Evaluation of Permeate Quality in Pilot Scale Membrane Distillation Systems
Abstract
1. Introduction
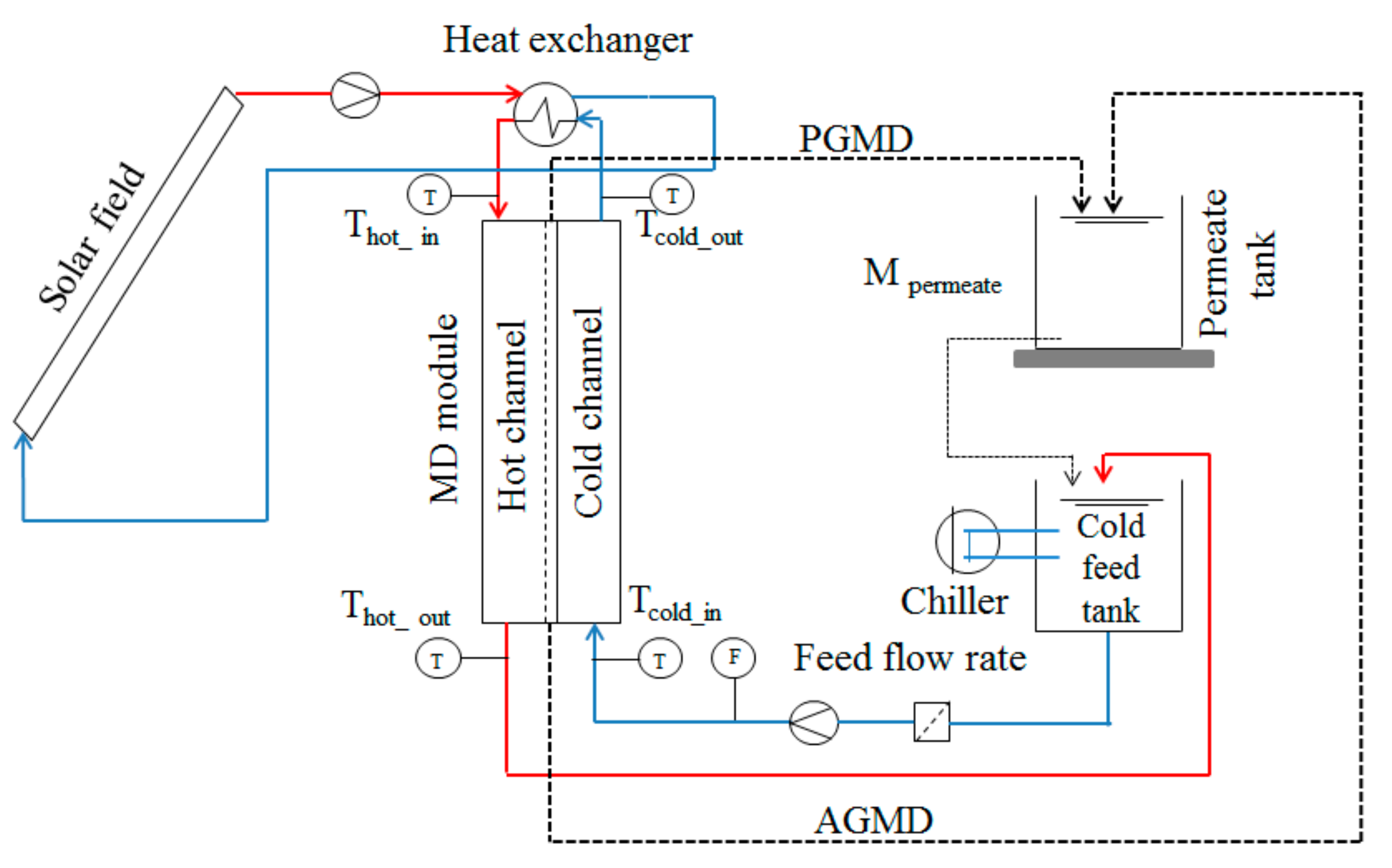
2. Materials and Methods
3. Results and Discussion
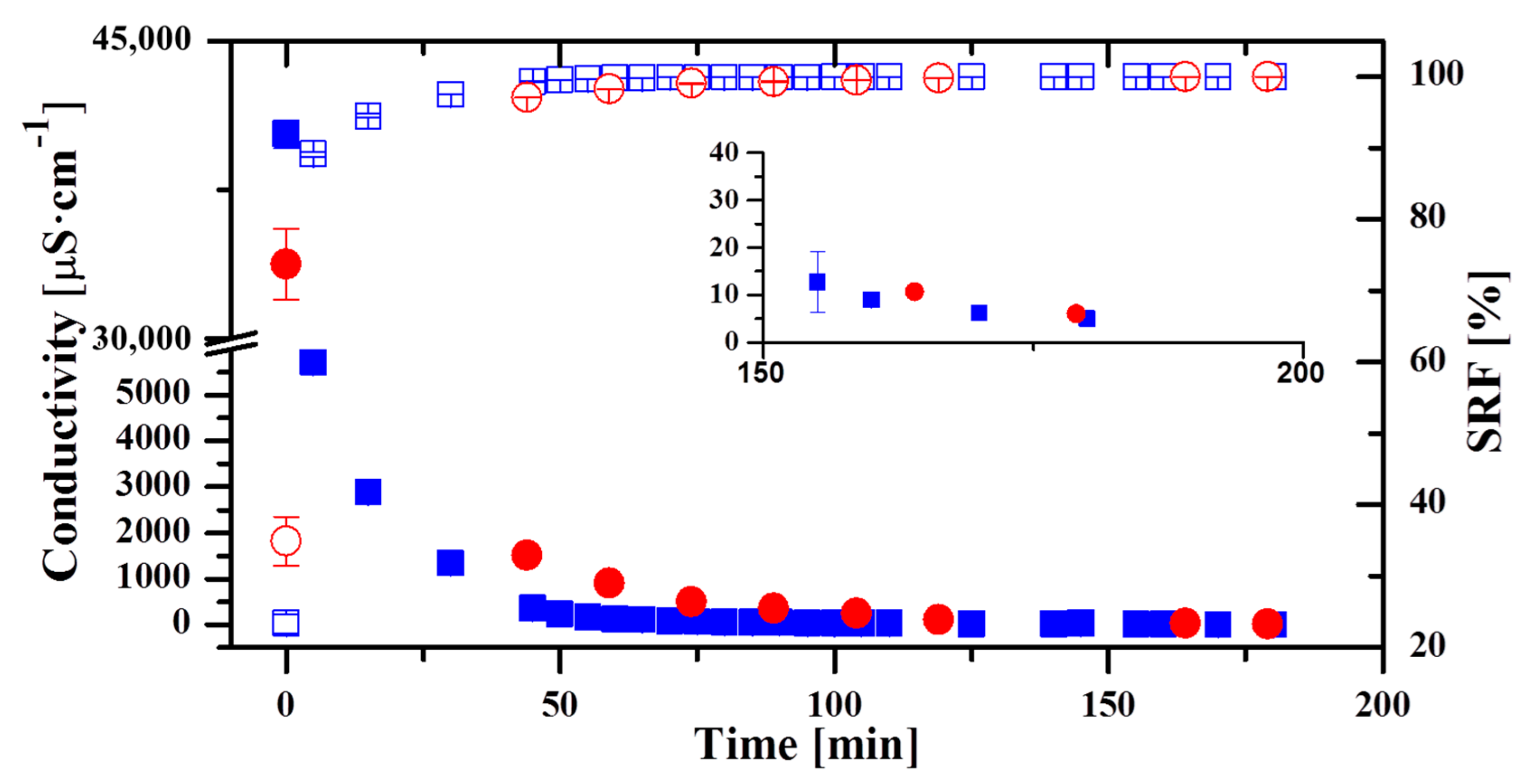
3.1. Evolution of the Quality of Permeate along the Operation with the Oryx 150 Module
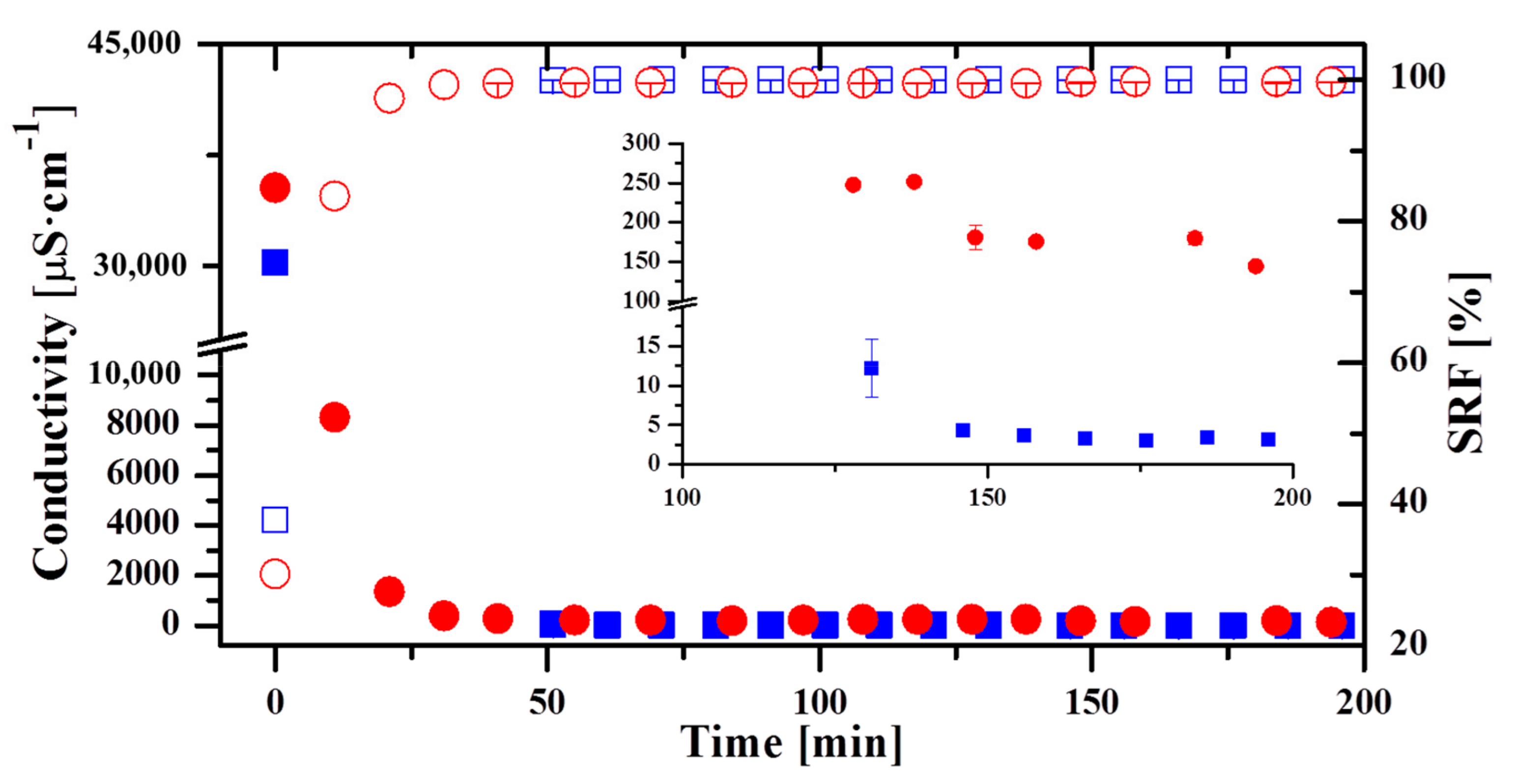
3.2. Evolution of the Permeate Quality along the Operation with Aquastill Modules
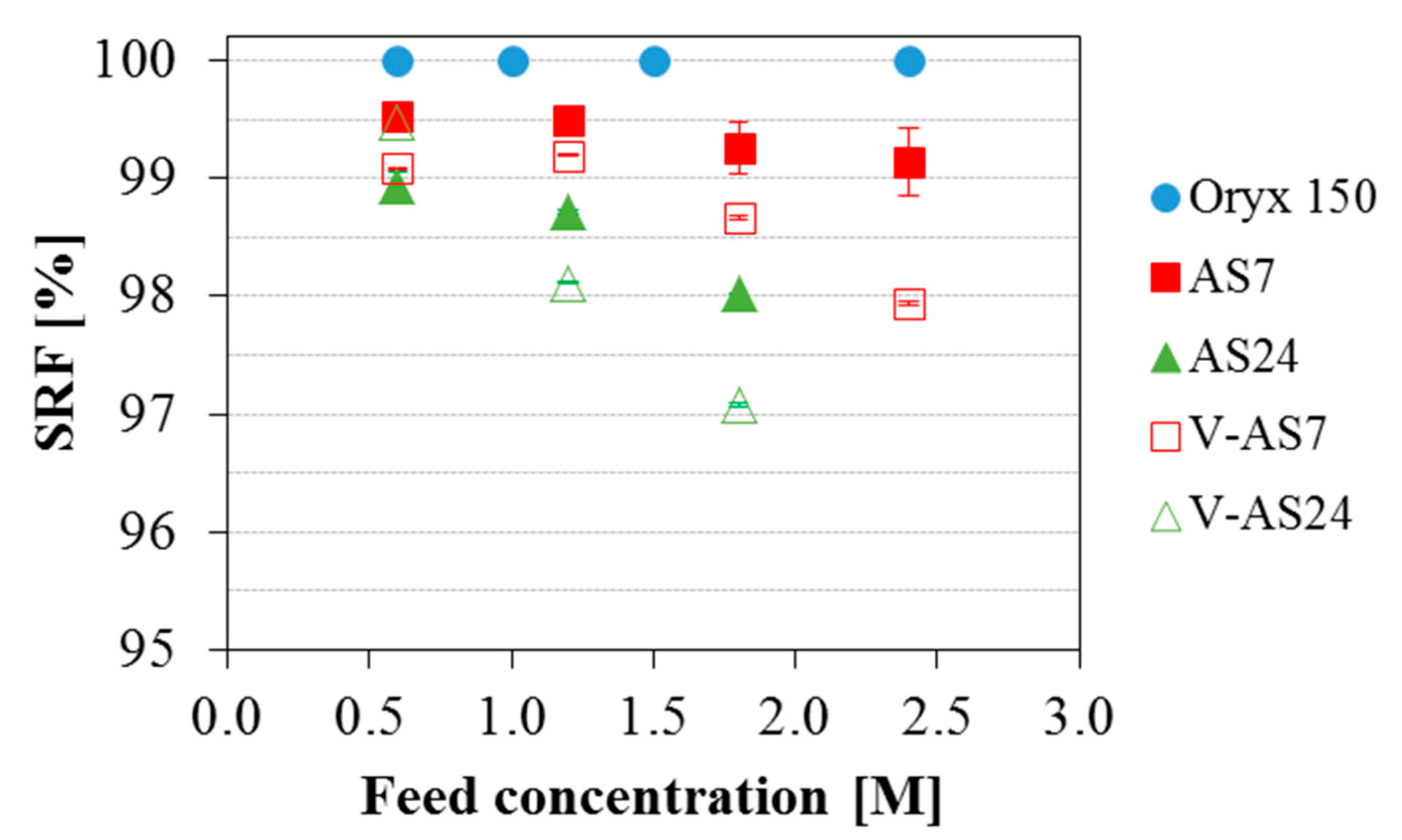
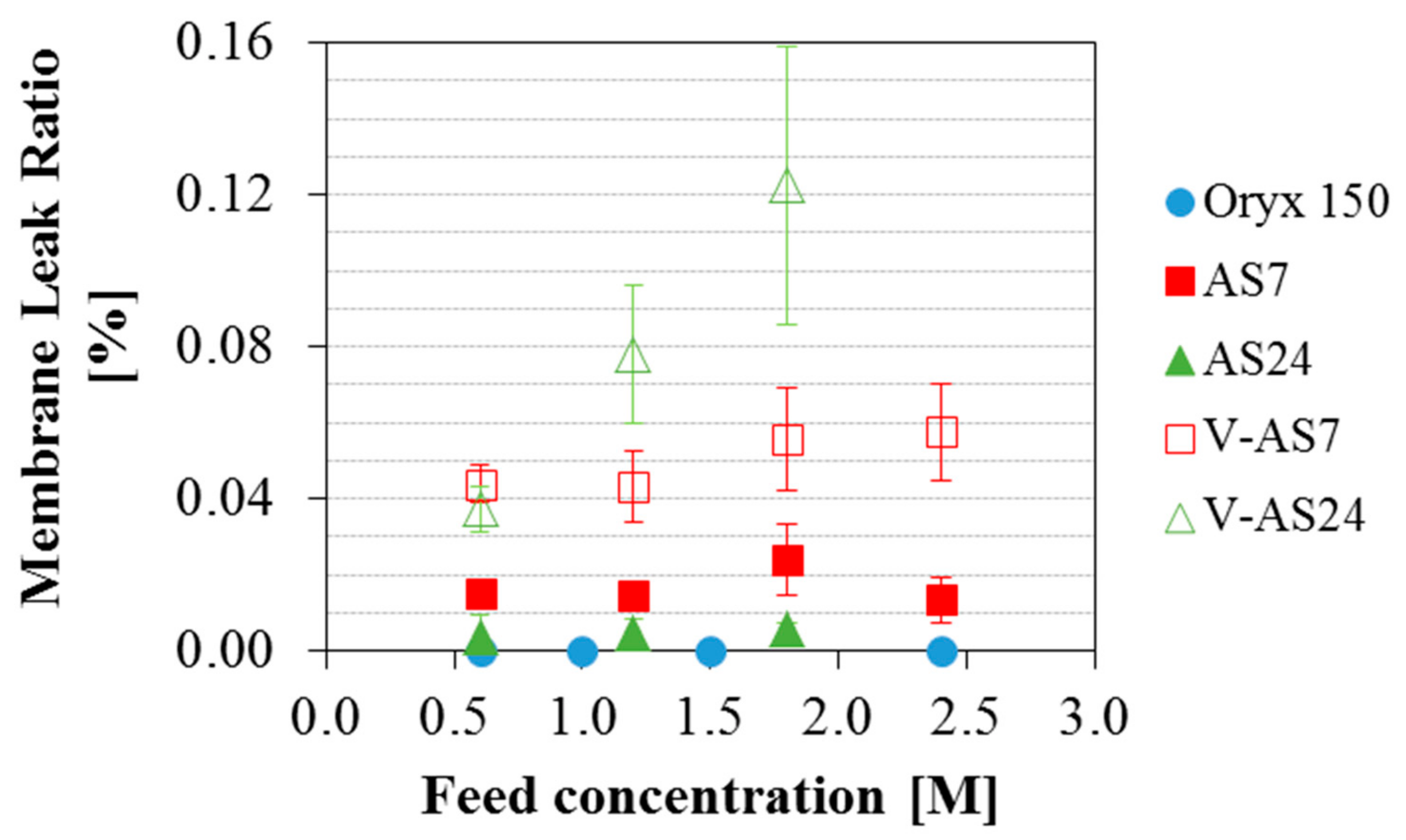
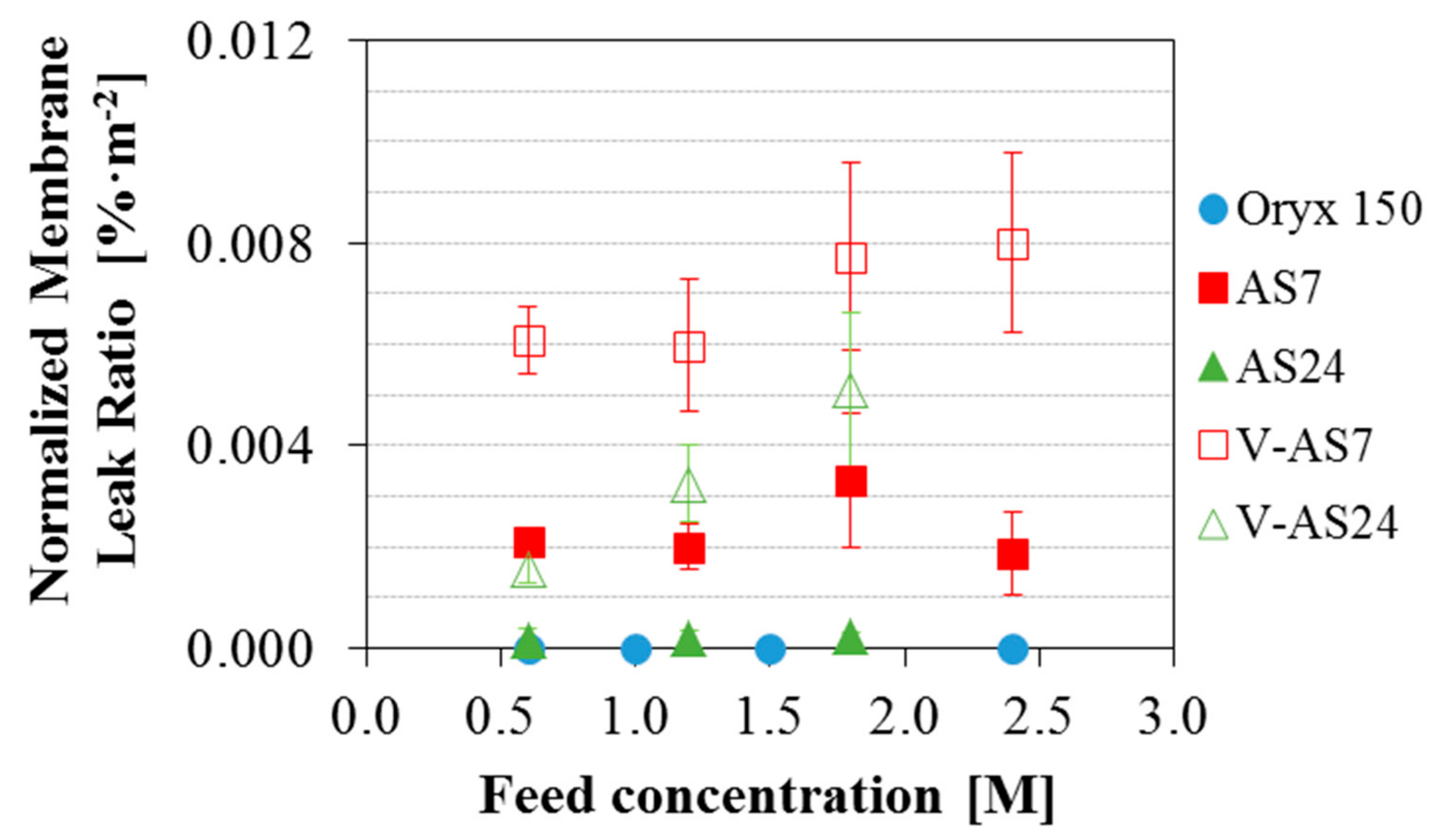
3.3. Effect of the Feed Salinity on the Permeate Quality
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Acronyms
| AGMD | Air gap membrane distillation |
| CSG | Coal seam gas |
| DIC | Dissolved inorganic carbon |
| DTC | Dissolved total carbon |
| GOR | Gained output ratio |
| LDPE | Low density polyethylene |
| MD | Membrane distillation |
| MVC | Mechanical vapour compression |
| PP | Polypropylene |
| PTFE | Polytetrafluoroethylene |
| RO | Reverse osmosis |
| SRF | Salt rejection factor |
| V-AGMD | Vacuum-enhanced air gap membrane distillation |
| WHO | VWorld health organization |
Nomenclature
| C EC | Concentration (g·L−1) Electrical conductivity (mS·cm−1) |
| F | Feed flow rate (L·h−1) |
| T | Temperature (°C) |
| S | Salinity (M) |
Subscripts
| cold | Cold channel |
| f | Feed |
| hot | Hot channel |
| p | Permeate |
Appendix A

References
- Shatat, M.; Worall, M.; Riffat, S. Opportunities for solar water desalination worldwide: Review. Sustain. Cities Soc. 2013, 9, 67–80. [Google Scholar] [CrossRef]
