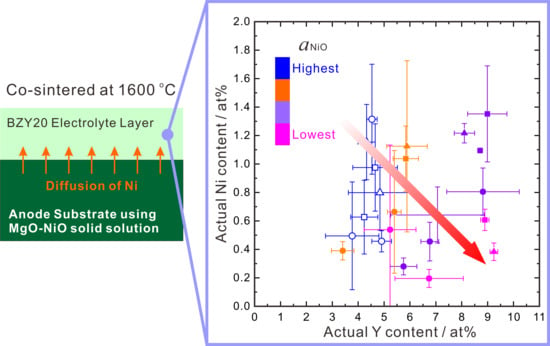
Correlation between Concentrations of Ni and Y in Y-Doped BaZrO3 Electrolyte in Co-Sintered Cells: A Case of Controlled NiO Activity by Using MgO-NiO Solid Solution as Anode Substrate
Abstract
1. Introduction
2. Experimental Methods
2.1. Material Preparation
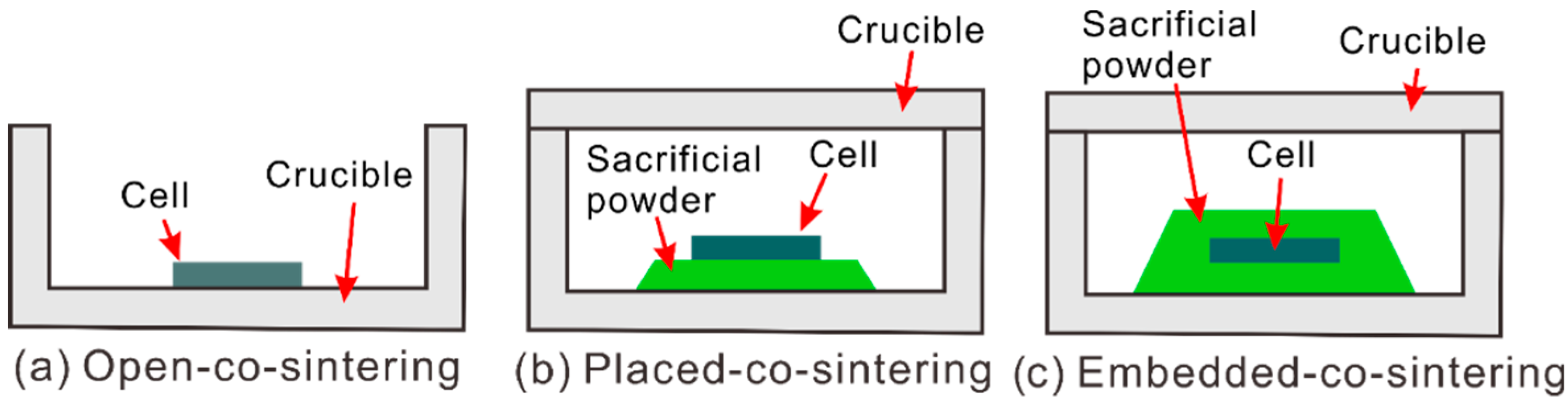
2.2. Half Cell Fabrication
2.3. Characterization
