Exploring Submerged Forward Osmosis for Water Recovery and Pre-Concentration of Wastewater before Anaerobic Digestion: A Pilot Scale Study
Abstract
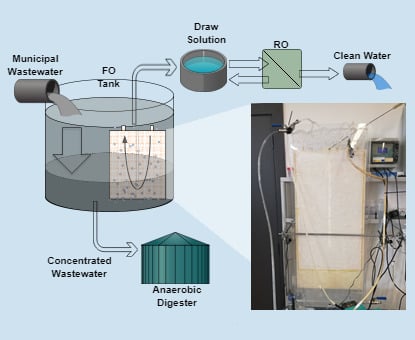
1. Introduction
2. Materials and Methods
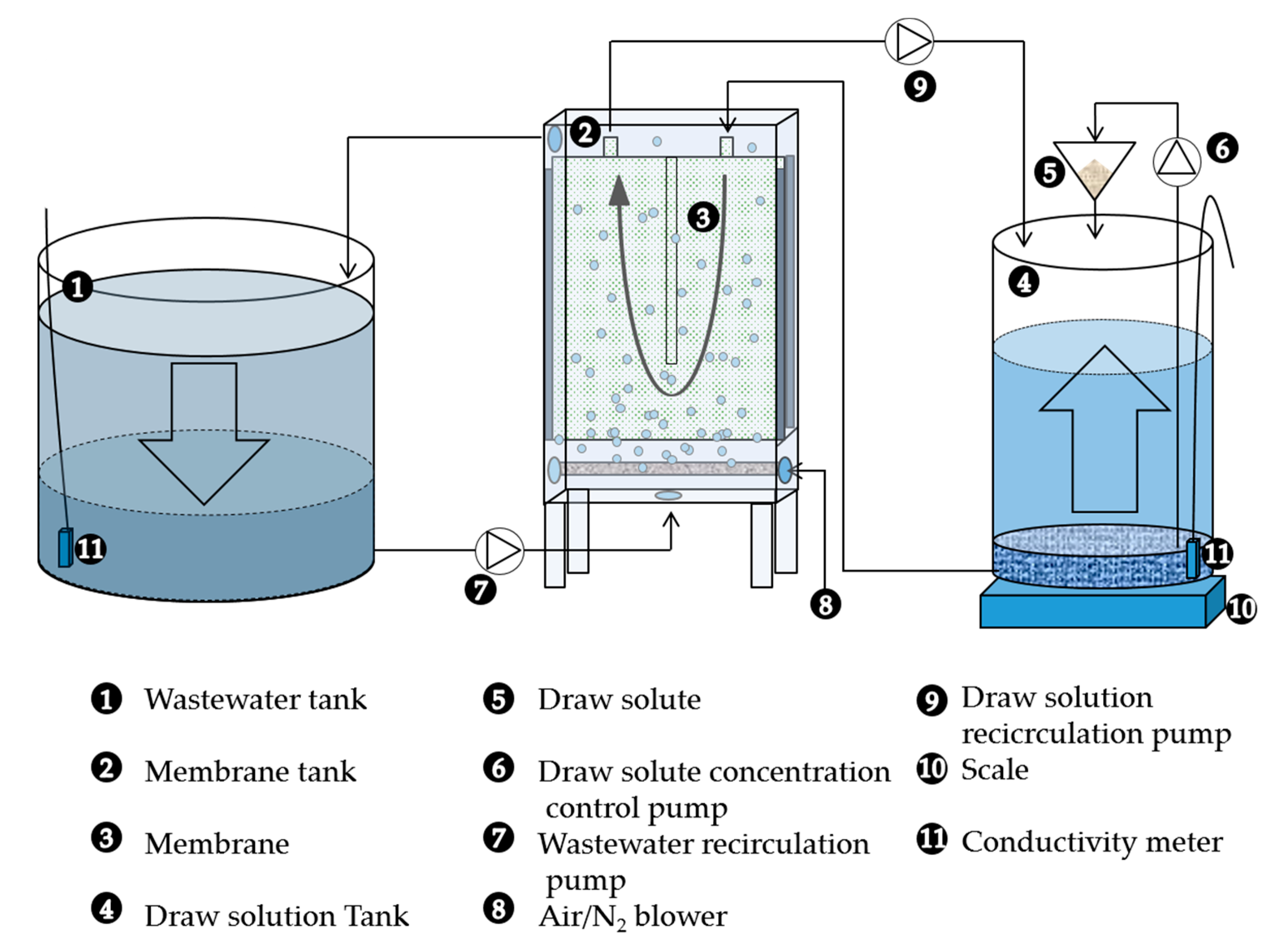
2.1. Pilot Set-up and Operating Conditions
2.2. Filtration Tests
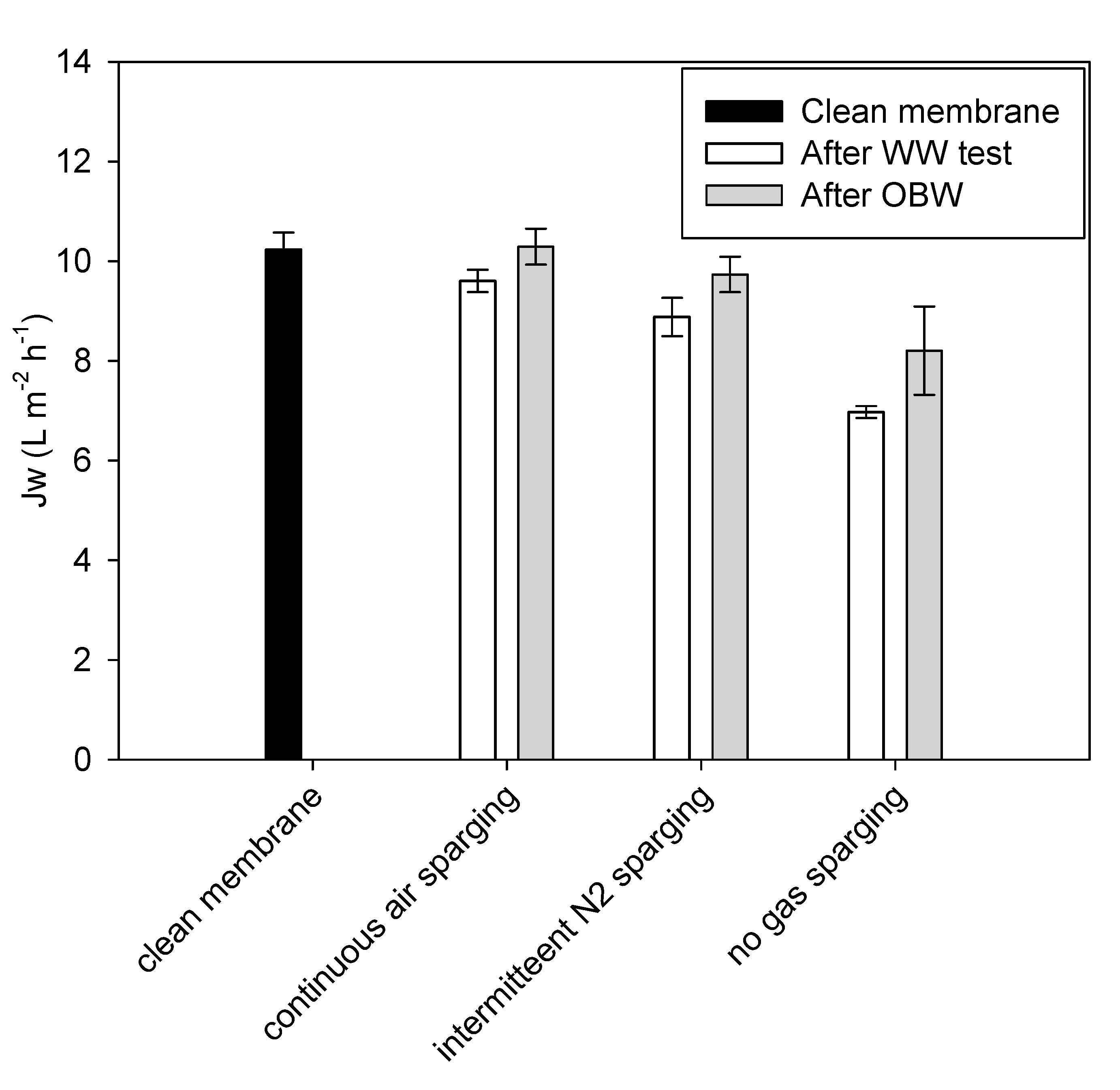
2.3. Assessment of Membrane Integrity and Performance
2.4. Chemical Analysis
3. Results and Discussion
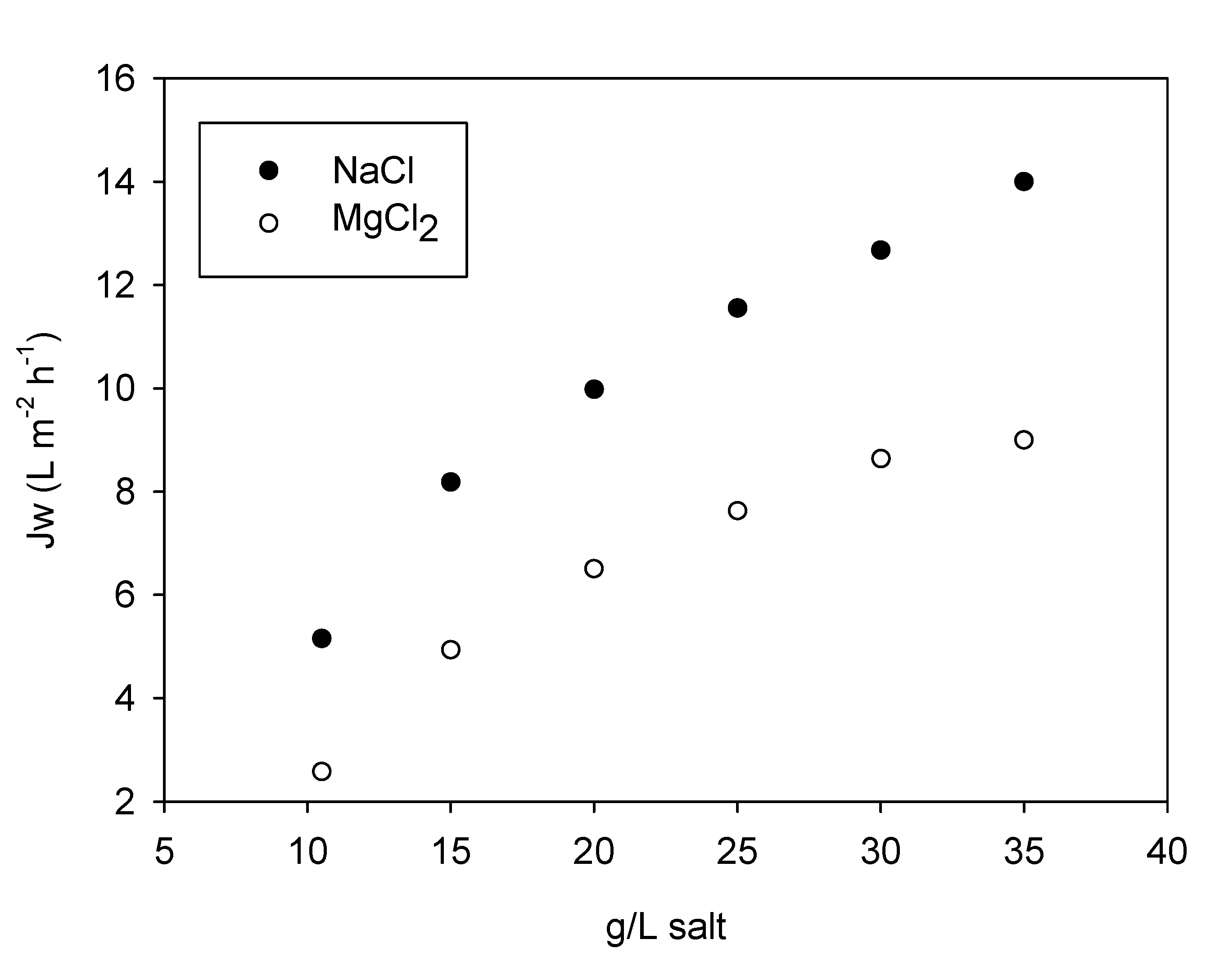
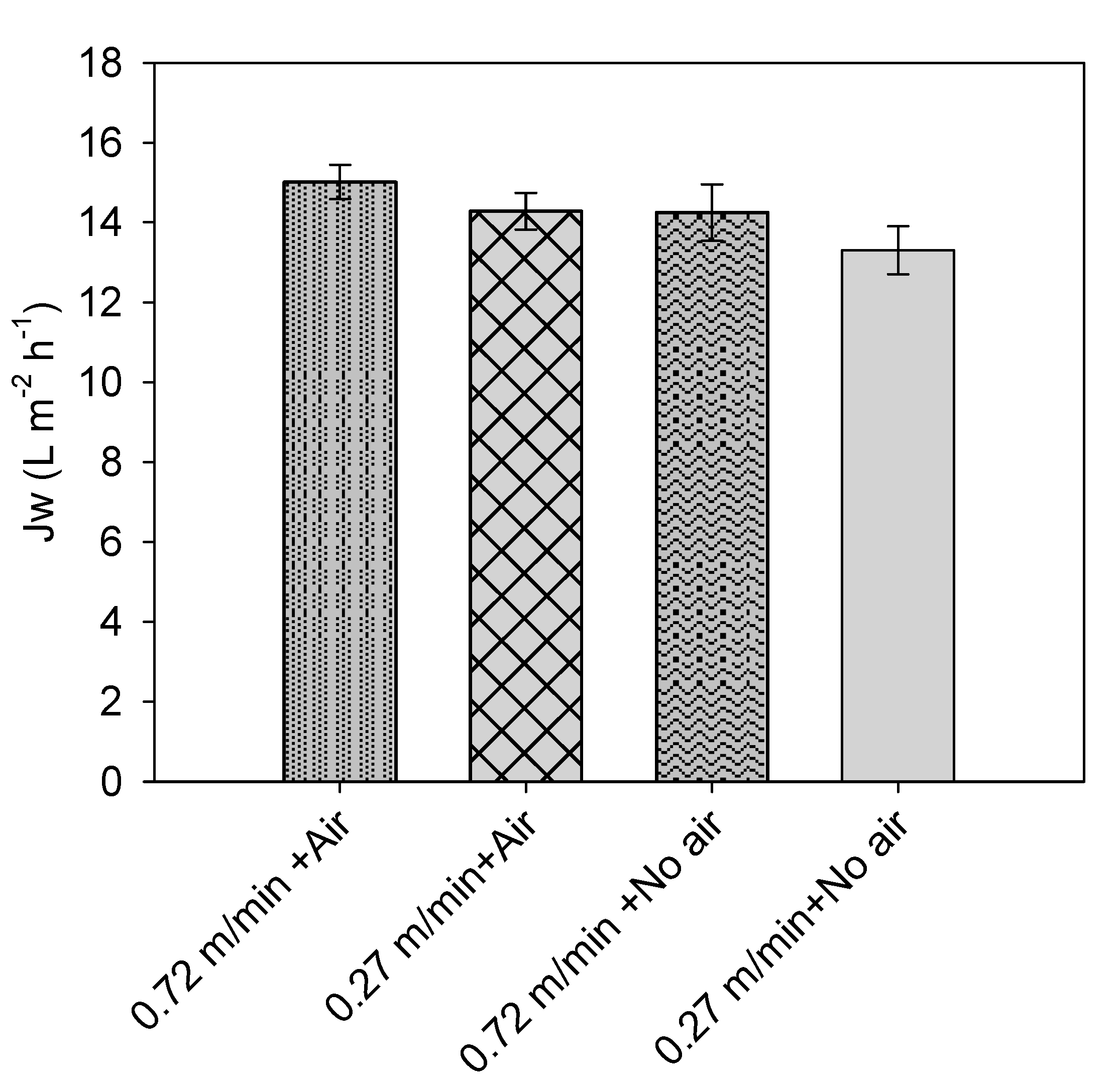
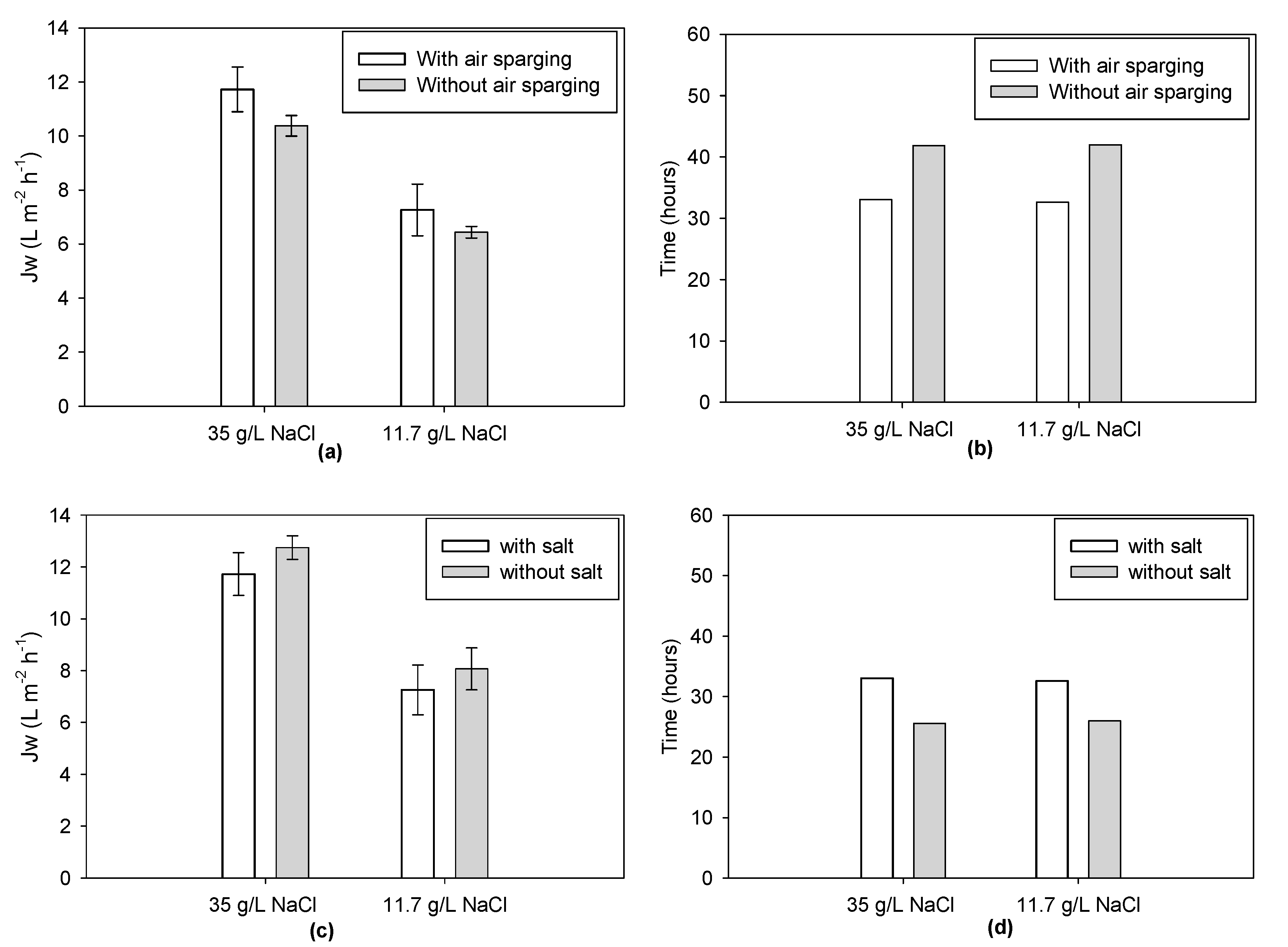
3.1. Tests with Tap Water
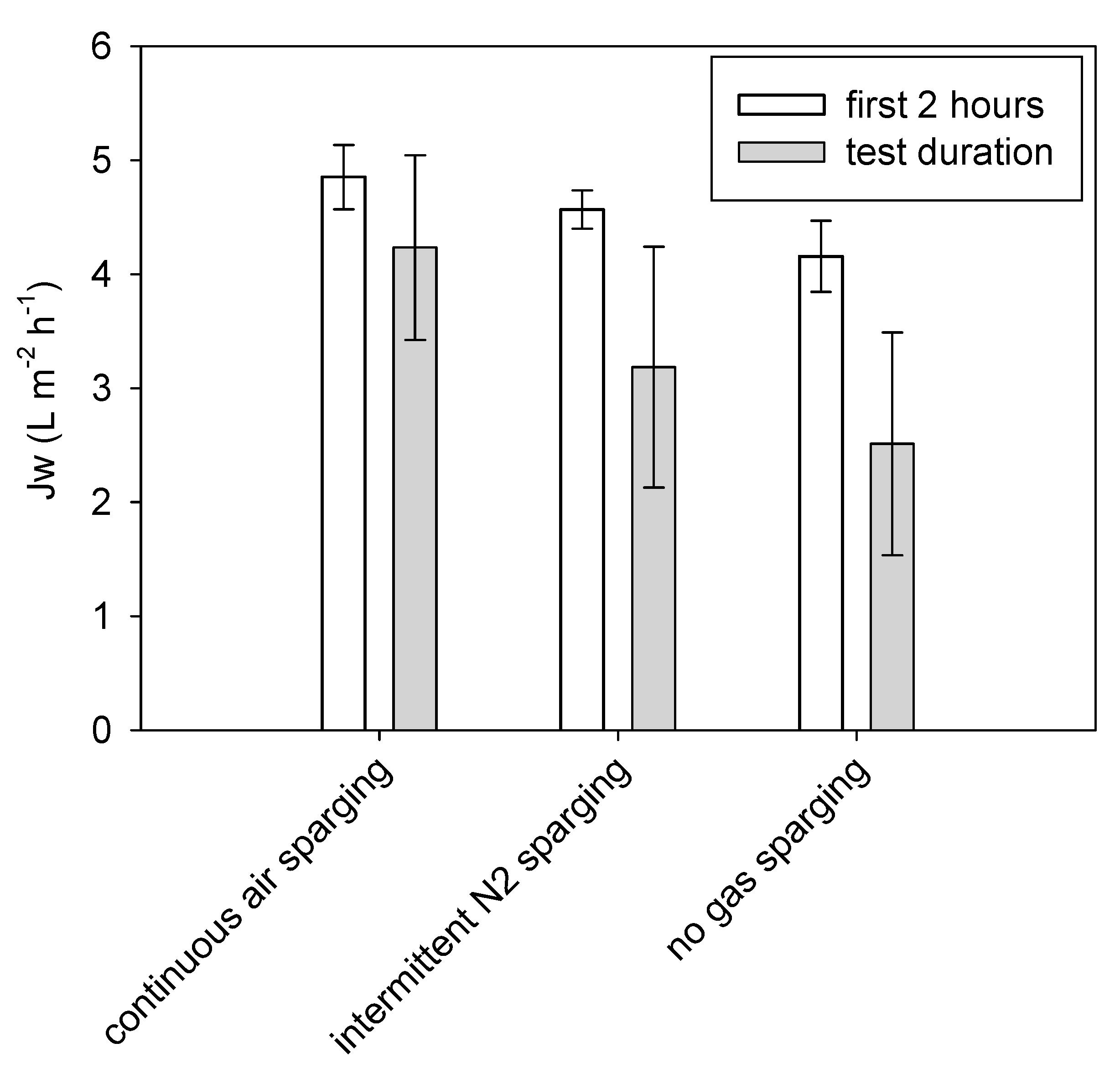
3.2. Tests with Municipal WW
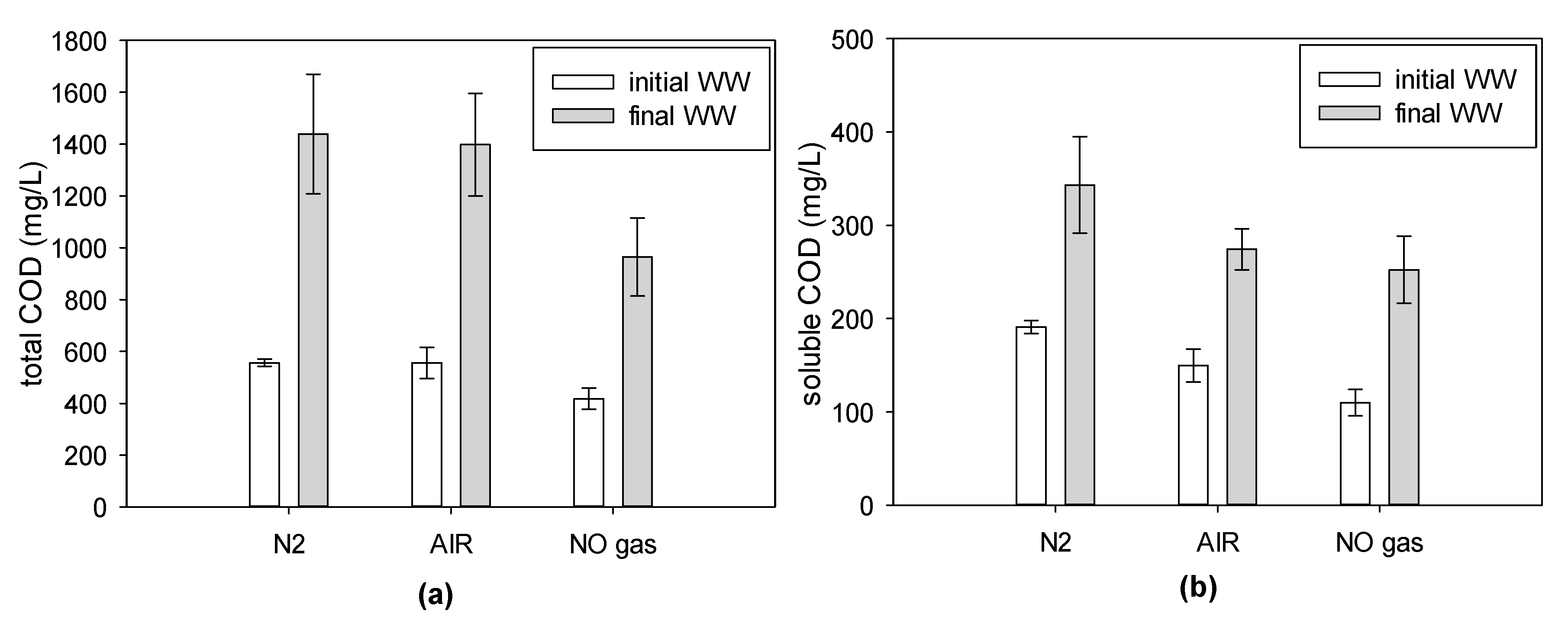
3.2.1. COD Concentration during the Filtration Batch
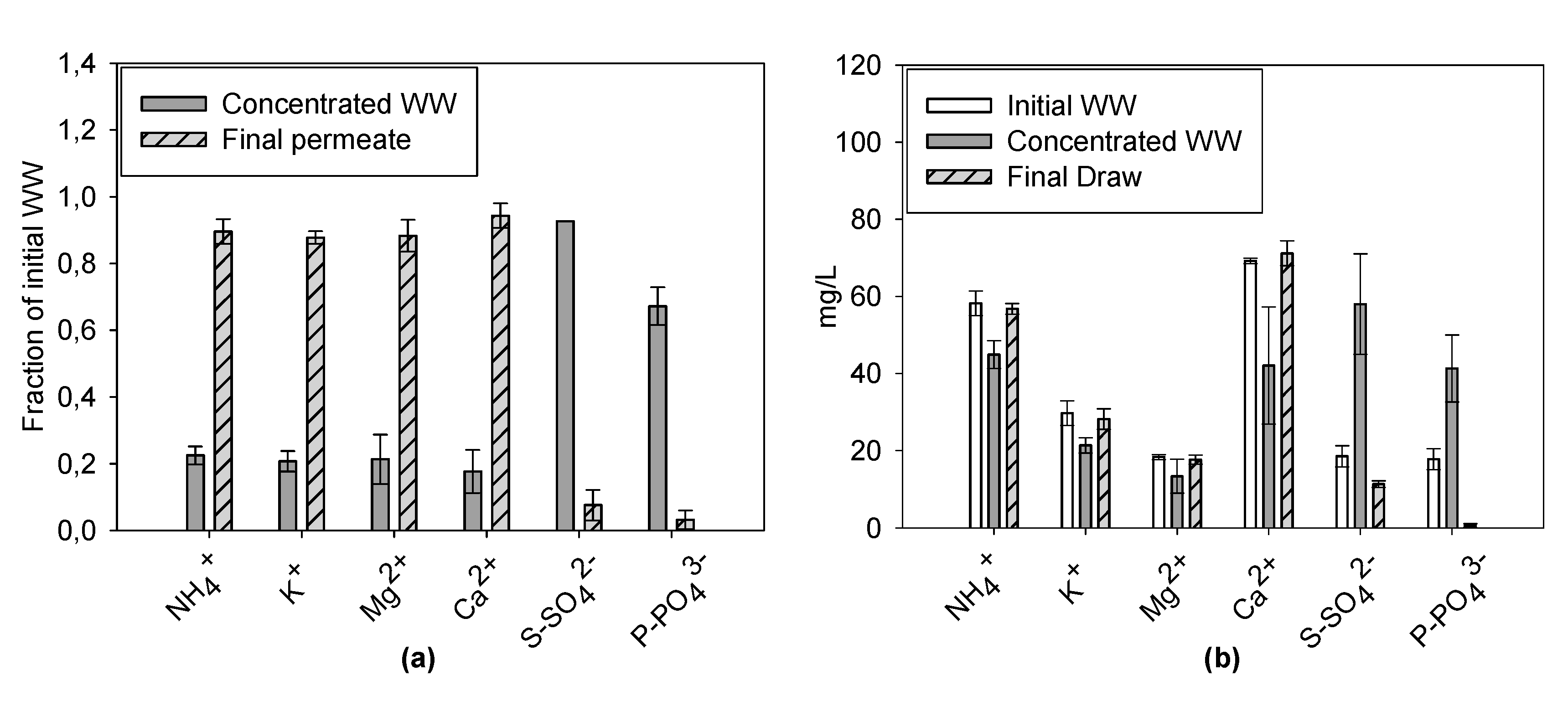
3.2.2. Ion Concentrations during Filtration Batch
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sowers, J.; Vengosh, A.; Weinthal, E. Climate change, water resources, and the politics of adaptation in the Middle East and North Africa. Clim. Chang. 2011, 104, 599–627. [Google Scholar] [CrossRef]