- Sarai Atab, M.; Smallbone, A.J.; Roskilly, A.D. An operational and economic study of a reverse osmosis desalination system for potable water and land irrigation. Desalination 2016, 397, 174–184. [Google Scholar] [CrossRef]
- Monnot, M.; Ngvyên, H.T.K.; Laborie, S.; Cabassud, C. Seawater reverse osmosis desalination plant at community-scale: Role of an innovative pretreatment on process performances and intensification. Chem. Eng. Process. Process Intensif. 2017, 113, 42–55. [Google Scholar] [CrossRef]
- Shalaby, S.M. Reverse Osmosis desalination powered by photovoltaic and solar Rankine cycle power systems: A review. Renew. Sustain. Energy Rev. 2017, 73, 789–797. [Google Scholar] [CrossRef]
- Roberts, D.A.; Johnston, E.L.; Knott, N.A. Impacts of desalination plant discharges on the marine environment. A critical review of published studies. Water Res. 2010, 44, 5117–5128. [Google Scholar] [CrossRef]
- Nakoa, K.; Rahaoui, K.; Date, A.; Akbarzadeh, A. Sustainable zero liquid discharge desalination (SZLDD). Sol. Energy 2016, 135, 337–347. [Google Scholar] [CrossRef]
- Macedonio, F.; Drioli, E. Zero Liquid Discharge in Desalination. In Sustainable Membrane Technology for Water and Wastewater Treatment. Green Chemistry and Sustainable Technology; Figoli, A., Criscuoli, A., Eds.; Springer: Singapore, 2017; pp. 221–241. [Google Scholar]