3. Results
3.1. Co-Sintered Half Cells
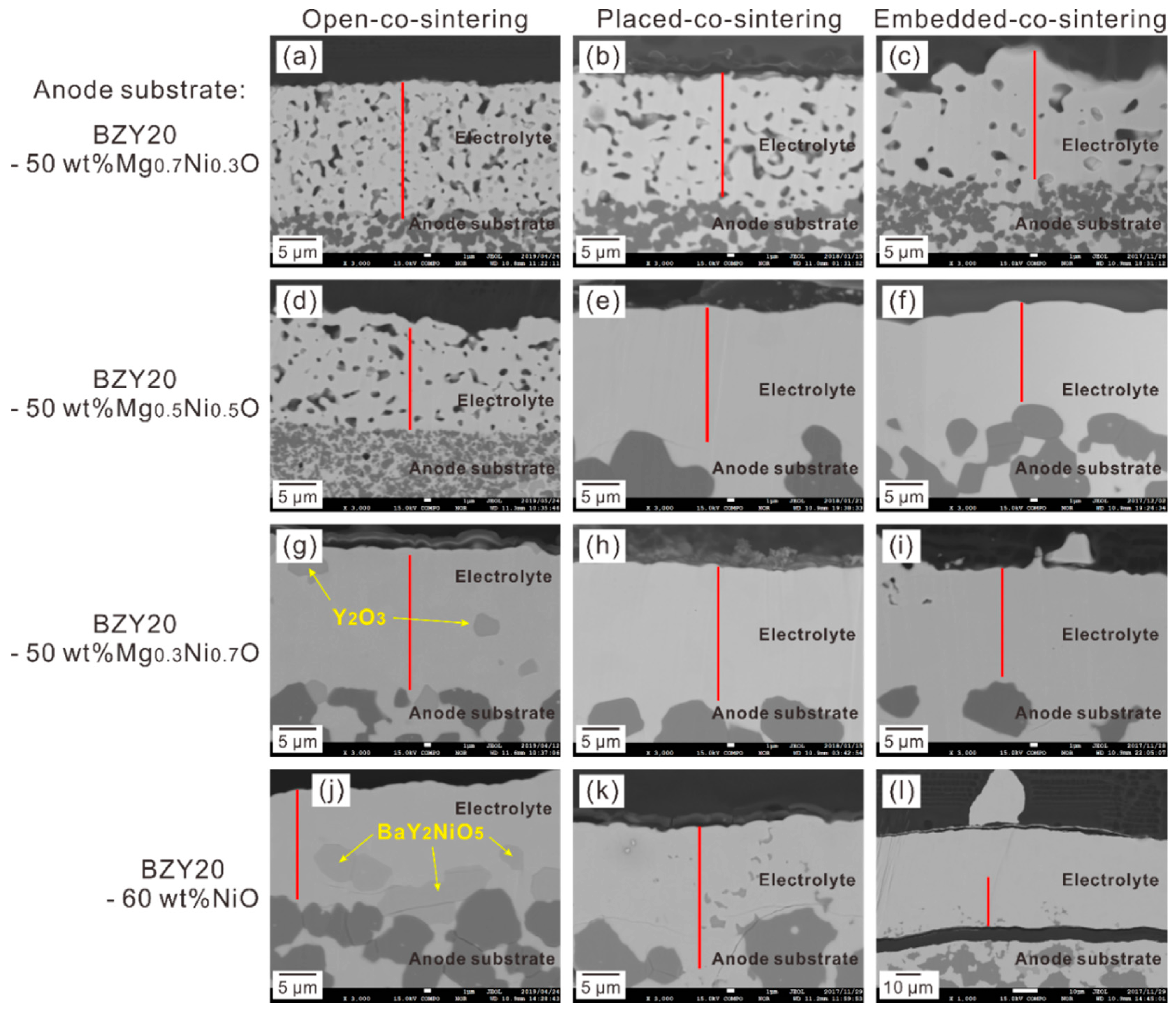
3.1.1. Morphology of Cross-Section Area
3.1.2. Phase Purity in Electrolyte
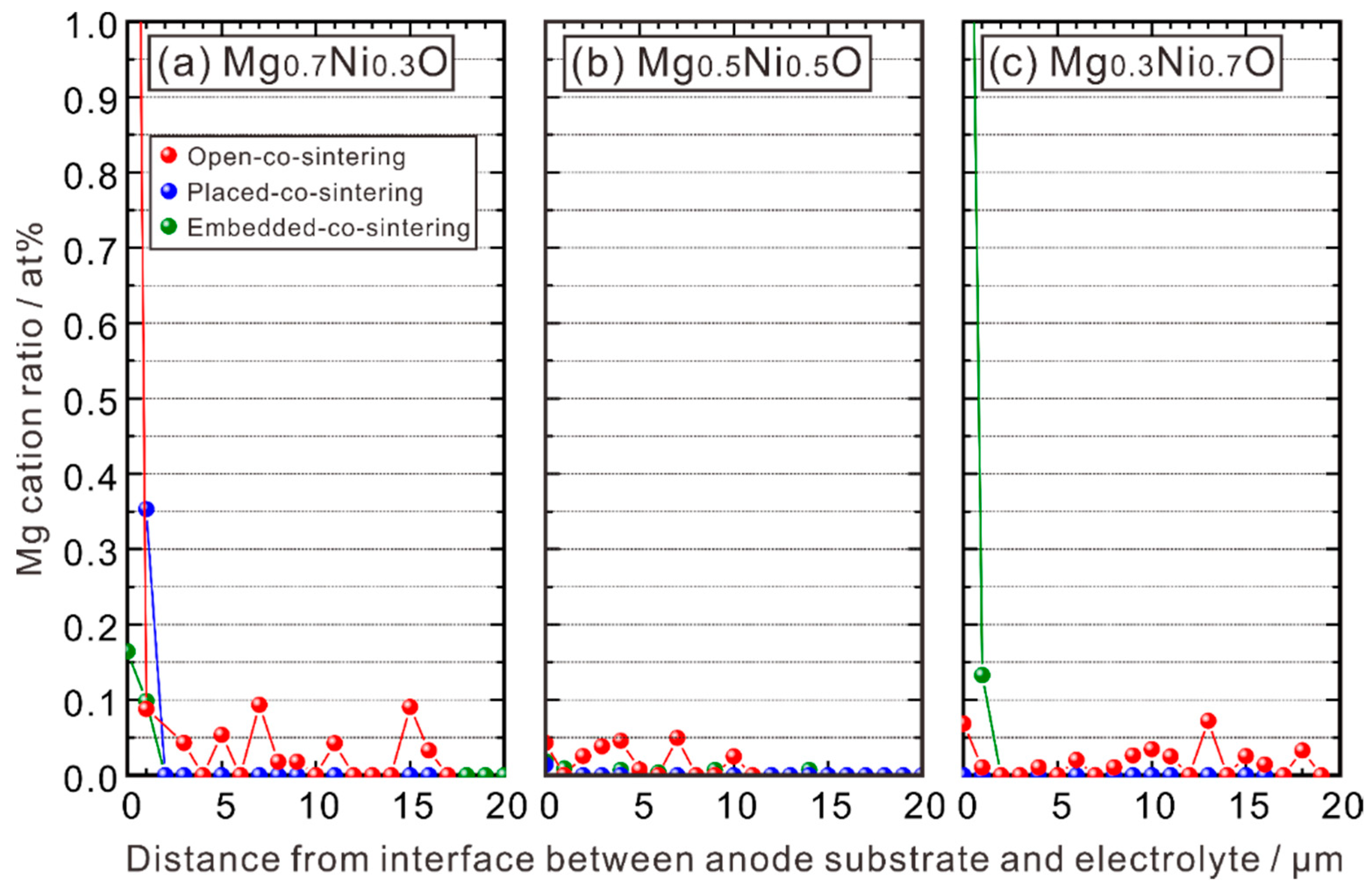
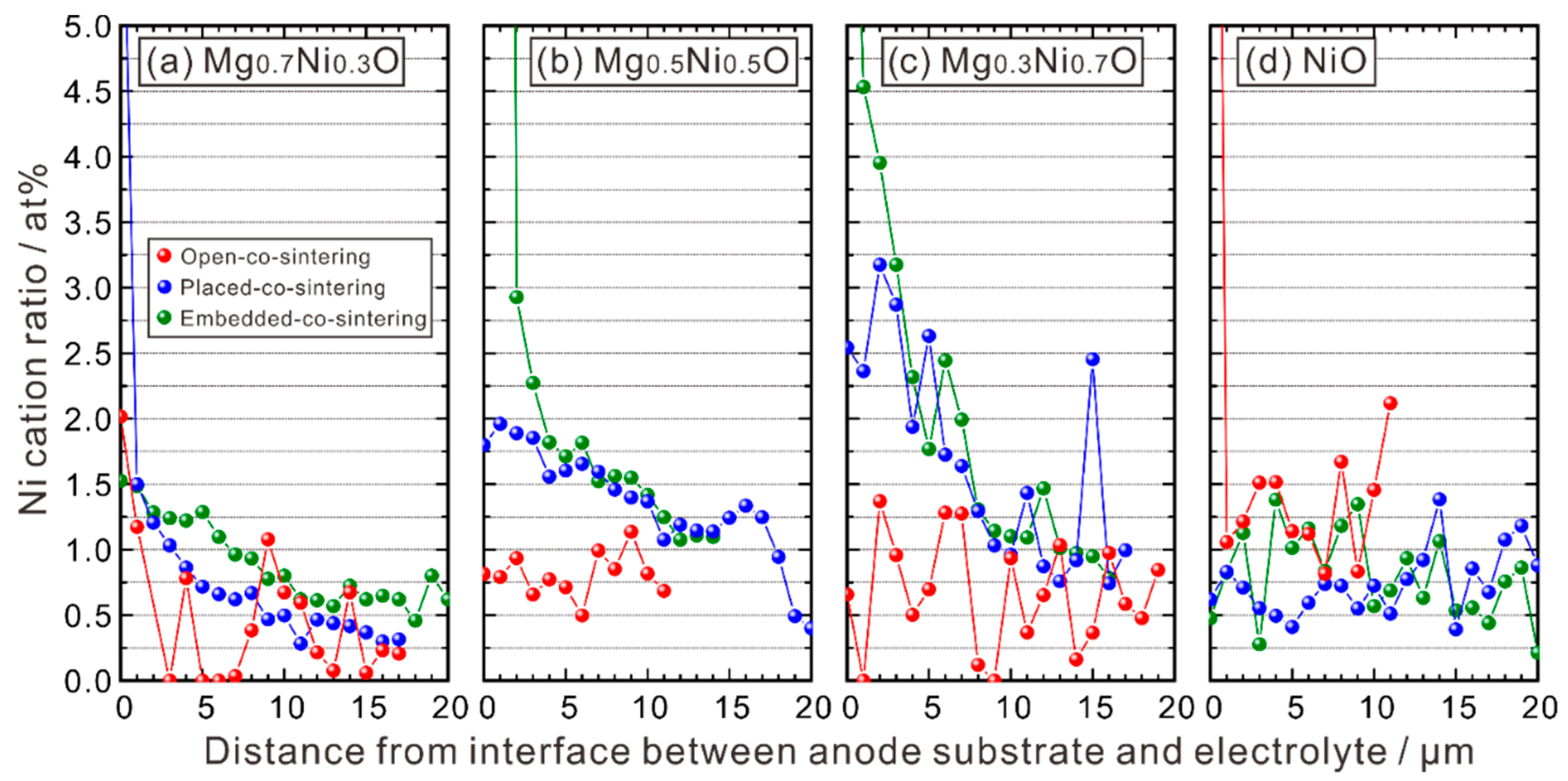
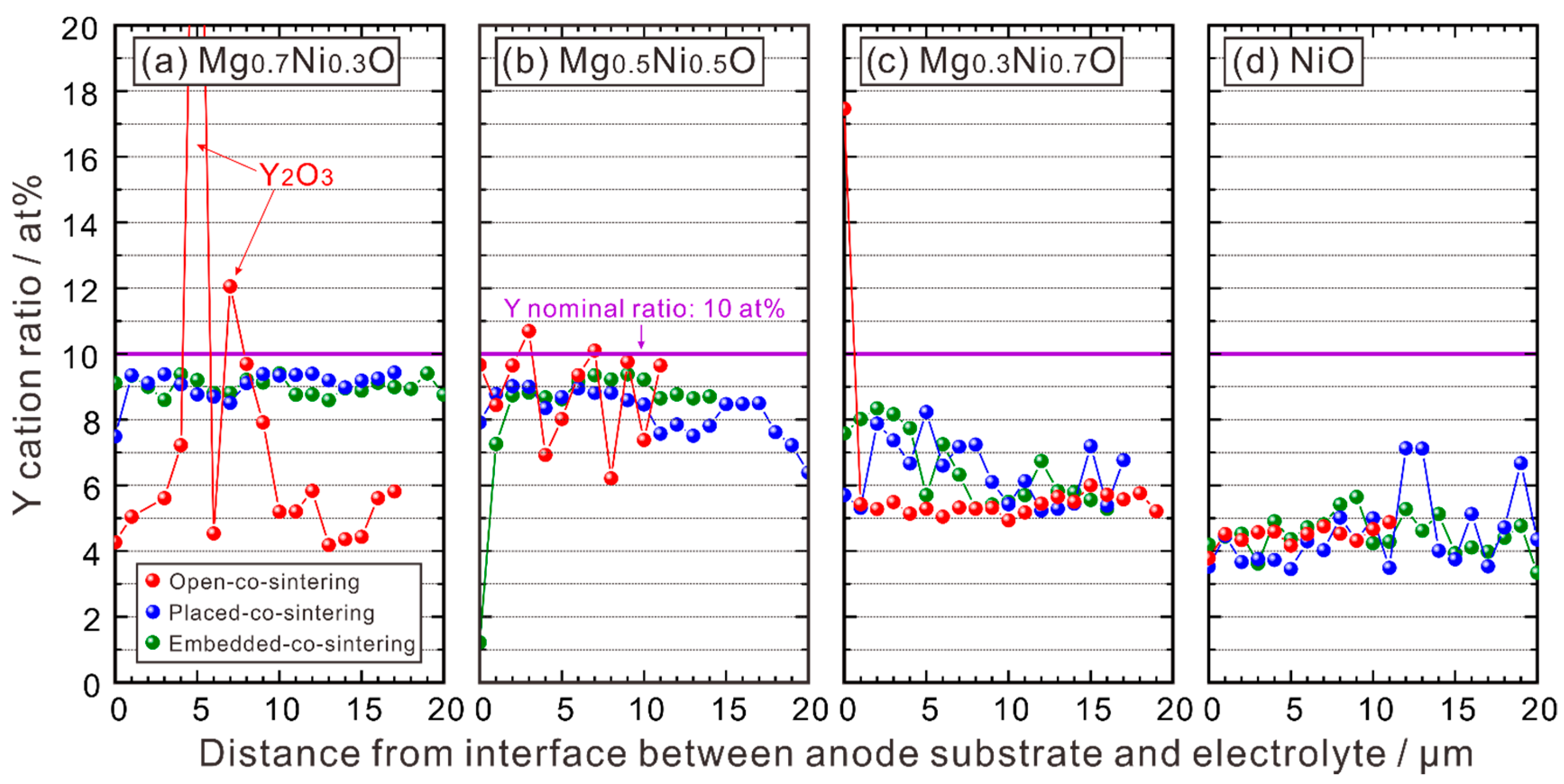
3.1.3. EPMA-WDS Line Analysis
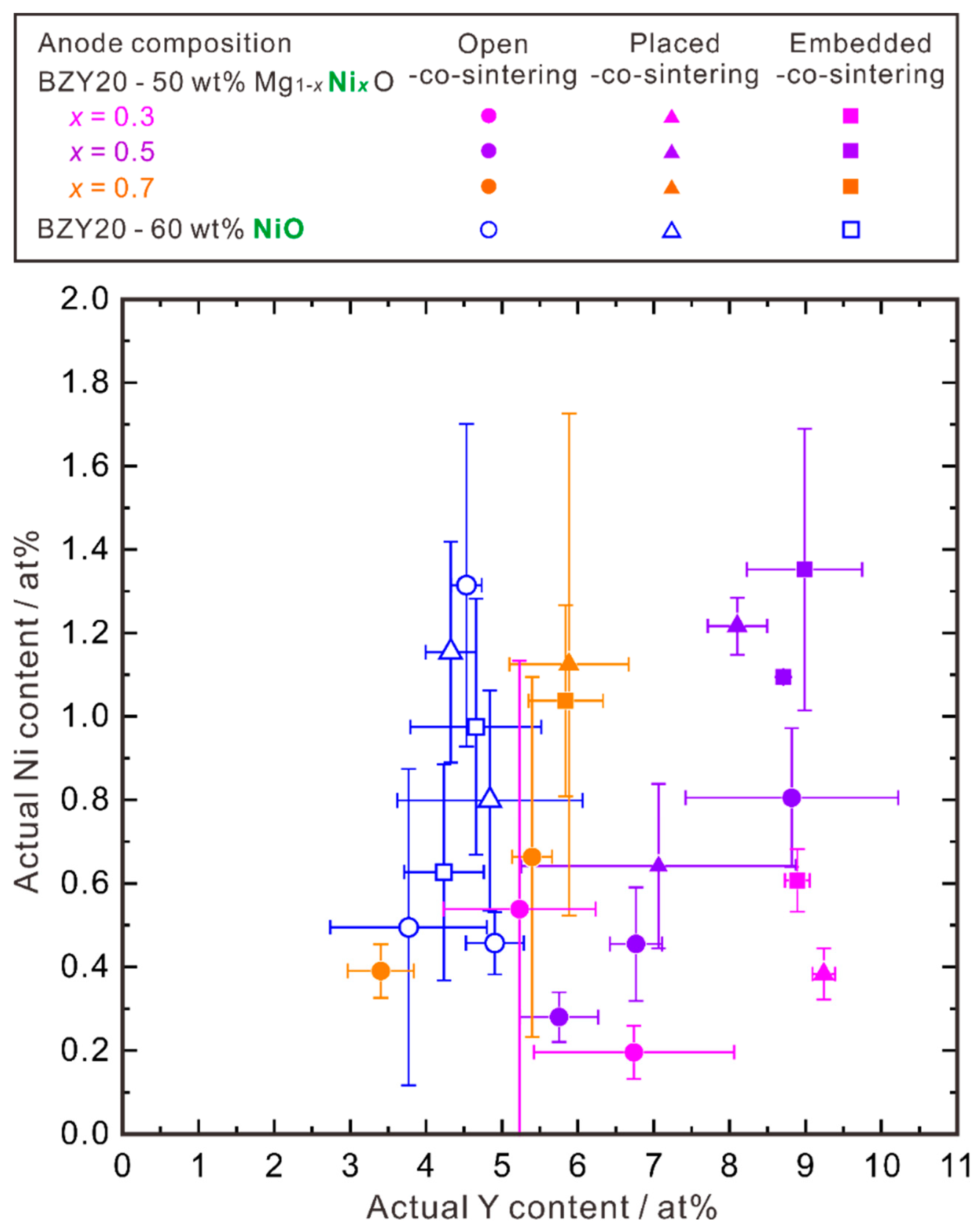
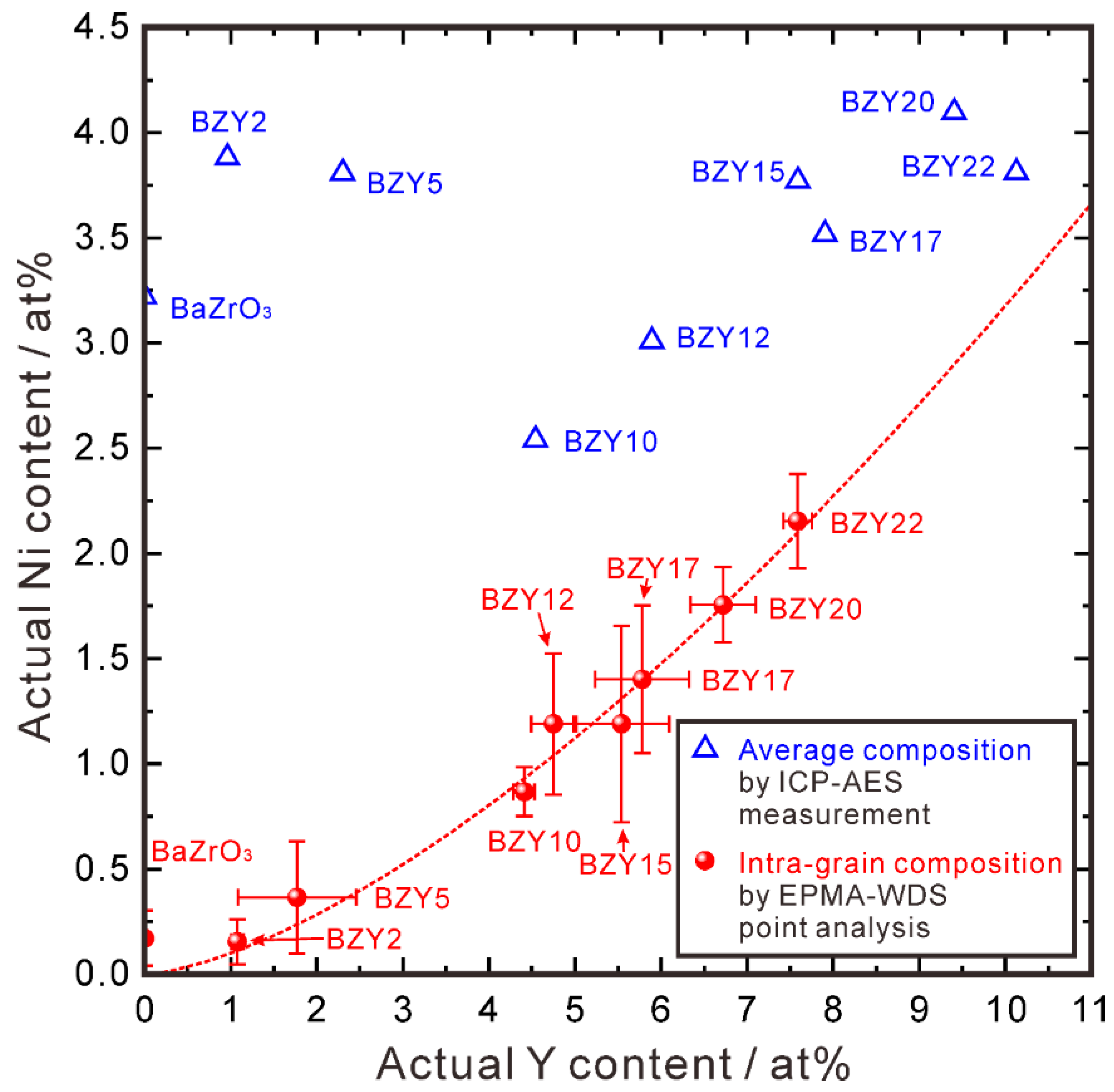