- Gao, D.-W.; Hu, Q.; Yao, C.; Ren, N.-Q. Treatment of domestic wastewater by an integrated anaerobic fluidized-bed membrane bioreactor under moderate to low temperature conditions. Bioresour. Technol. 2014, 159, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Yang, S.; Li, Y.-Y.; Wen, W.; Wang, X.C.; Chen, R. Application of anaerobic membrane bioreactors to municipal wastewater treatment at ambient temperature: A review of achievements, challenges, and perspectives. Bioresour. Technol. 2018, 267, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives future perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Korenak, J.; Basu, S.; Balakrishnan, M.; Hélix-Nielsen, C.; Petrinic, I. Forward Osmosis in Wastewater Treatment Processes. Acta Chim. Slov. 2017, 64, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.A.; She, Q.; Fane, A.G.; Field, R.W. Exploring the Differences between Forward Osmosis and Reverse Osmosis Fouling. J. Membr. Sci. 2018, 565, 241–253. [Google Scholar] [CrossRef]
- Holloway, R.; Childress, A.; Dennett, K.; Cath, T. Forward osmosis for concentration of anaerobic digester centrate. Water Res. 2007, 41, 4005–4014. [Google Scholar] [CrossRef]
- Onoda, S.; Masumori, Y.; Miyoshi, T.; Yasukawa, M.; Takahashi, T.; Matsuyama, H. Rejection of nutrients contained in an anaerobic digestion effluent using a forward osmosis membrane. Desalin. Water Treat. 2016, 57, 15748–15758. [Google Scholar] [CrossRef]
- Kim, Y.; Li, S.; Chekli, L.; Woo, Y.C.; Wei, C.-H.; Phuntsho, S.; Ghaffour, N.; Leiknes, T.; Shon, H.K. Assessing the removal of organic micro-pollutants from anaerobic membrane bioreactor effluent by fertilizer-drawn forward osmosis. J. Membr. Sci. 2017, 533, 84–95. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Y.; Cao, C.; Zhang, J.; Ng, J.-W.; Tang, C. Performance of a submerged anaerobic membrane bioreactor with forward osmosis membrane for low-strength wastewater treatment. Water Res. 2014, 50, 114–123. [Google Scholar] [CrossRef]
