Jahr, H.; Federlin, K.; Bretzel, R.G. Endotoxin impairs the engraftment of rat islets transplanted beneath the kidney capsule of C57BL/6-mice. J. Mol. Med. 1999, 77, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Jahr, H.; Pfeiffer, G.; Hering, B.J.; Federlin, K.; Bretzel, R.G. Endotoxin-mediated activation of cytokine production in human PBMCs by collagenase and Ficoll. J. Mol. Med. 1999, 77, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Hering, B.J.; Kandaswamy, R.; Harmon, J.V.; Ansite, J.D.; Clemmings, S.M.; Sakai, T.; Paraskevas, S.; Eckman, P.M.; Sageshima, J.; Nakano, M.; et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am. J. Transplant. 2004, 4, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Mita, A.; Ricordi, C.; Messinger, S.; Miki, A.; Misawa, R.; Barker, S.; Molano, R.D.; Haertter, R.; Khan, A.; Miyagawa, S.; et al. Antiproinflammatory effects of iodixanol (OptiPrep)-based density gradient purification on human islet preparations. Cell Transplant. 2010, 19, 1537–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanley, S.; Liu, S.; Lipsett, M.; Castellarin, M.; Rosenberg, L.; Tchervenkov, J.; Paraskevas, S. Tumor necrosis factor-alpha production by human islets leads to postisolation cell death. Transplantation 2006, 82, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Deeds, M.C.; Armstrong, A.; Gastineau, D.; Kudva, Y. Utilization of a test gradient enhances islet recovery from deceased donor pancreases. Cytotherapy 2007, 9, 630–636. [Google Scholar] [CrossRef]
- Lake, S.P.; Chamberlain, J.; Walczak, K.; Bell, P.R.; James, R.F. A test gradient system for optimizing density gradient isolation of pancreatic islets. Transplantation 1989, 48, 354–357. [Google Scholar]
