Role of Cellulose Micro and Nano Crystals in Thin Film and Support Layer of Nanocomposite Membranes for Brackish Water Desalination
Abstract
1. Introduction
2. Experimental Work
2.1. Materials
2.2. Cellulose Micro and Nano Crystal Characterizations
2.3. Preparation of the Support Layer
2.4. Preparation of the Thin Membrane
2.5. Characterizations of the Thin Film Membrane
3. Results and Discussion
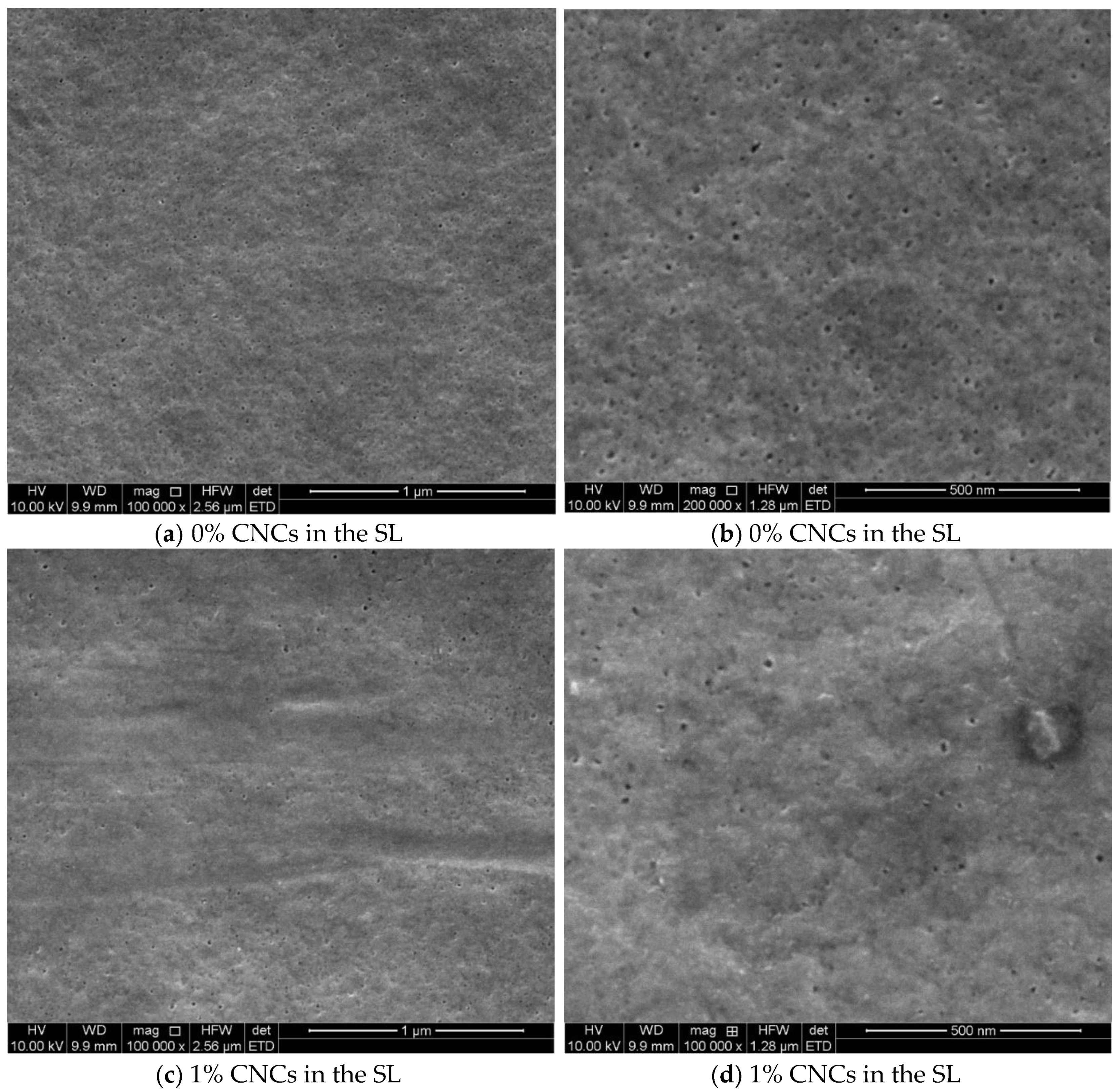
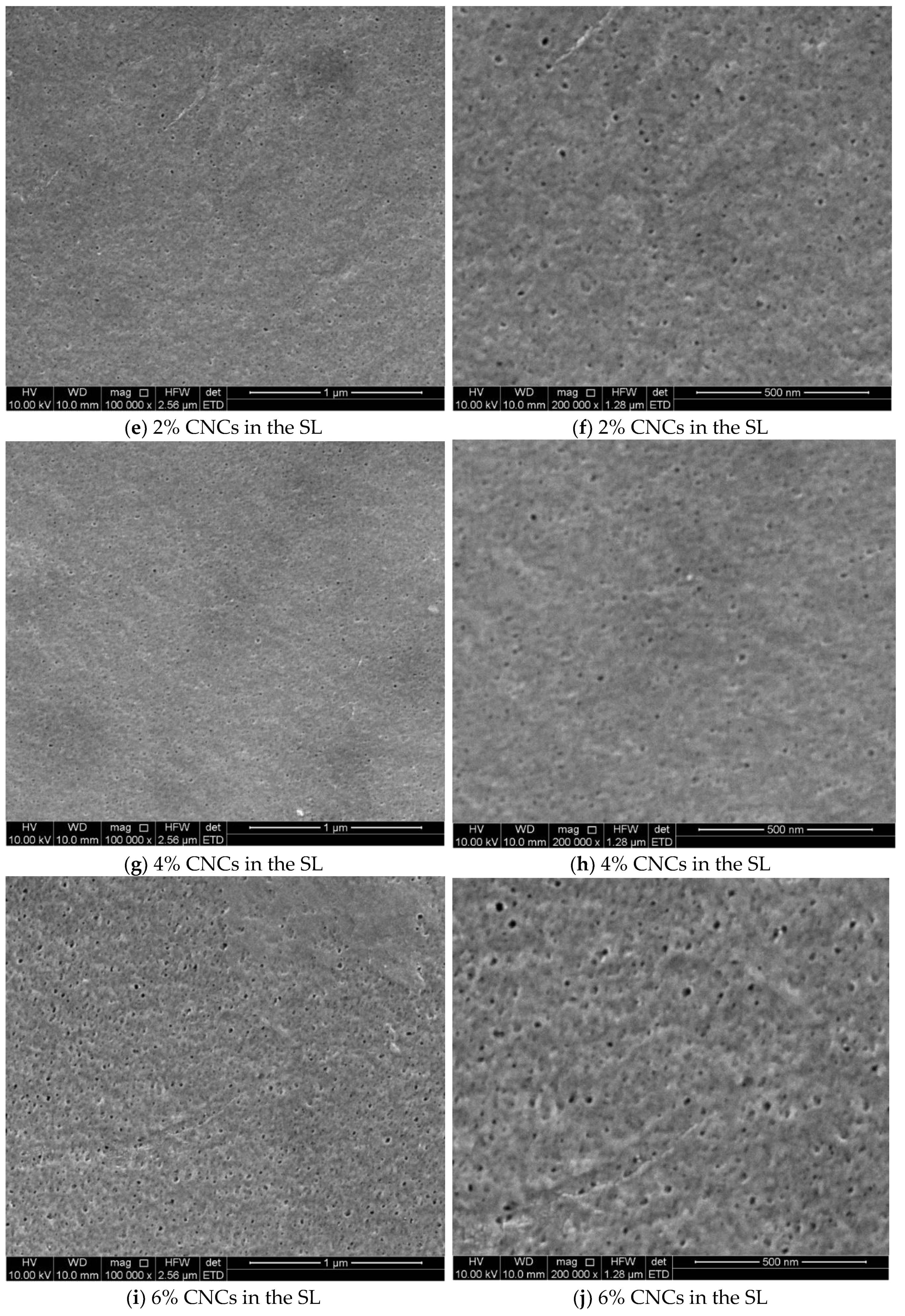
3.1. Cellulose Micro and Nano Crystal Properties
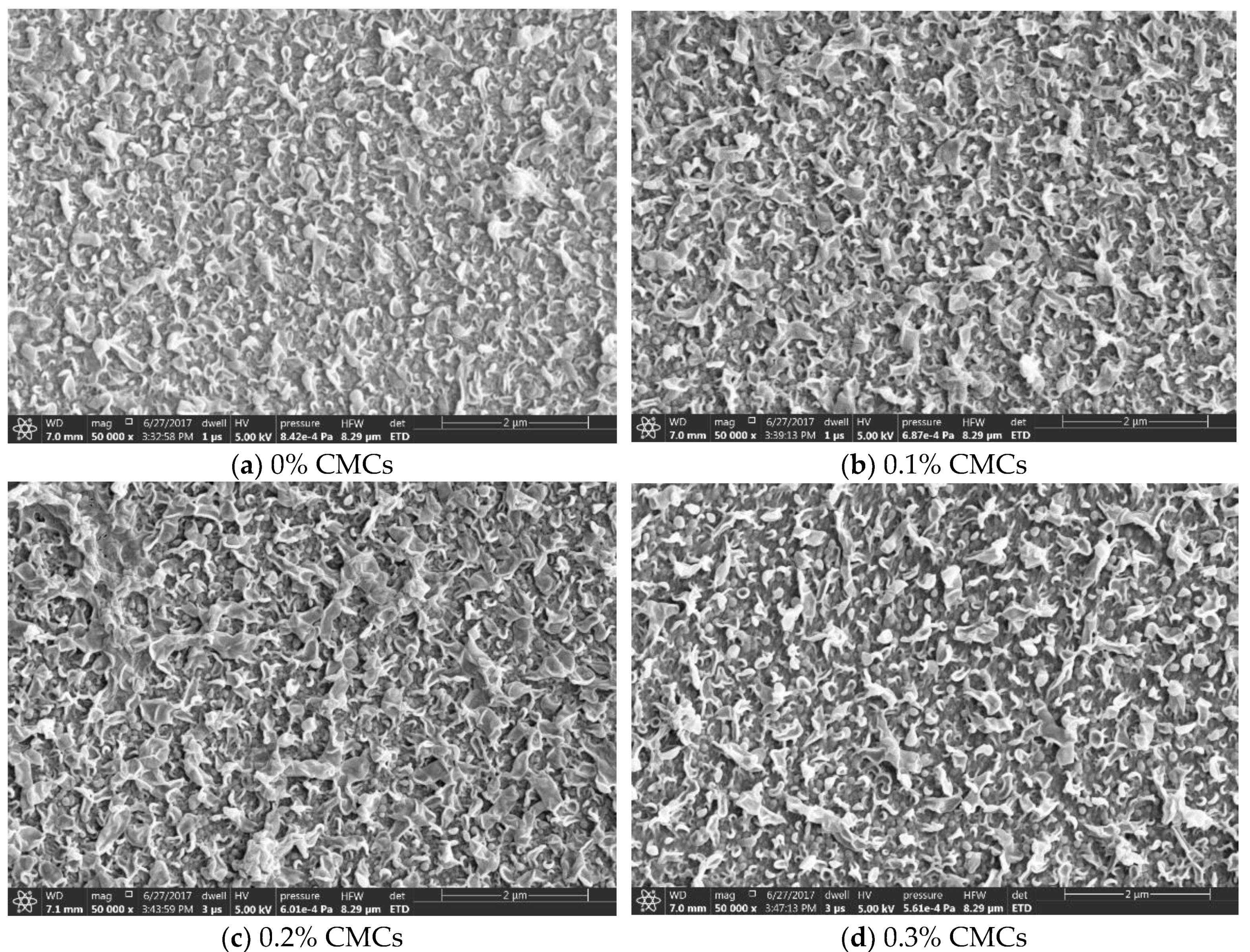
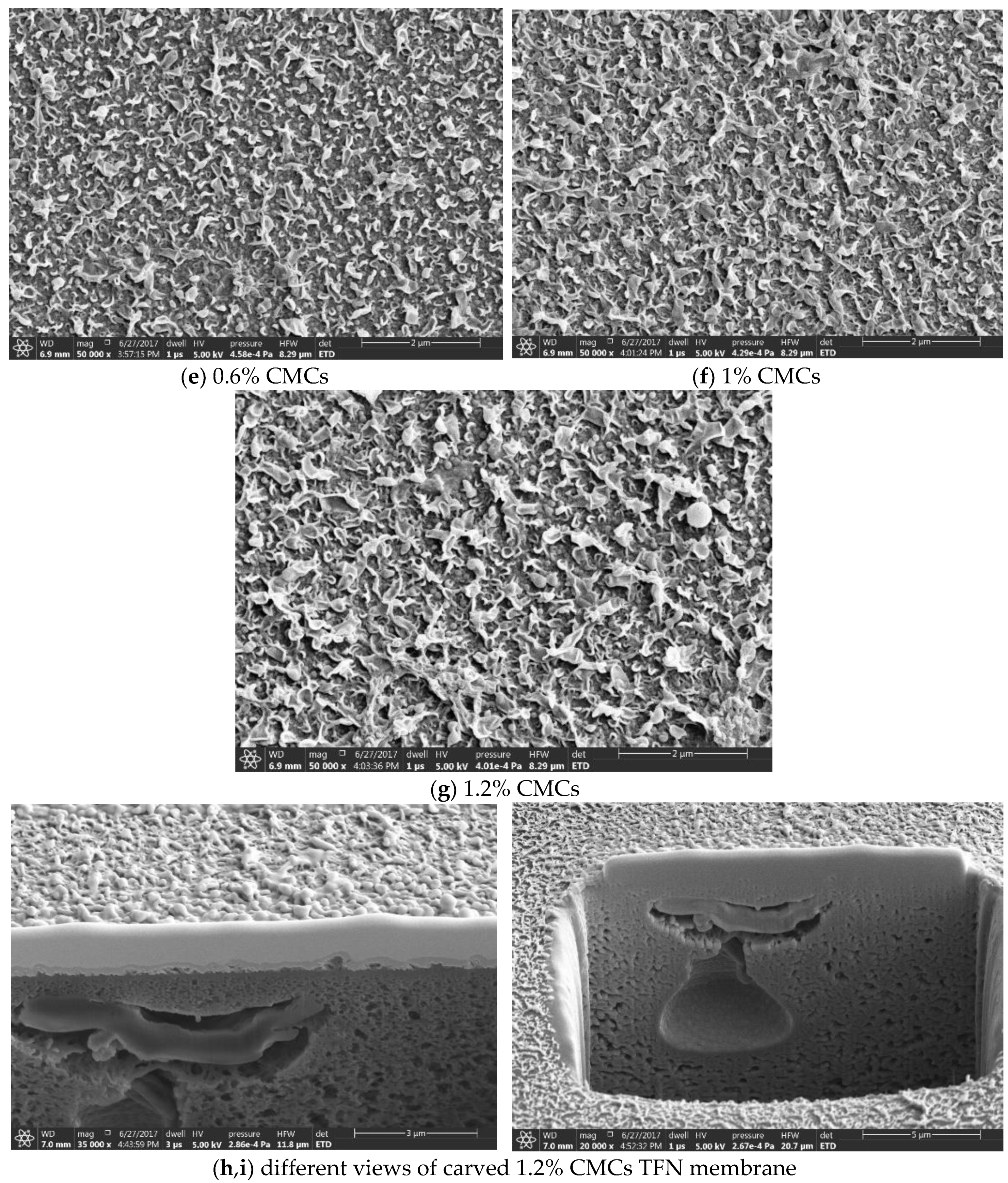
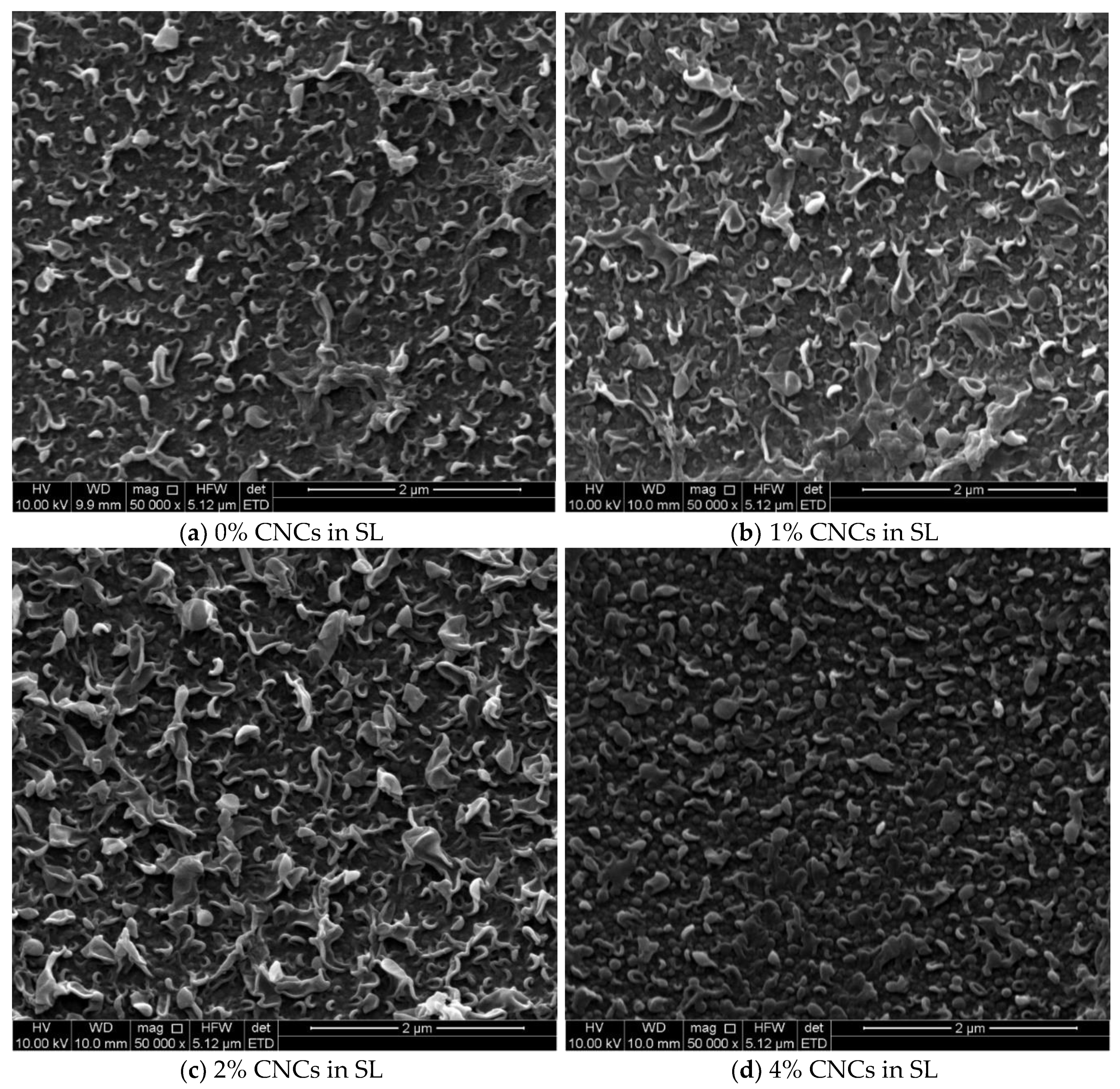
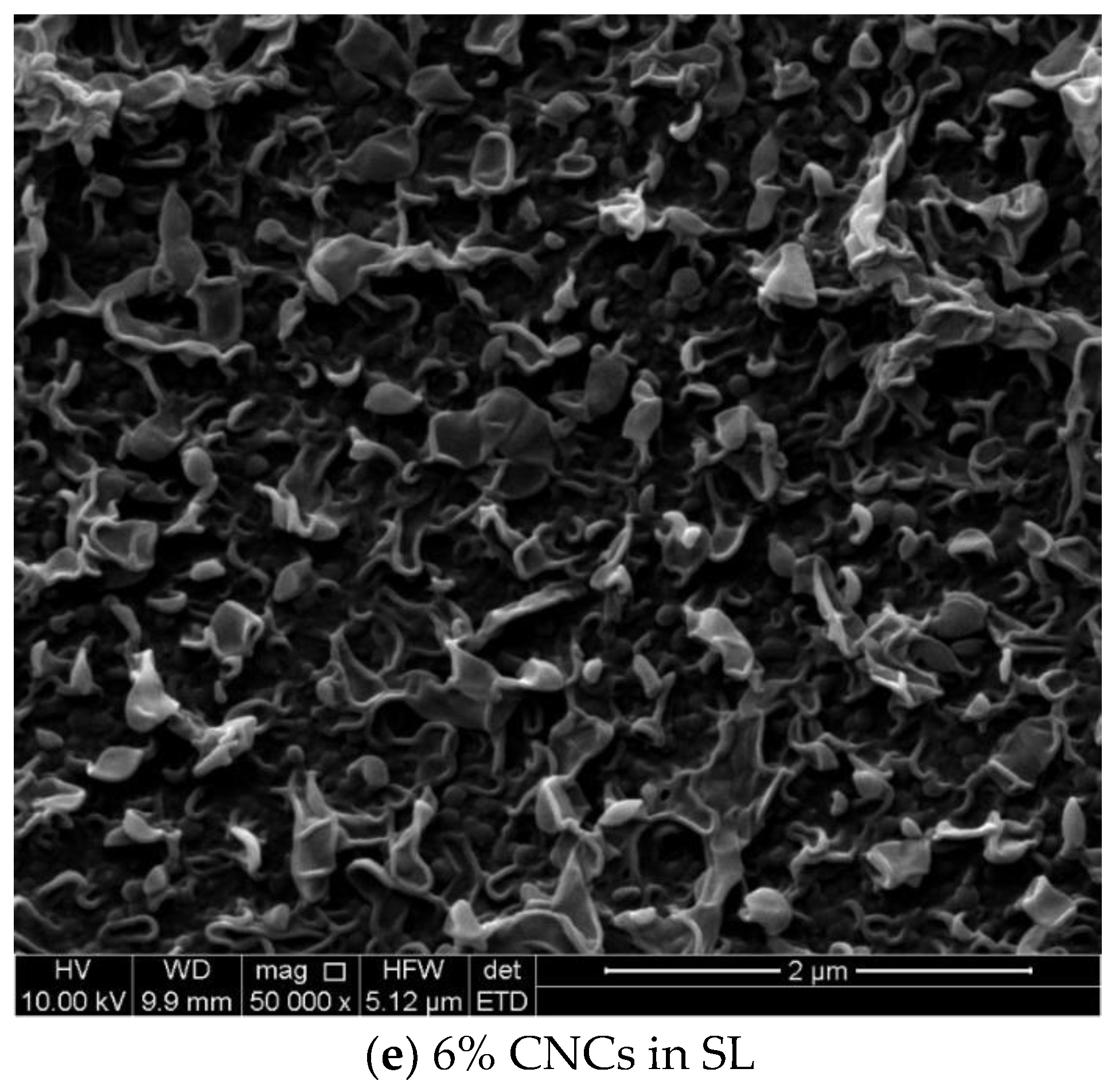
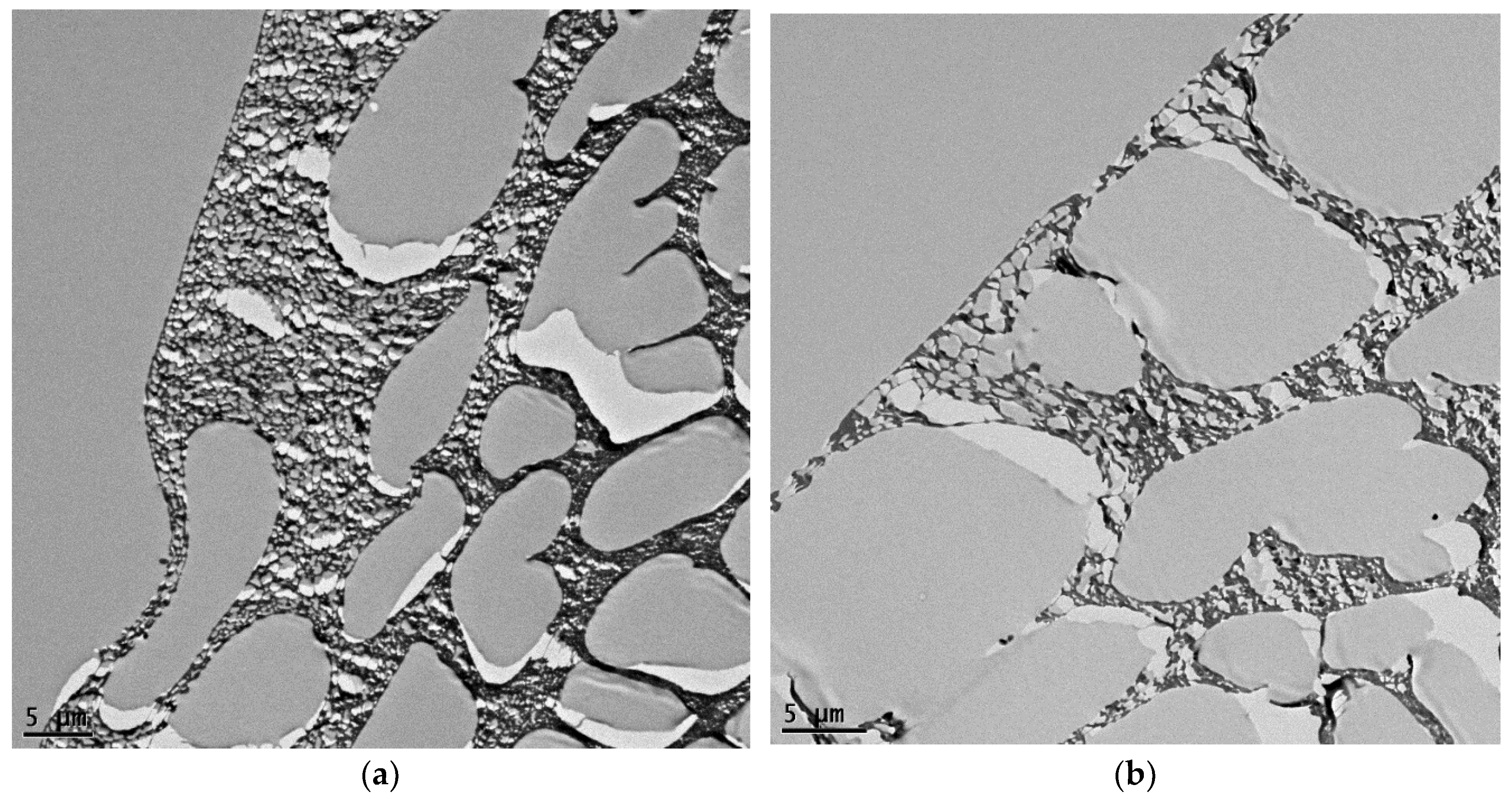
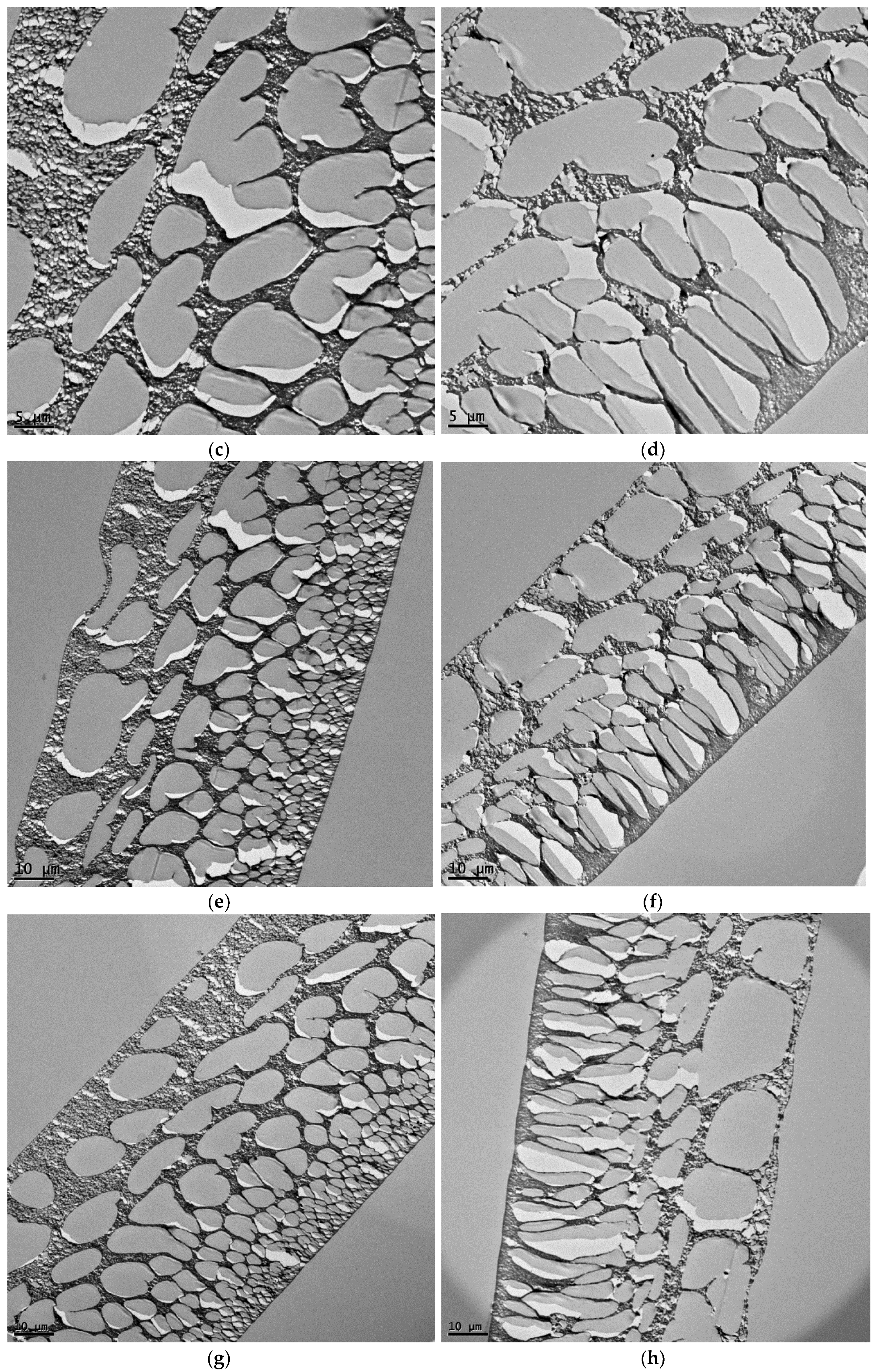
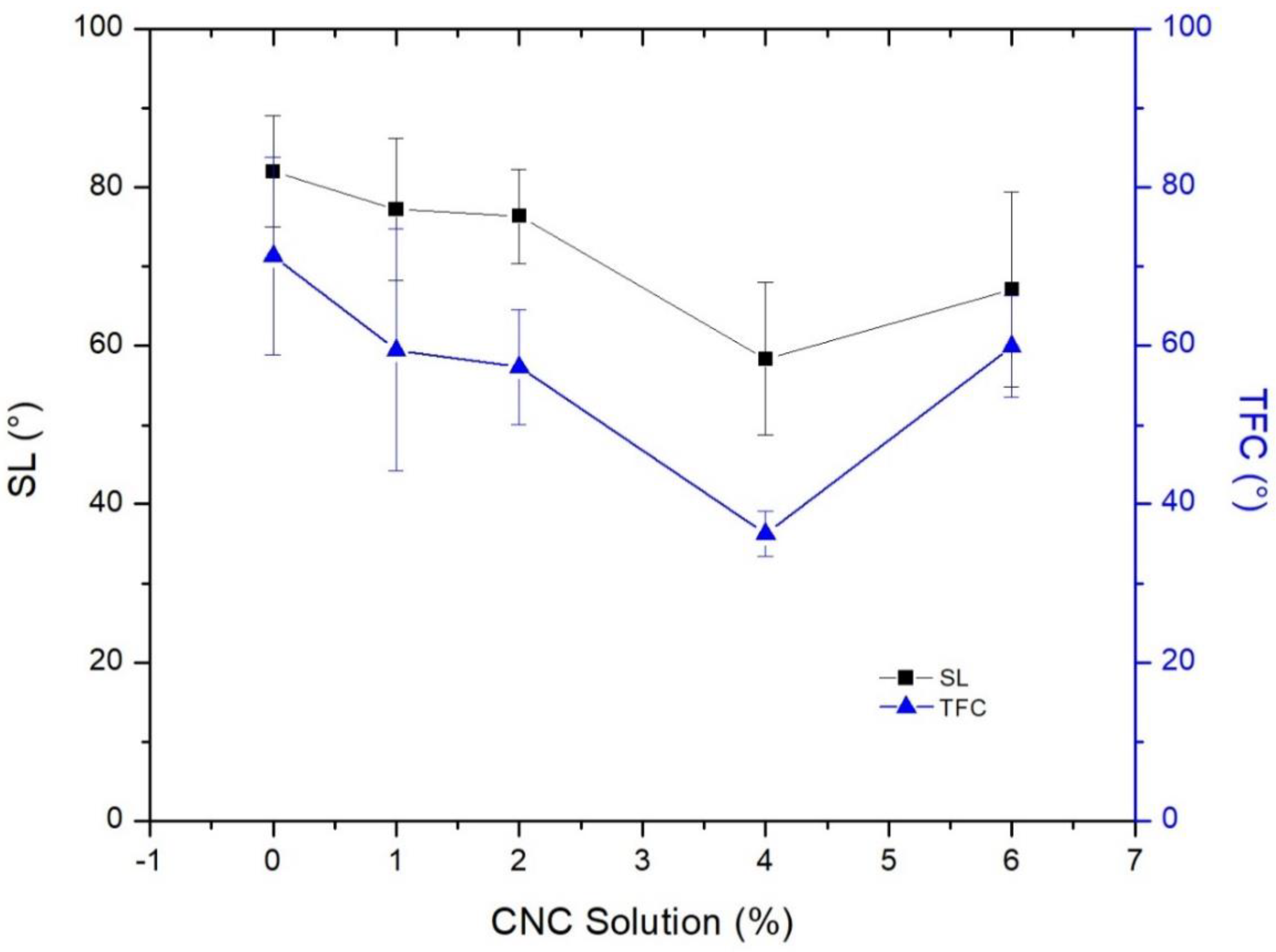
3.2. The Thin Film Membranes Properties
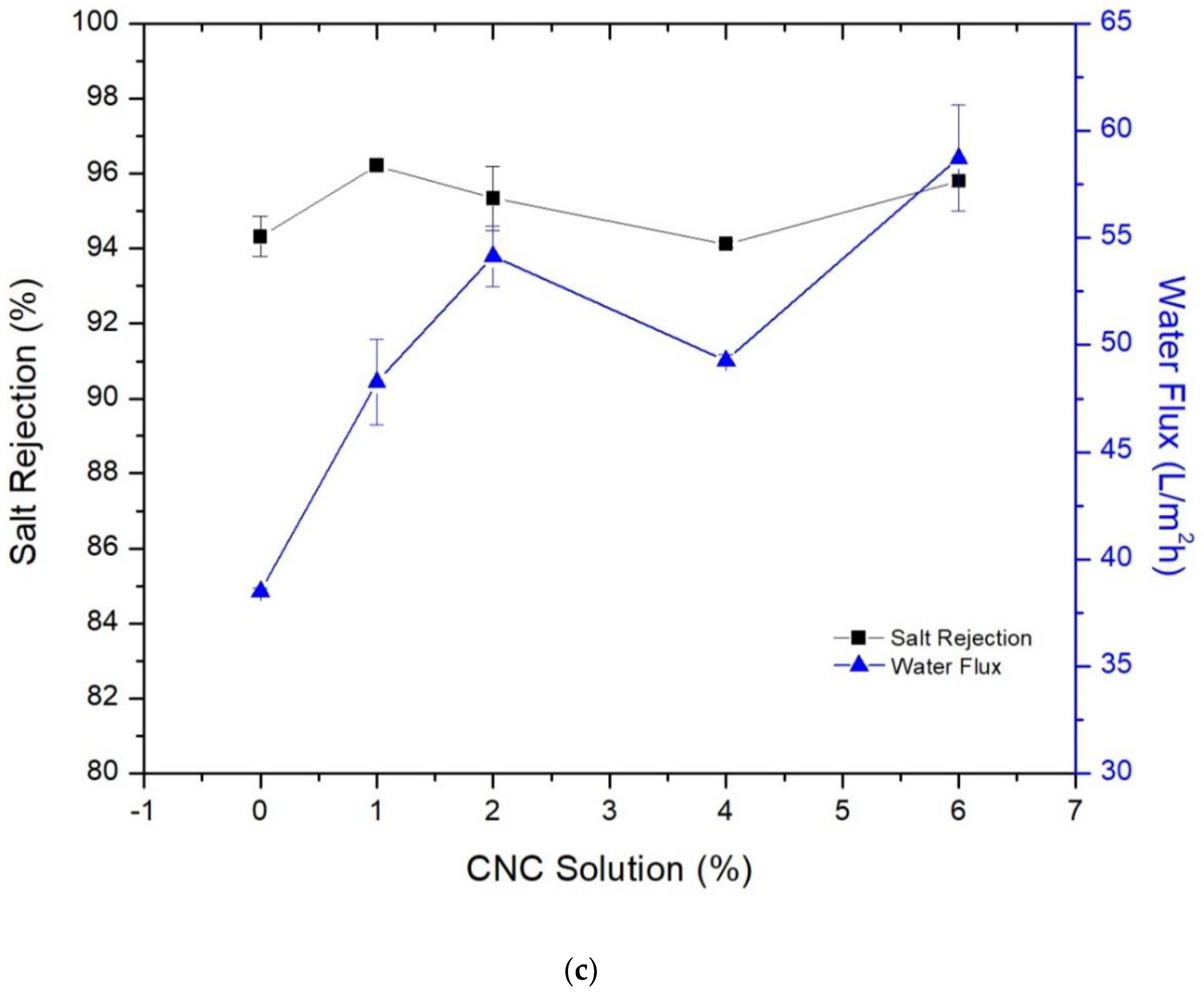
3.3. Membrane’s Performance in Desalination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| ATR FT-IR | Attenuated total reflection Fourier transform infrared |
| CaCl2 | Calcium chloride |
| CMCs | Cellulose micro crystals |
| CNCs | Cellulose nano crystals |
| CNFs | Cellulose nanofibers |