- Ji, X.; Curcio, E.; Al Obaidini, S.; Di Profio, G.; Fontananova, E.; Drioli, E. Membrane Distillation—Crystallization of seawater reverse osmosis brines. Sep. Purif. Technol. 2010, 71, 76–82. [Google Scholar] [CrossRef]
- Drioli, E.; Curcio, E. Progress in membrane crystallization. Curr. Opin. Chem. Eng. 2012, 1, 178–182. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Membrane crystallization for salts recovery from brine—An experimental and theoretical analysis. Desalin. Water Treat. 2015, 57, 7593–7603. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Integrated Membrane Desalination Systems with Membrane Crystallization Units for Resource Recovery: A new approach for mining from the sea. Crystals 2016, 6, 36–48. [Google Scholar] [CrossRef]
- Ruiz Salmón, I.; Luis, P. Membrane crystallization via membrane distillation. Chem. Eng. Process. Process Intensif. 2018, 123, 258–271. [Google Scholar] [CrossRef]
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Tong, T.; Elimelech, M. The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Singh, Y.B.; Amy, G.L.; Ghaffour, N. Effect of operating parameters and membrane characteristics on air gap membrane distillation performance for the treatment of highly saline water. J. Membr. Sci. 2016, 512, 73–82. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hilal, N. Air gap membrane distillation: A detailed study of high saline solution. Desalination 2017, 403, 179–186. [Google Scholar] [CrossRef]