3.1.4. Relationship between Ni and Y Concentrations
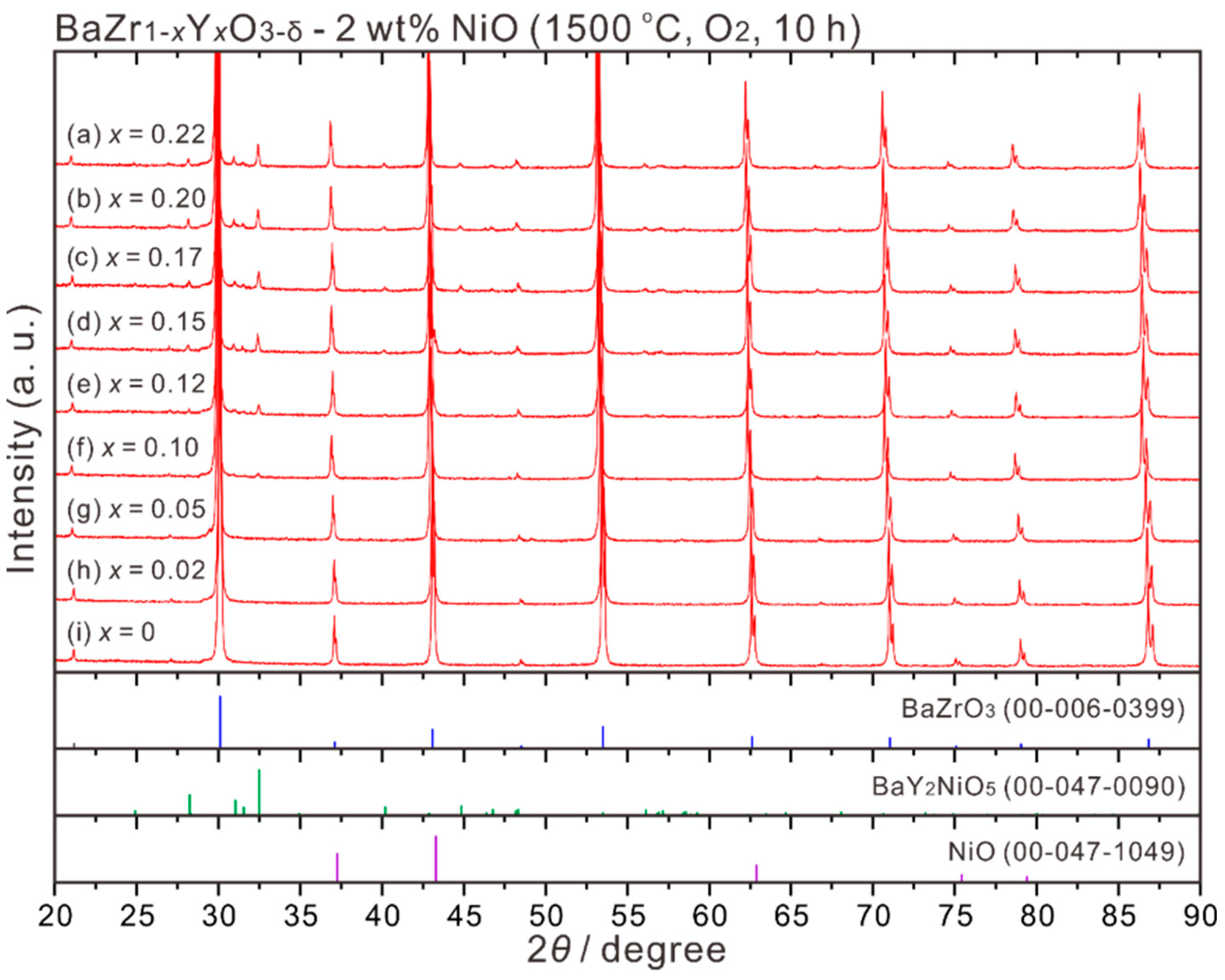
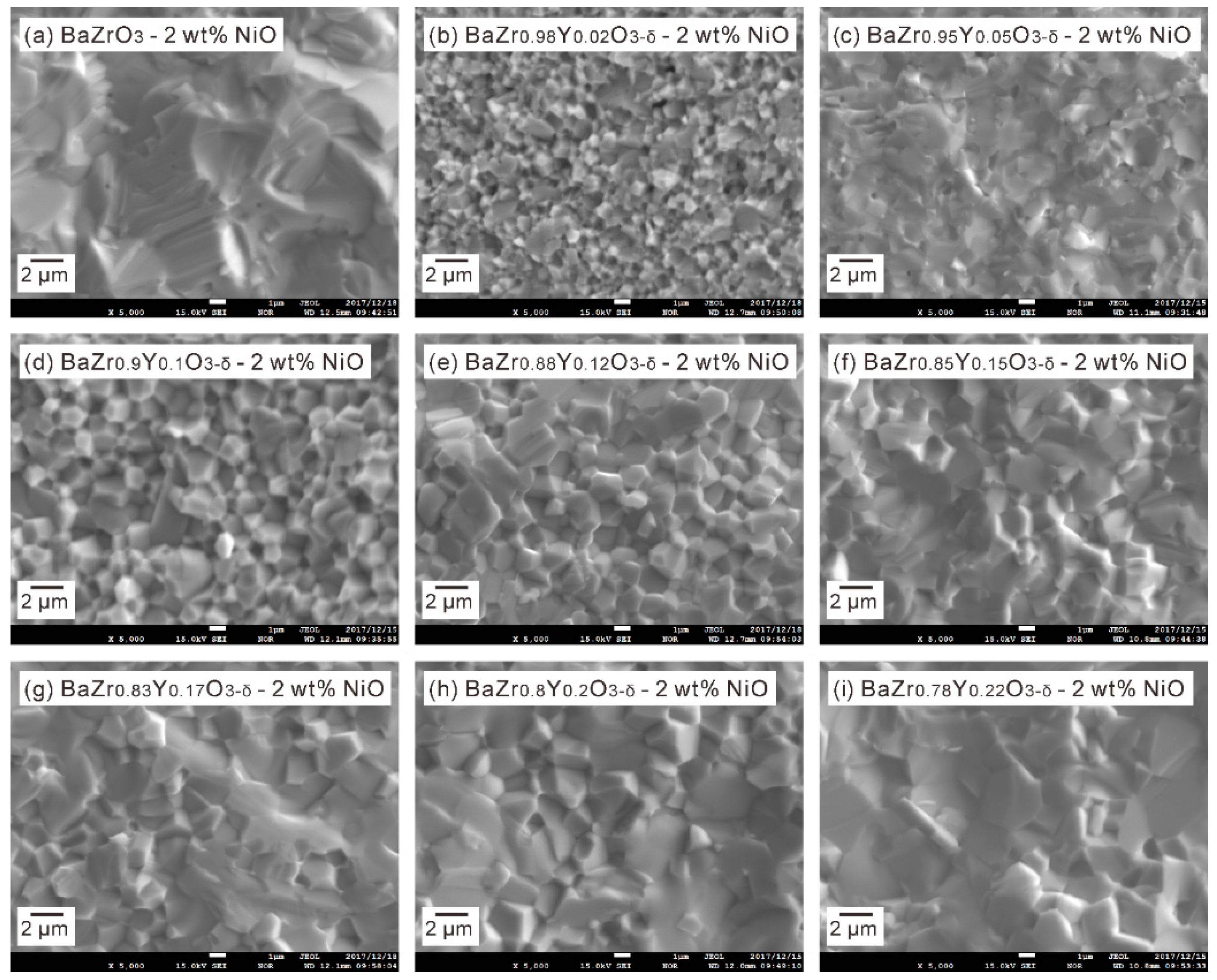
3.2. BaZr1−xYxO3−δ (x = 0–0.22)-2 wt % NiO Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technology. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Hernandez-Sanchez, R.; Haile, S.M. High total proton conductivity in large-grained yttrium-doped barium zirconate. Chem. Mater. 