- Gray, D.W.; McShane, P.; Grant, A.; Morris, P.J. A method for isolation of islets of Langerhans from the human pancreas. Diabetes 1984, 33, 1055–1061. [Google Scholar] [CrossRef]
- Scharp, D.W.; Lacy, P.E.; Finke, E.; Olack, B. Low-temperature culture of human islets isolated by the distention method and purified with Ficoll or Percoll gradients. Surgery 1987, 102, 869–879. [Google Scholar]
- Shintaku, H.; Okitsu, T.; Kawano, S.; Matsumoto, S.; Suzuki, T.; Kanno, I.; Kotera, H. Effects of fluid dynamic stress on fracturing of cell-aggregated tissue during purification for islets of Langerhans transplantation. J. Phys. D Appl. Phys. 2008, 41, 115507. [Google Scholar] [CrossRef]
- Fritschy, W.M.; van Suylichem, P.T.; Wolters, G.H.; van Schilfgaarde, R. Comparison of top and bottom loading of a dextran gradient for rat pancreatic islet purification. Diabetes Res. 1992, 19, 91–95. [Google Scholar] [PubMed]
- Huang, G.C.; Zhao, M.; Jones, P.; Persaud, S.; Ramracheya, R.; Löbner, K.; Christie, M.R.; Banga, J.P.; Peakman, M.; Sirinivsan, P.; et al. The development of new density gradient media for purifying human islets and islet-quality assessments. Transplantation 2004, 77, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.S.; Chadwick, D.; Contractor, H.; Rose, S.; Chamberlain, R.; Clayton, H.; Bell, P.R.; James, R.F.; London, N.J. Storage of human pancreatic digest in University of Wisconsin solution significantly improves subsequent islet purification. Br. J. Surg. 1992, 79, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Ichii, H.; Pileggi, A.; Molano, R.D.; Baidal, D.A.; Khan, A.; Kuroda, Y.; Inverardi, L.; Goss, J.A.; Alejandro, R.; Ricordi, C. Rescue purification maximizes the use of human islet preparations for transplantation. Am. J. Transplant. 