- Li, J.; Guan, Y.; Cheng, F.; Liu, Y. Treatment of high salinity brines by direct contact membrane distillation: Effect of membrane characteristics and salinity. Chemosphere 2015, 140, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Eykens, L.; Hitsov, I.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Nopens, I.; Van der Bruggen, B. Influence of membrane thickness and process conditions on direct contact membrane distillation at different salinities. J. Membr. Sci. 2016, 498, 353–364. [Google Scholar] [CrossRef]
- Safavi, M.; Mohammadi, T. High-salinity water desalination using VMD. Chem. Eng. J. 2009, 149, 191–195. [Google Scholar] [CrossRef]
- Adham, S.; Hussain, A.; Minier Matar, J.; Dores, R.; Janson, A. Application of Membrane Distillation for desalting brines from thermal desalination plants. Desalination 2013, 314, 101–108. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Choi, Y.; Vigneswaran, S. Membrane distillation for wastewater reverse osmosis concentrate treatment with water reuse potential. J. Membr. Sci. 2017, 524, 565–575. [Google Scholar] [CrossRef]
- Naidu, G.; Choi, Y.; Jeong, S.; Hwang, T.M.; Vigneswaran, S.J. Experiments and modeling of a vacuum membrane distillation for high saline water. Ind. Eng. Chem. 2014, 20, 2174–2183. [Google Scholar] [CrossRef]
- Gryta, M. Concentration of NaCl solution by membrane distillation integrated with crystallization. Sep. Sci. Technol. 2002, 37, 3535–3558. [Google Scholar] [CrossRef]