- Kim, Y.; Chekli, L.; Shim, W.-G.; Phuntsho, S.; Li, S.; Ghaffour, N.; Leiknes, T.; Shon, H.K. Selection of suitable fertilizer draw solute for a novel fertilizer-drawn forwardosmosis-anaerobic membrane bioreactor hybrid system. Bioresour. Technol. 2016, 210, 26–34. [Google Scholar] [CrossRef]
- Chang, H.-M.; Chen, S.-S.; Lu, M.-Y.; Duong, C.C.; Nguyen, N.C.; Chang, W.-S.; Ray, S.S. Mesophilic microfiltration–anaerobic osmotic membrane bioreactor–membrane distillation hybrid system for phosphorus recovery. J. Chem. Technol. Biotechnol. 2018, 94, 1230–1239. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, J.; Zhao, Z.; Shi, W.; Liu, D.; Cui, F. Membrane fouling of forward osmosis (FO) membrane for municipal wastewater treatment: A comparison between direct FO and OMBR. Water Res. 2016, 104, 330–339. [Google Scholar] [CrossRef]
- Hu, T.; Wang, X.; Wang, C.; Li, X.; Ren, Y. Impacts of inorganic draw solutes on the performance of thin- film composite forward osmosis membrane in a micro filtration assisted anaerobic osmotic membrane bioreactor. RSC Adv. 2017, 7, 16057–16063. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Drewes, J.E.; Nghiem, L.D. Forward osmosis as a platform for resource recovery from municipal wastewater—A critical assessment of the literature. J. Membr. Sci. 2017, 529, 195–206. [Google Scholar] [CrossRef]
- Morrow, C.P.; McGaughey, A.L.; Hiibel, S.R.; Childress, A.E. Submerged or sidestream? The influence of module configuration on fouling and salinity in osmotic membrane bioreactors. J. Membr. Sci. 2018, 548, 583–592. [Google Scholar] [CrossRef]
- Yoon, S.-H. Membrane Bioreactor Processes Principles and Applications; CRC Press: Hoboken, NJ, USA, 2015. [Google Scholar]
- Blandin, G.; Gautier, C.; Toran, M.S.; Monclús, H.; Rodriguez-Roda, I.; Comas, J. Retrofitting membrane bioreactor (MBR) into osmotic membrane bioreactor (OMBR): A pilot scale study. Chem. Eng. J. 2018, 339, 268–277. [Google Scholar] [CrossRef]