2009, 21, 2755–2762. [Google Scholar] [CrossRef]
- Han, D.; Shinoda, K.; Sato, S.; Majima, M.; Uda, T. Correlation between electroconductive and structural properties of proton conductive acceptor-doped barium zirconate. J. Mater. Chem. A 2015, 3, 1243–1250. [Google Scholar] [CrossRef]
- Han, D.; Uda, T. The best composition of an Y-doped BaZrO3 electrolyte: Selection criteria from transport properties, microstructure, and phase behavior. J. Mater. Chem. A 2018, 6, 18571–18582. [Google Scholar] [CrossRef]
- Katahira, K.; Kohchi, Y.; Shimura, T.; Iwahara, H. Protonic conduction in Zr-substituted BaCeO3. Solid State Ion. 2000, 138, 91–98. [Google Scholar] [CrossRef]
- Guo, Y.; Lin, Y.; Shi, H.; Ran, R.; Shao, Z. A high electrochemical performance proton conductor electrolyte with CO2 Tolerance. Chin. J. Catal. 2009, 30, 479–481. [Google Scholar] [CrossRef]
- Ricote, S.; Bonanos, N.; Caboche, G. Water vapour solubility and conductivity study of the proton conductor BaCe0.9-xZrxY0.1O3-δ. Solid State Ion. 2009, 180, 990–997. [Google Scholar] [CrossRef]
- Han, D.; Uemura, S.; Hiraiwa, C.; Majima, M.; Uda, T. Detrimental effect of sintering additives on conducting ceramics: Yttrium-doped barium zirconate. ChemSusChem 2018, 11, 4102–4113. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Kuramitsu, A.; Onishi, T.; Noda, Y.; Majima, M.; Uda, T. Fabrication of protonic ceramic fuel cells via infiltration with Ni nanoparticles: A new strategy to suppress NiO diffusion increase open circuit voltage. Solid State Ion. Submitted.