- Tun, C.M.; Fane, A.G.; Matheickal, J.T.; Sheikholeslami, R. Membrane distillation crystallization of concentrated salts-flux and crystal formation. J. Membr. Sci. 2005, 257, 144–155. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Minier-Matar, J.; Hussain, A.; Janson, A.; Benyahia, F.; Adham, S. Field evaluation ofmembrane distillation technologies for desalination of highly saline brines. Desalination 2014, 351, 101–108. [Google Scholar] [CrossRef]
- Duong, H.C.; Chivas, A.R.; Nelemans, B.; Duke, M.; Gray, S.; Cath, T.Y.; Nghiem, L.D. Treatment of RO brine from CSG produced water by spiral-wound air gap membrane distillation—A pilot study. Desalination 2015, 366, 121–129. [Google Scholar] [CrossRef]
- Winter, D.; Koschikowski, J.; Wieghaus, M. Desalination using membrane distillation: Experimental studies on full scale spiral wound module. J. Membr. Sci. 2011, 375, 104–112. [Google Scholar] [CrossRef]
- Ruiz-Aguirre, A.; Alarcón-Padilla, D.-C.; Zaragoza, G. Productivity analysis of two spiral-wound membrane distillation prototypes coupled with solar energy. Desalin. Water Treat. 2015, 55, 2777–2785. [Google Scholar] [CrossRef]
- Schwantes, R.; Bauer, L.; Chavan, K.; Dücker, D.; Felsmann, C.; Pfafferott, J. Air gap membrane distillation for hypersaline brine concentration: Operational analysis of a full-scale module–New strategies for wetting mitigation. Desalination 2018, 444, 13–25. [Google Scholar] [CrossRef]
- Ruiz-Aguirre, A.; Andrés-Mañas, J.A.; Fernández-Sevilla, J.M.; Zaragoza, G. Modeling and optimization of a commercial permeate gap spiral wound membrane distillation module for seawater desalination. Desalination 2017, 419, 160–168. [Google Scholar] [CrossRef]