2005, 5, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Naziruddin, B.; Shimoda, M.; Chujo, D.; Takita, M.; Sugimoto, K.; Itoh, T.; Onaca, N.; Levy, M.F.; Matsumoto, S. A Combined Continuous Density/Osmolality Gradient for Supplemental Purification of Human Islets. Cell Med. 2012, 3, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, R.; Nadeau, D. Adjustment of the osmolality of Percoll for the isopycnic separation of cells and cell organelles. Anal. Biochem. 1984, 141, 322–328. [Google Scholar] [CrossRef]
- Chadwick, D.R.; Robertson, G.S.; Rose, S.; Contractor, H.; Bell, P.R.; James, R.F.; London, N.J. Islet purification: Optimisation of islet isolation solutions. Transplant. Proc. 1994, 26, 816–817. [Google Scholar]
- London, N.J.; Toomey, P.; Contractor, H.; Thirdborough, S.T.; James, R.F.; Bell, P.R. The effect of osmolality and glucose concentration on the purity of human islet isolates. Transplant. Proc. 1992, 24, 1002. [Google Scholar]
- Noguchi, H.; Ueda, M.; Hayashi, S.; Kobayashi, N.; Okitsu, T.; Iwanaga, Y.; Nagata, H.; Nakai, Y.; Matsumoto, S. Ductal injection of preservation solution increases islet yields in islet isolation and improves islet graft function. Cell Transplant. 2008, 17, 69–81. [Google Scholar] [CrossRef] [Green Version]


| Continuous/ Discontinuous | COBE2991/ Bottle | Solutions | Year | Reference |
|---|---|---|---|---|
| Discontinuous | 50 mL tube | Ficoll | 1984 | [26] |
| Discontinuous | 250 mL bottle | Ficoll | 1988 | [26] |