| CSA | (1s)-(+)-10-camphorsulfonic acid |
| DI | Deionized |
| DMF | N,N-dimethylformamide |
| FO | Forward osmosis |
| FTIR | Fourier-transform infrared spectroscopy |
| MF | Micro-filtration |
| MMMs | Mixed matrix membranes |
| MOFs | Metal–organic frameworks |
| MPD | m-phenyldiamine |
| NaCl | Sodium chloride |
| NMP | 1-Methyl-2-pyrrolidinone |
| PSU | Polysulfone |
| PVP | Polyvinylpyrrolidone |
| RO | Reverse osmosis |
| SEM | Scanning electron microscope |
| SL | Support layer |
| TEA | Triethylamine |
| TEM | Transmission electron microscopy |
| TEMPO | 2,2,6,6 Tetramethylpiperidine-1-oxyl |
| TFC | Thin film composite |
| TFN | Thin film nanocomposite |
| TMC | Trimesoyl chloride |
| UF | Ultra-filtration |
| XRD | X-ray powder diffraction |
References
- Kummu, M.; Ward, P.J.; De Moel, H.; Varis, O. Is physical water scarcity a new phenomenon? Global assessment of water shortage over the last two millennia. Environ. Res. Lett. 2010, 5, 034006. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Kadhom, M.; Deng, B. Metal-organic frameworks (MOFs) in water filtration membranes for desalination and other applications. Appl. Mater. Today 2018, 11, 219–230. [Google Scholar] [CrossRef]