- Ruiz-Aguirre, A.; Andrés-Mañas, J.A.; Fernández-Sevilla, J.M.; Zaragoza, G. Experimental characterization and optimization of multi-channel spiral wound air gap membrane distillation modules for seawater desalination. Sep. Purif. Technol. 2018, 205, 212–222. [Google Scholar] [CrossRef]
- Gil, J.D.; Ruiz-Aguirre, A.; Roca, L.; Zaragoza, G.; Berenguel, M. Prediction models to analyse the performance of a commercial-scale membrane distillation unit for desalting brines from RO plants. Desalination 2018, 445, 15–28. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO Press: Geneva, Switzerland, 2011; p. 223. [Google Scholar]
- Guillén-Burrieza, E.; Zaragoza, G.; Miralles-Cuevas, S.; Blanco, J. Experimental evaluation of two pilot-scale membrane distillation modules used for solar desalination. J. Membr. Sci. 2012, 409, 264–275. [Google Scholar] [CrossRef]
- Sanmartino, J.A.; Khayet, M.; García-Payo, M.C.; El Bakouri, H.; Riaza, A. Desalination and concentration of saline aqueous solutions up to supersaturation by air gap membrane distillation and crystallization fouling. Desalination 2016, 393, 39–51. [Google Scholar] [CrossRef]
- Nguyen, Q.M.; Lee, S. Fouling analysis and control in a DCMD process for SWRO brine. Desalination 2015, 367, 21–27. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Application of a porous composite hydrophobic/hydrophilic membrane in desalination by air gap and liquid gap membrane distillation: A comparative study. Sep. Purif. Technol. 2014, 133, 176–186. [Google Scholar] [CrossRef]