- Blandin, G.; Rodriguez-Roda, I.; Comas, J. Submerged Osmotic Processes: Design and Operation to Mitigate Mass Transfer Limitations. Membranes (Basel) 2018, 8, 72. [Google Scholar] [CrossRef]
- Kim, J., Blandin. Practical considerations for operability of an 8″ spiral wound forward osmosis module: Hydrodynamics, fouling behaviour and cleaning strategy. Desalination 2017, 404, 249–258. [Google Scholar] [CrossRef]
- Sauchelli, M.; Pellegrino, G.; D’Haese, A.; Rodriguez-Roda, I.; Gernjak, W. Transport of trace organic compounds through novel forward osmosis membranes: Role of membrane properties and the draw solution. Water Res. 2018, 141, 65–73. [Google Scholar] [CrossRef]
- Blandin, G. Can osmotic membrane bioreactor be a realistic solution for water reuse? NPJ Clean Water 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Blandin, G.; Rossellóa, B.; Monsalvoc, V.M.; Batlle-Vilanovac, P.; Viñasc, J.M.; Rogallac, F.; Comas, J. Volatile fatty acids concentration in real wastewater by forward osmosis. J. Membr. Sci. 2019, 575, 60–70. [Google Scholar] [CrossRef]
- Xue, W.; Tobino, T.; Nakajima, F.; Yamamoto, K. Seawater-driven forward osmosis for enriching nitrogen and phosphorous in treated municipal wastewater: Effect of membrane properties and feed solution chemistry. Water Res. 2015, 69, 120–130. [Google Scholar] [CrossRef]
- Geise, G.M.; Paul, D.R.; Freeman, B.D. Fundamental water and salt transport properties of polymeric materials. Prog. Polym. Sci. 2014, 39, 1–42. [Google Scholar] [CrossRef]
- Sung, S.; Liu, T. Ammonia inhibition on thermophilic anaerobic digestion. Chemosphere 2003, 53, 43–52. [Google Scholar] [CrossRef]
- Sun, W.; Shi, J.; Chen, C.; Li, N.; Xu, Z.; Li, J.; Lv, H.; Qian, X.; Zhao, L. A review on organic–inorganic hybrid nanocomposite membranes: A versatile tool to overcome the barriers of forward osmosis. RSC Adv. 2018, 8, 10040–10056. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, F.; Pijuan, M.; Rodriguez-Roda, I.; Blandin, G. Exploring Submerged Forward Osmosis for Water Recovery and Pre-Concentration of Wastewater before Anaerobic Digestion: A Pilot Scale Study. Membranes 2019, 9, 97. https://doi.org/10.3390/membranes9080097
Ferrari F, Pijuan M, Rodriguez-Roda I, Blandin G. Exploring Submerged Forward Osmosis for Water Recovery and Pre-Concentration of Wastewater before Anaerobic Digestion: A Pilot Scale Study. Membranes. 2019; 9(8):97. https://doi.org/10.3390/membranes9080097
Chicago/Turabian StyleFerrari, Federico, Maite Pijuan, Ignasi Rodriguez-Roda, and Gaetan Blandin. 2019. "Exploring Submerged Forward Osmosis for Water Recovery and Pre-Concentration of Wastewater before Anaerobic Digestion: A Pilot Scale Study" Membranes 9, no. 8: 97. https://doi.org/10.3390/membranes9080097
APA StyleFerrari, F., Pijuan, M., Rodriguez-Roda, I., & Blandin, G. (2019). Exploring Submerged Forward Osmosis for Water Recovery and Pre-Concentration of Wastewater before Anaerobic Digestion: A Pilot Scale Study. Membranes, 9(8), 97. https://doi.org/10.3390/membranes9080097