- Cadotte, J. Interfacially Synthesized Reverse Osmosis Membrane. U.S. Patent 4277344, 7 July 1981. [Google Scholar]
- Zhao, L.; Ho, W.W. Novel reverse osmosis membranes incorporated with a hydrophilic additive for seawater desalination. J. Membr. Sci. 2014, 455, 44–54. [Google Scholar] [CrossRef]
- Lau, W.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Chen, J.P.; Ismail, A. A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Kadhom, M.; Yin, J.; Deng, B. A Thin Film Nanocomposite Membrane with MCM-41 Silica Nanoparticles for Brackish Water Purification. Membranes 2016, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Kadhom, M.; Deng, B. Thin film nanocomposite membranes filled with bentonite nanoparticles for brackish water desalination: A novel water uptake concept. Microporous Mesoporous Mater. 2019, 279, 82–91. [Google Scholar] [CrossRef]
- Salehi, T.; Peyravi, M.; Jahanshahi, M.; Lau, W.; Rad, A. Impacts of zeolite nanoparticles on substrate properties of thin film nanocomposite membranes for engineered osmosis. J. Nanoparticle Res. 2018, 20, 113. [Google Scholar] [CrossRef]
- Kadhom, M.; Hu, W.; Deng, B. Thin Film Nanocomposite Membrane Filled with Metal-Organic Frameworks UiO-66 and MIL-125 Nanoparticles for Water Desalination. Membranes 2017, 7, 31. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, H.; Xu, M.; Zhuang, Z.; Xu, M.; Dai, H. High Yield Preparation Method of Thermally Stable Cellulose Nanofibers. BioResources 2014, 9, 1986–1997. [Google Scholar] [CrossRef]
- Abou-Zeid, R.E.; Khiari, R.; El-Wakil, N.; Dufresne, A. Current State and New Trends in the Use of Cellulose Nanomaterials for Wastewater Treatment. Biomacromolecules 2018, 20, 573–597. [Google Scholar] [CrossRef] [PubMed]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-Based Materials for Water Purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.; Grishkewich, N.; Tam, K.C.; Grishkewich, N. Cellulose nanomaterials: promising sustainable nanomaterials for application in water/wastewater treatment processes. Environ. Sci. Nano 2018, 5, 623–658. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Ding, A.; Ren, N.; Li, G.; Liang, H. Surface coating of UF membranes to improve antifouling properties: a comparison study between cellulose nanocrystals (CNCs) and cellulose nanofibrils (CNFs). Chemosphere 2019, 217, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Daraei, P.; Ghaemi, N.; Ghari, H.S. An ultra-antifouling polyethersulfone membrane embedded with cellulose nanocrystals for improved dye and salt removal from water. Cellulose 2017, 24, 915–929. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Bai, H.; Zhang, L.; Qu, P.; Bai, L. Preperation and characteristics of polysulfone dialysis composite membranes modified with nanocrystalline cellulose. BioResources 2011, 6, 1670–1680. [Google Scholar]
- Wang, D. A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose 2019, 26, 687–701. [Google Scholar] [CrossRef]
- Asempour, F.; Emadzadeh, D.; Matsuura, T.; Kruczek, B. Synthesis and characterization of novel Cellulose Nanocrystals-based Thin Film Nanocomposite membranes for reverse osmosis applications. Desalination 2018, 439, 179–187. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Bossa, N.; Ding, A.; Ren, N.; Li, G.; Liang, H.; Wiesner, M.R. Incorporation of Cellulose Nanocrystals (CNCs) into the Polyamide Layer of Thin-Film Composite (TFC) Nanofiltration Membranes for Enhanced Separation Performance and Antifouling Properties. Environ. Sci. Technol. 2018, 52, 11178–11187. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liu, Y.; Ding, A.; Ren, N.; Li, G.; Liang, H. Fabrication and characterization of thin-film composite (TFC) nanofiltration membranes incorporated with cellulose nanocrystals (CNCs) for enhanced desalination performance and dye removal. Chem. Eng. J. 2019, 358, 1519–1528. [Google Scholar] [CrossRef]
- Smith, E.D.; Hendren, K.D.; Haag, J.V.; Foster, E.J.; Martin, S.M. Functionalized Cellulose Nanocrystal Nanocomposite Membranes with Controlled Interfacial Transport for Improved Reverse Osmosis Performance. Nanomaterials 2019, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.-H.; Hoek, E.M.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Dong, Y.; Zhao, X.; Jiang, Z.; Zhang, R.; Zhao, J. Separation performance of thin-film composite nanofiltration membrane through interfacial polymerization using different amine monomers. Desalination 2014, 333, 59–65. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, S.; Shi, W.; Wang, W.; Wang, X.; Zhang, Z.; Li, L.; Zhang, B.; Bao, X. A novel polyesteramide thin film composite nanofiltration membrane prepared by interfacial polymerization of serinol and trimesoyl chloride (TMC) catalyzed by 4-dimethylaminopyridine (DMAP). J. Membr. Sci. 2017, 542, 68–80. [Google Scholar] [CrossRef]