- Peñate, B.; García-Rodríguez, L. Current trends and future prospects in the design of seawater reverse osmosis desalination technology. Desalination 2012, 284, 1–8. [Google Scholar] [CrossRef]
- Schwantes, R.; Cipollina, A.; Gross, F.; Koschikowski, J.; Pfeifle, D.; Rolletschek, M.; Subiela, V. Membrane distillation: Solar and waste heat driven demonstration plants for desalination. Desalination 2013, 323, 93–106. [Google Scholar] [CrossRef]
- Kovač, N.; Glavaš, N.; Donelec, M.; Šmuc, N.R.; Šlejkovec, Z. Chemical Composition of Natural Sea Salt from the Sečovlje Salina (Gulf of Trieste, northern Adriatic). Acta Chim. Slov. 2013, 60, 706–714. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 96th ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Washburn, E.W.; West, C.J. International Critical Tables of Numerical Data, Physics, Chemistry and Technology; McGraw Hill: New York, NY, USA, 1986. [Google Scholar]

| Characteristics | Oryx 150 | AS7/AS24 |
|---|---|---|
| Configuration: | PGMD | AGMD and V-AGMD |
| Channels length: | 7 m | 1.5 m/5 m |
| Channels thickness: | 3.2 mm | 2 mm. |
| Membrane surface area: | 10 m2 | 7.2 m2/24 m2 |
| Membrane material: | active layer: PTFE; support: PP | active layer: LDPE 1 |
| Active layer thickness: | 70 µm | 76 µm |
| Active layer pore size: | 0.2 µm | 0.3 µm |
| Active layer porosity: | 80% | 85% |
| Support layer thickness: | 280 µm | - |
| Support layer porosity: | 50% | - |
| Gap thickness: | 1 mm | 0.88 mm |
| Vacuum pressure: | - | 150–250 mbar abs |