| Discontinuous | COBE2991 | Bovine serum albumin | 1989 | [29] |
| Continuous | tube | Bovine serum albumin (Mini-continuous gradient: 11 mL) | 1993 | [31] |
| Continuous | COBE2991 | Bovine serum albumin | 1993 | [32] |
| Continuous | COBE2991 | Ficoll | 1998 | [28] |
| Continuous | COBE2991 | Ficoll | 2000 | [1] |
| Continuous | COBE2991 | Iodixanol + MK solution | 2009 | [25] |
| Continuous | COBE2991 | Iodixanol + cold storage/purification stock solution | 2011 | [6] |
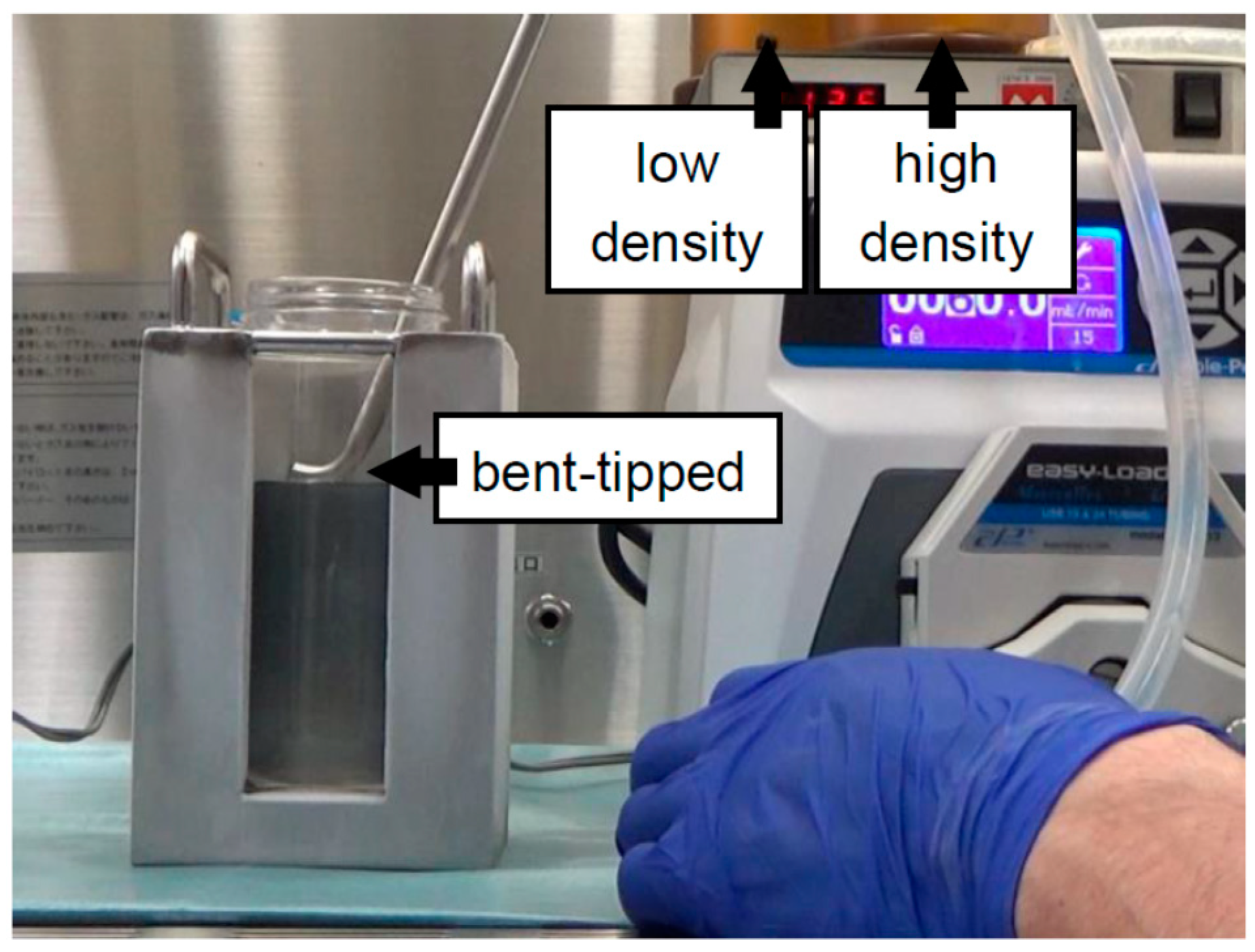
| Continuous | 500 mL bottle | Iodixanol + MK solution (Figure 2) | 2012 | [18] |
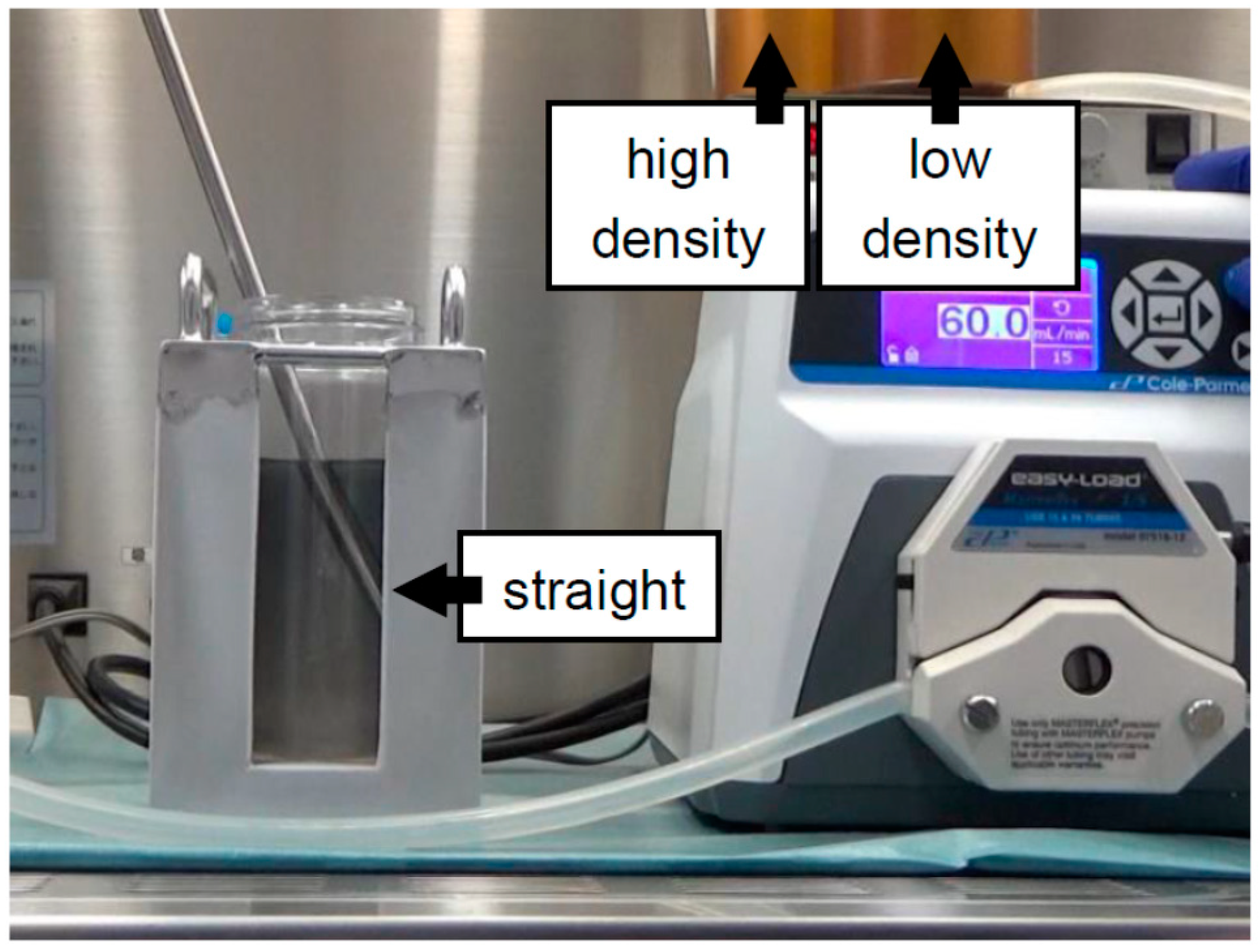
| Continuous | 500 mL bottle | Iodixanol + MK solution (Figure 3) | 2016 | [19] |
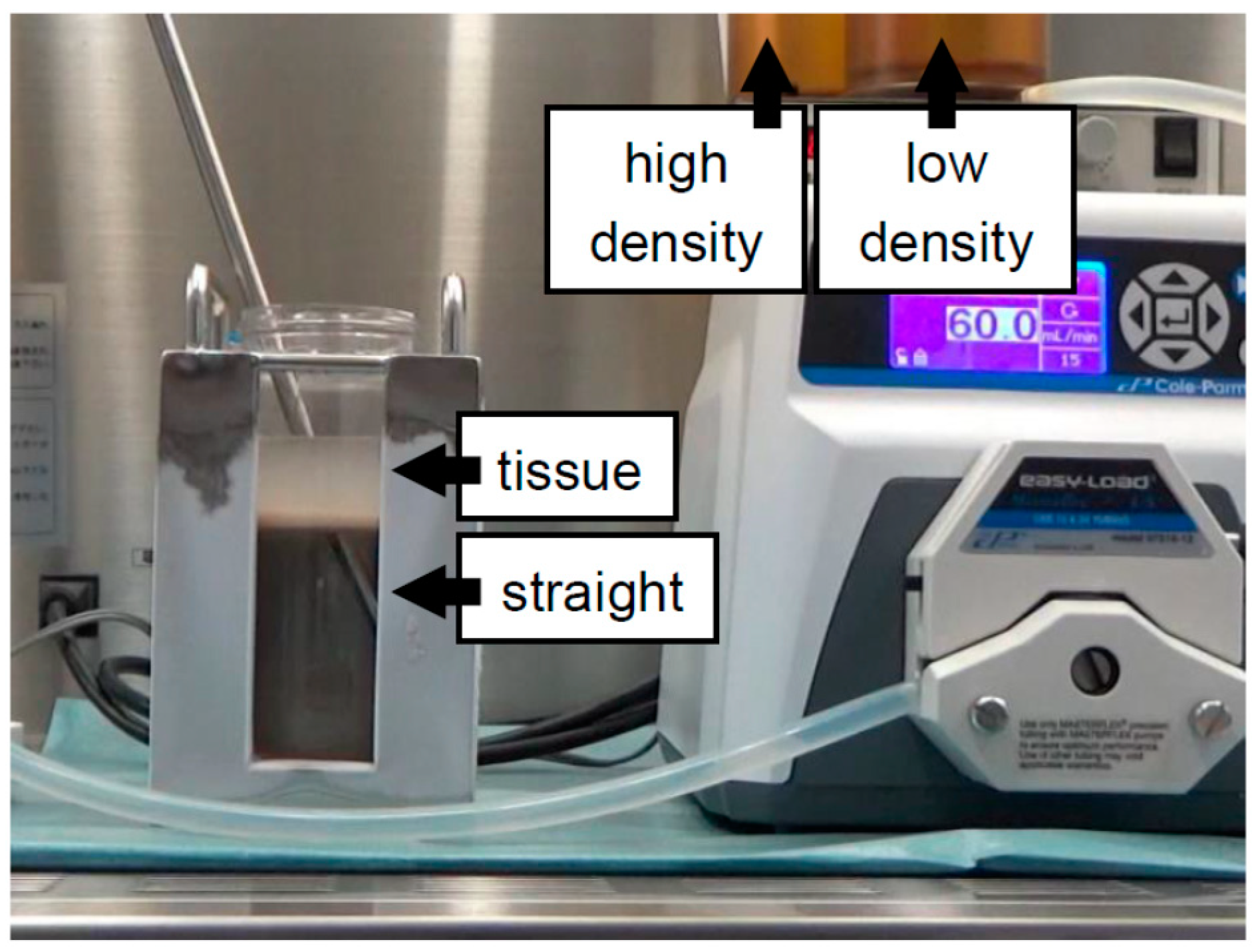
| Continuous | 500 mL bottle | Iodixanol + MK solution (Figure 4) | 2018 | [22] |
| Continuous | 500 mL bottle | Iodixanol + MK solution (Figure 5) | 2018 | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noguchi, H. Pancreatic Islet Purification from Large Mammals and Humans Using a COBE 2991 Cell Processor versus Large Plastic Bottles. J. Clin. Med. 2021, 10, 10. https://doi.org/10.3390/jcm10010010
Noguchi H. Pancreatic Islet Purification from Large Mammals and Humans Using a COBE 2991 Cell Processor versus Large Plastic Bottles. Journal of Clinical Medicine. 2021; 10(1):10. https://doi.org/10.3390/jcm10010010
Chicago/Turabian StyleNoguchi, Hirofumi. 2021. "Pancreatic Islet Purification from Large Mammals and Humans Using a COBE 2991 Cell Processor versus Large Plastic Bottles" Journal of Clinical Medicine 10, no. 1: 10. https://doi.org/10.3390/jcm10010010