- Ueno, K.; Hatada, N.; Han, D.; Uda, T. Thermodynamic maximum of Y doping level in barium zirconate in co-sintering with NiO. J. Mater. Chem. A 2019, 7, 7232–7241. [Google Scholar] [CrossRef]
- Han, D.; Shinoda, K.; Tsukimoto, S.; Takeuchi, H.; Hiraiwa, C.; Majima, M.; Uda, T. Origins of structural and electrochemical influence on Y-doped BaZrO3 heat-treated with NiO additive. J. Mater. Chem. A 2560, 2, 12552–12560. [Google Scholar] [CrossRef]
- Dai, H.; Kou, H.; Wang, H.; Bi, L. Electrochemical performance of protonic ceramic fuel cells with stable BaZrO3-based electrolyte: A mini-review. Electrochem. Commun. 2018, 96, 11–15. [Google Scholar] [CrossRef]
- Chen, M.; Chen, M.; Wang, K.; Xu, Q. Densification and electrical conducting behavior of BaZr0.9Y0.1O3-δ proton conducting ceramics with NiO additive. J. Alloy Compd. 2019, 781, 857–865. [Google Scholar] [CrossRef]
- Han, D.; Hatada, N.; Uda, T. Chemical expansion of yttrium-doped barium zirconate and correlation with proton concentration and conductivity. J. Am. Ceram. Soc. 2016, 99, 3745–3753. [Google Scholar] [CrossRef]
- Shirane, Y.; Nabika, S.; Sakamoto, S.; Nakashima, I. Activity measurement in the oxide solid solutions of NiO-MgO and NiO-MgO-SiO2 systems in the temperature range between 1073 and 1273 K. Int. J. Min. Process. 1987, 19, 237–251. [Google Scholar] [CrossRef]
- Fukui, T.; Okumura, K.; Yamamoto, Y.; Kubo, Y. Development of SOFC electrode using NiO-MgO solid solution. Resour. Process. 1991, 38, 21–25. [Google Scholar] [CrossRef]
- Han, D.; Nose, Y.; Shinoda, K.; Uda, T. Site selectivity of dopants in BaZr1-yMyO3-δ (M = Sc, Y, Sm, Eu, Dy) and measurement of their water contents and conductivities. Solid State Ion. 2012, 213, 2–7. [Google Scholar] [CrossRef]
- Han, D.; Otani, Y.; Noda, Y.; Onishi, T.; Majima, M.; Uda, T. Strategy to improve phase compatibility between proton conductive BaZr0.8Y0.2O3-δ and nickel oxide. RSC Adv. 2016, 6, 19288–19297. [Google Scholar] [CrossRef]
- Onishi, T.; Han, D.; Noda, Y.; Hatada, N.; Majima, M.; Uda, T. Evaluation of performance and durability of Ni-BZY cermet electrodes with BZY electrolyte. Solid State Ion. 2018, 317, 127–135. [Google Scholar] [CrossRef]
- Babilo, P.; Uda, T.; Haile, S.M. Processing of yttrium-doped barium zirconate for high proton conductivity. J. Mater. Res. 2007, 22, 1322–1330. [Google Scholar] [CrossRef]
- Tong, J.; Clark, D.; Bernau, L.; Sanders, M.; O’Hayre, R. Solid-state reactive sintering mechanism for large-grained yttrium-doped barium zirconate proton conducting ceramics. J. Mater. Chem. 2010, 20, 6333–6341. [Google Scholar] [CrossRef]
- Narendar, N.; Mather, G.C.; Dias, P.A.N.; Fagg, D.P. The importance of phase impurity in Ni-BaZr0.85Y0.15O3-δ cermet anodes—Novel nitrate-free combustion route and electrochemical study. RSC Adv. 2013, 3, 859–869. [Google Scholar] [CrossRef]
- Oyama, Y.; Kojima, A.; Li, X.; Cervera, R.B.; Tanaka, K.; Yamaguchi, S. Phase relation in the BaO–ZrO2–YO1.5 system: Presence of separate BaZrO3 phases and complexity in phase formation. Solid State Ion. 2011, 197, 1–12. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hernandez-Sanchez, R.; Haile, S.M. Cation non-stoichiometry in yttrium-doped barium zirconate: Phase behavior, microstructure, and proton conductivity. J. Mater. Chem. 2010, 20, 8158–8166. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Kuno, K.; Uda, T. Correlation between Concentrations of Ni and Y in Y-Doped BaZrO3 Electrolyte in Co-Sintered Cells: A Case of Controlled NiO Activity by Using MgO-NiO Solid Solution as Anode Substrate. Membranes 2019, 9, 95. https://doi.org/10.3390/membranes9080095
Han D, Kuno K, Uda T. Correlation between Concentrations of Ni and Y in Y-Doped BaZrO3 Electrolyte in Co-Sintered Cells: A Case of Controlled NiO Activity by Using MgO-NiO Solid Solution as Anode Substrate. Membranes. 2019; 9(8):95. https://doi.org/10.3390/membranes9080095
Chicago/Turabian StyleHan, Donglin, Kenji Kuno, and Tetsuya Uda. 2019. "Correlation between Concentrations of Ni and Y in Y-Doped BaZrO3 Electrolyte in Co-Sintered Cells: A Case of Controlled NiO Activity by Using MgO-NiO Solid Solution as Anode Substrate" Membranes 9, no. 8: 95. https://doi.org/10.3390/membranes9080095
APA StyleHan, D., Kuno, K., & Uda, T. (2019). Correlation between Concentrations of Ni and Y in Y-Doped BaZrO3 Electrolyte in Co-Sintered Cells: A Case of Controlled NiO Activity by Using MgO-NiO Solid Solution as Anode Substrate. Membranes, 9(8), 95. https://doi.org/10.3390/membranes9080095