| Variable (Unit) | Module | Range of Operation |
|---|---|---|
| Tevap (°C) | All modules | 60–80 |
| Tcond (°C) | All modules | 20–30 |
| F (L·h−1) * | Oryx 150 | 400–600 |
| AS7, AS24 | 400–1100 | |
| S (M) ** | Oryx 150 | 0.6–1; 1–1.5; 1.5–2.4 |
| AS7 | 0.6–1.2; 1.2–1.8; 1.8–2.4 | |
| AS24 | 0.6–1.2; 1.2–1.8 |
| Day | F (L·h−1) | Thot (°C) | Tcold (°C) | Pflux (L·h−1·m−2) |
|---|---|---|---|---|
| 1 | 500 | 70 | 30 | 1.5 |
| 2 | 400 | 60 | 20 | 0.9 |
| Module | F (L·h−1) | Thot (°C) | Tcold (°C) | Pflux (L·h−1·m−2) |
|---|---|---|---|---|
| AS7 | 500 | 70 | 20 | 2.7 |
| AS24 | 600 | 80 | 20 | 1.1 |
| AS24 | 600 | 80 | 20 | 1.3 |
| AS24 | 600 | 70 | 20 | 1.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Aguirre, A.; Andrés-Mañas, J.A.; Zaragoza, G. Evaluation of Permeate Quality in Pilot Scale Membrane Distillation Systems. Membranes 2019, 9, 69. https://doi.org/10.3390/membranes9060069
Ruiz-Aguirre A, Andrés-Mañas JA, Zaragoza G. Evaluation of Permeate Quality in Pilot Scale Membrane Distillation Systems. Membranes. 2019; 9(6):69. https://doi.org/10.3390/membranes9060069
Chicago/Turabian StyleRuiz-Aguirre, Alba, Juan A. Andrés-Mañas, and Guillermo Zaragoza. 2019. "Evaluation of Permeate Quality in Pilot Scale Membrane Distillation Systems" Membranes 9, no. 6: 69. https://doi.org/10.3390/membranes9060069
APA StyleRuiz-Aguirre, A., Andrés-Mañas, J. A., & Zaragoza, G. (2019). Evaluation of Permeate Quality in Pilot Scale Membrane Distillation Systems. Membranes, 9(6), 69. https://doi.org/10.3390/membranes9060069