- Baroña, G.N.B.; Lim, J.; Choi, M.; Jung, B. Interfacial polymerization of polyamide-aluminosilicate SWNT nanocomposite membranes for reverse osmosis. Desalination 2013, 325, 138–147. [Google Scholar] [CrossRef]
- Kumar, S.; Nandi, B.K.; Guria, C.; Mandal, A. Oil Removal from Produced Water by Ultrafiltration using Polysulfone Membrane. Braz. J. Chem. Eng. 2017, 34, 583–596. [Google Scholar] [CrossRef]
- Bai, H.; Wang, X.; Zhou, Y.; Zhangn, L. Preparation and characterization of poly(vinylidenefluoride) composite membranes blended with nano-crystalline cellulose. Prog. Nat. Sci. Mater. Int. 2012, 22, 250–257. [Google Scholar] [CrossRef]
- Kadhom, M.; Deng, B. Synthesis of high-performance thin film composite (TFC) membranes by controlling the preparation conditions: Technical notes. J. Water Process. Eng. 2019, 30, 100542. [Google Scholar] [CrossRef]
- Al-Furaiji, M.; Benes, N.; Nijmeijer, A.; McCutcheon, J. Use of a Forward Osmosis−Membrane Distillation Integrated Process in the Treatment of High-Salinity Oily Wastewater. Ind. Eng. Chem. Res. 2019, 58, 956–962. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadhom, M.; Albayati, N.; Salih, S.; Al-Furaiji, M.; Bayati, M.; Deng, B. Role of Cellulose Micro and Nano Crystals in Thin Film and Support Layer of Nanocomposite Membranes for Brackish Water Desalination. Membranes 2019, 9, 101. https://doi.org/10.3390/membranes9080101
Kadhom M, Albayati N, Salih S, Al-Furaiji M, Bayati M, Deng B. Role of Cellulose Micro and Nano Crystals in Thin Film and Support Layer of Nanocomposite Membranes for Brackish Water Desalination. Membranes. 2019; 9(8):101. https://doi.org/10.3390/membranes9080101
Chicago/Turabian StyleKadhom, Mohammed, Noor Albayati, Suhaib Salih, Mustafa Al-Furaiji, Mohamed Bayati, and Baolin Deng. 2019. "Role of Cellulose Micro and Nano Crystals in Thin Film and Support Layer of Nanocomposite Membranes for Brackish Water Desalination" Membranes 9, no. 8: 101. https://doi.org/10.3390/membranes9080101
APA StyleKadhom, M., Albayati, N., Salih, S., Al-Furaiji, M., Bayati, M., & Deng, B. (2019). Role of Cellulose Micro and Nano Crystals in Thin Film and Support Layer of Nanocomposite Membranes for Brackish Water Desalination. Membranes, 9(8), 101. https://doi.org/10.3390/membranes9080101






