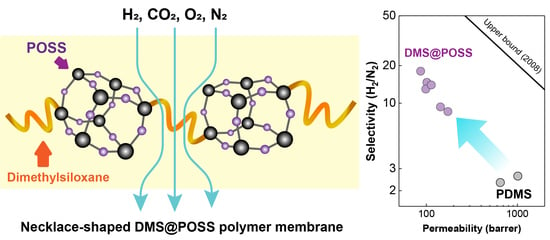
Study of Gases Permeation in Necklace-Shaped Dimethylsiloxane Polymers Bearing POSS Cages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membranes Fabrication
2.2. Characterization
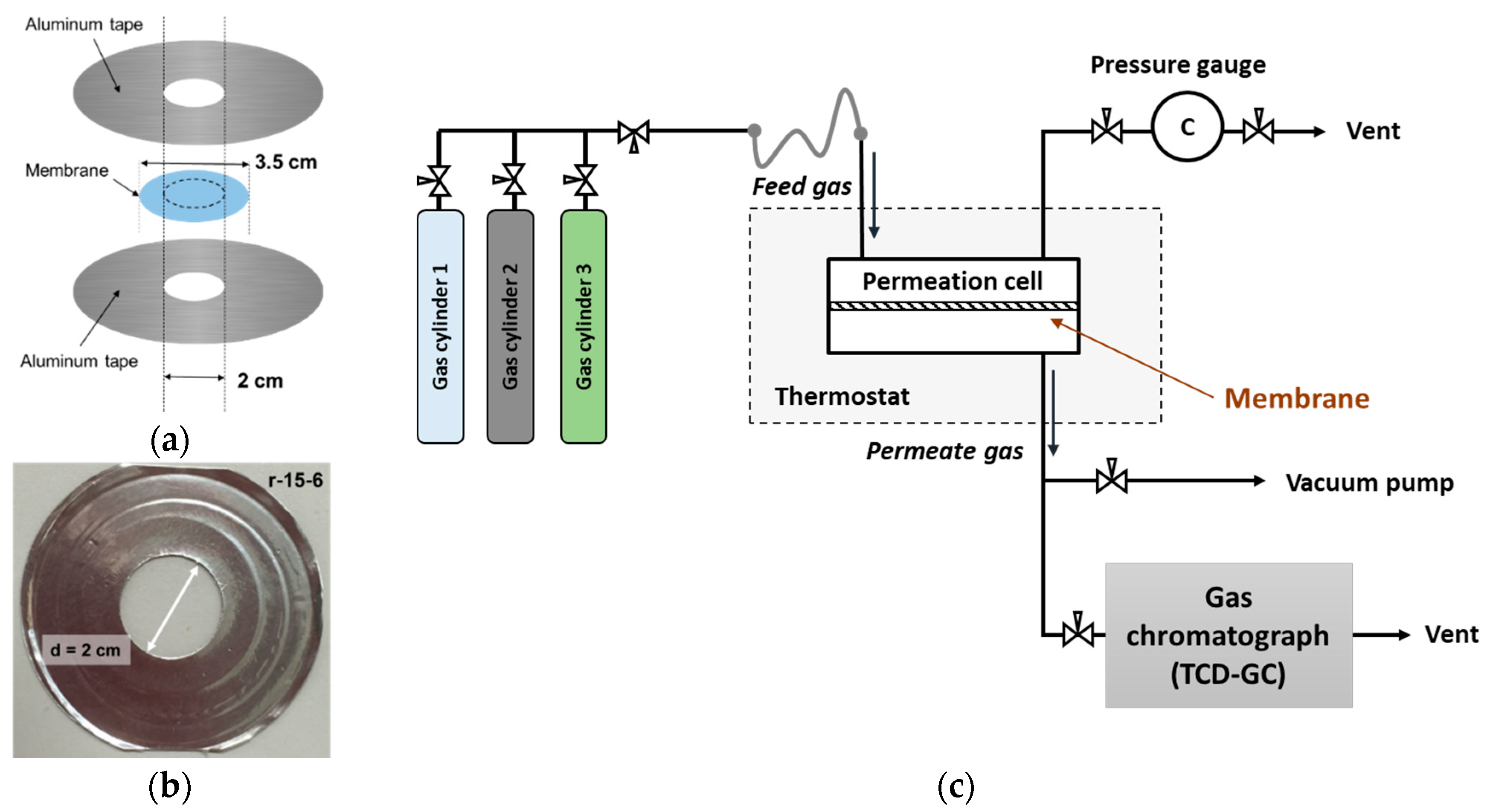
2.3. Gas Permeability Measurement
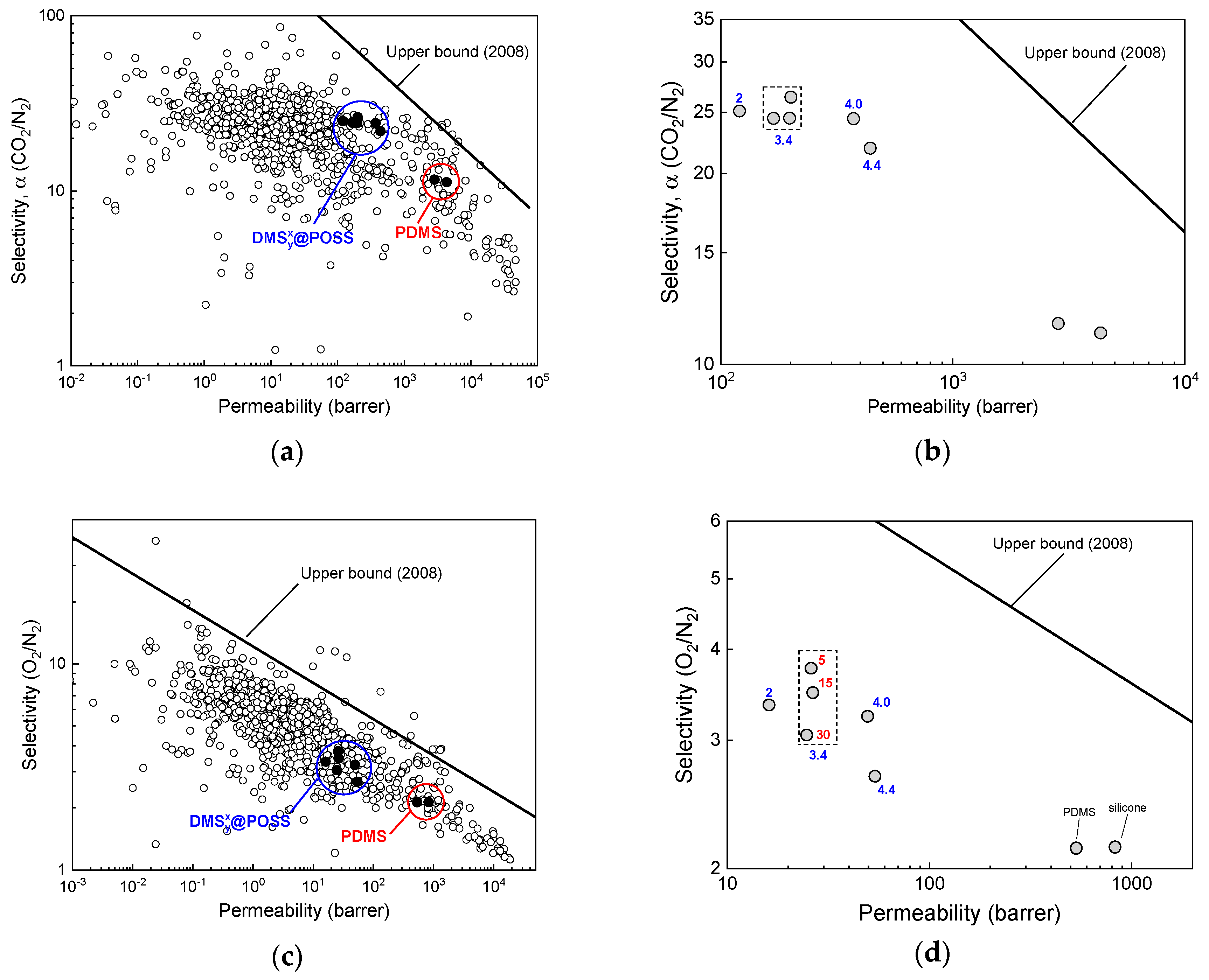
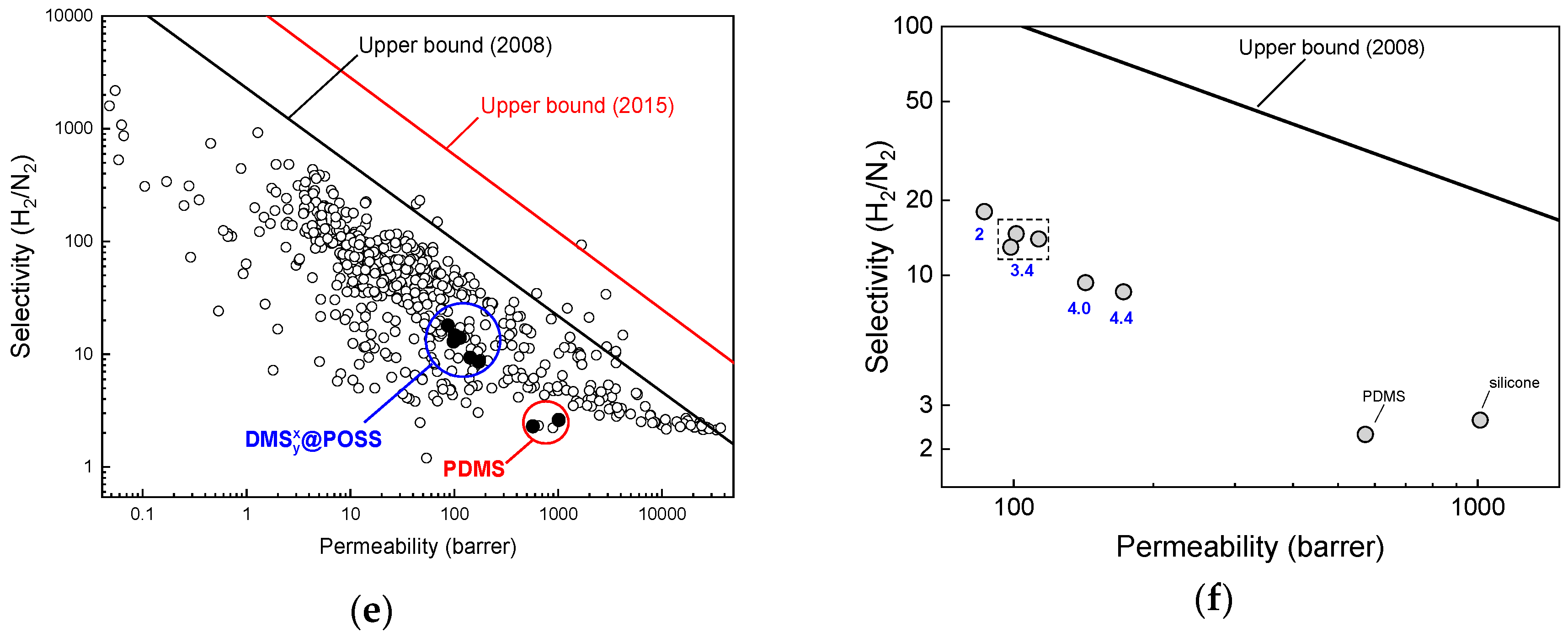
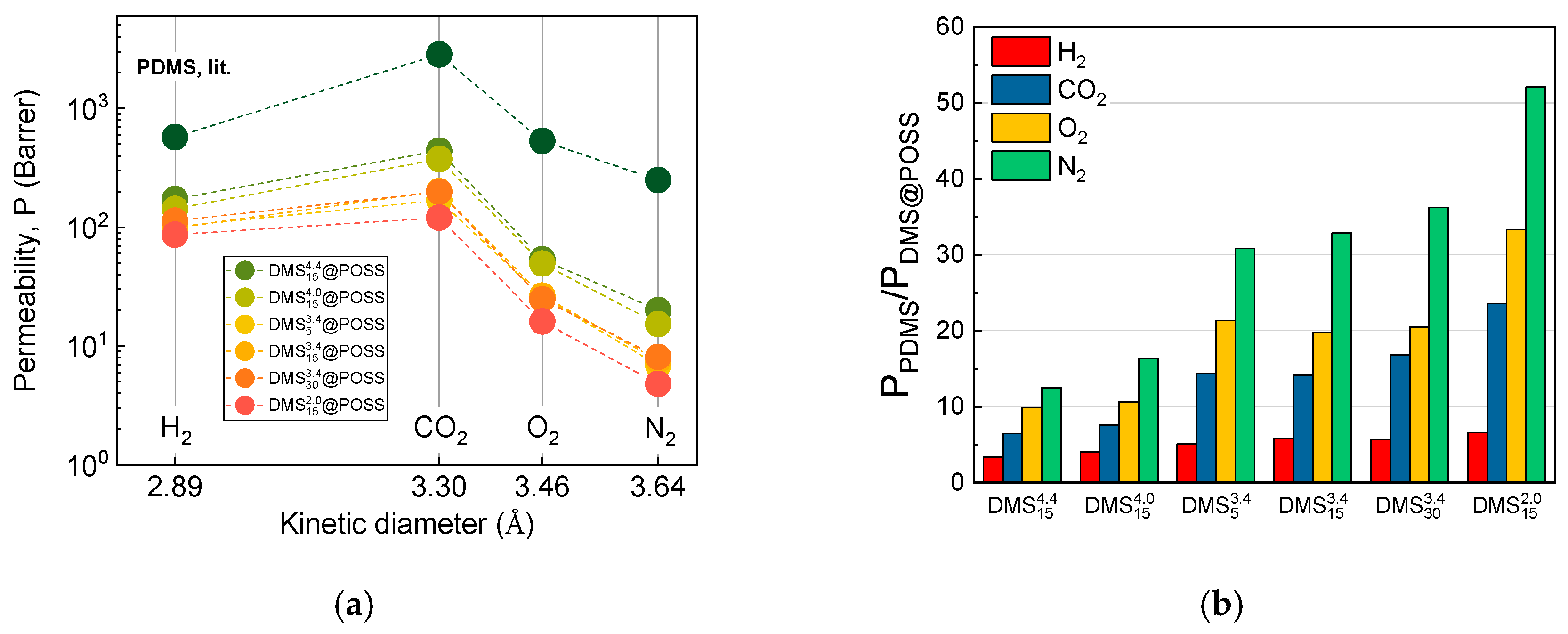
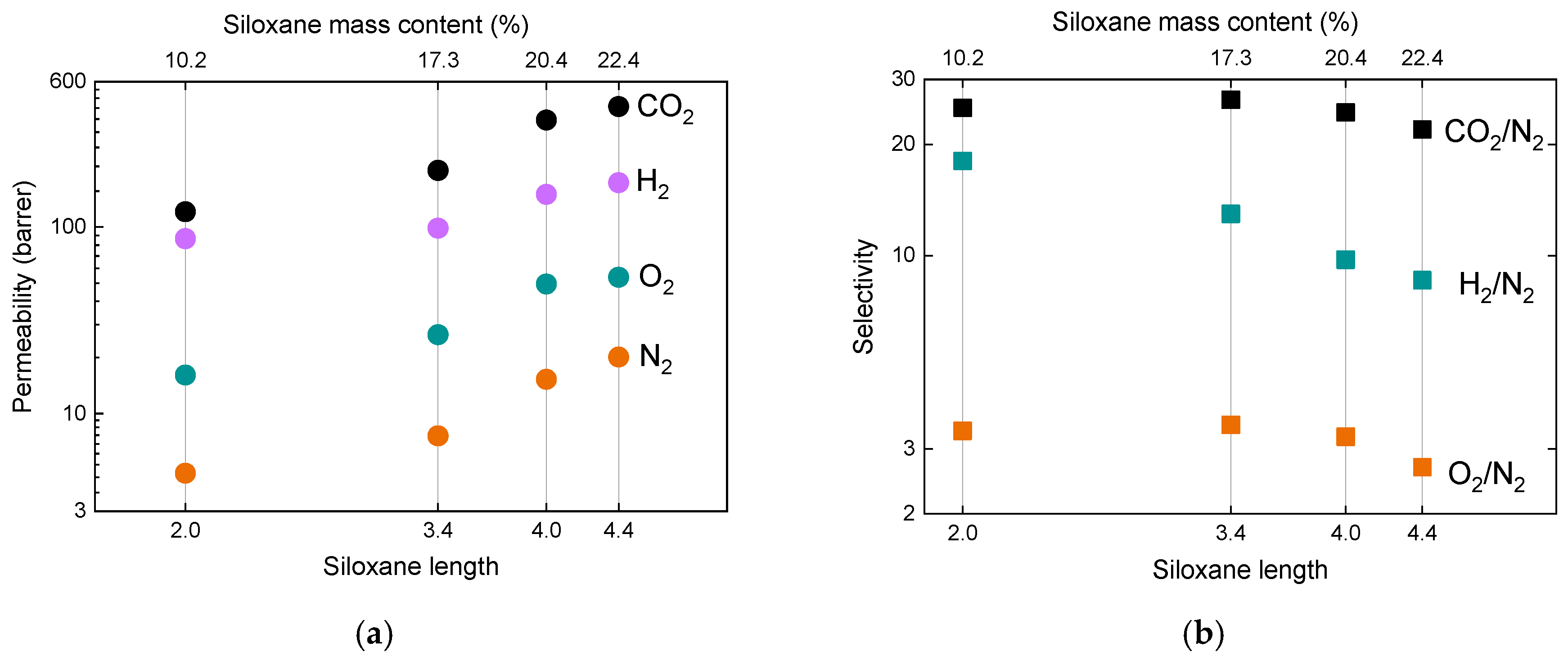
3. Results
3.1. Membranes Characterization
3.2. Gases Permeation at Room Temperature
3.3. Influence of the Temperature on the Gas Transport in Membranes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Blanco, I. The rediscovery of POSS: A molecule rather than a filler. Polymers 2018, 10, 3390. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, K.; Reddy, B.S.R. Structure-gas transport property relationships of poly(dimethylsiloxane-urethane) nanocomposite membranes. J. Memb. Sci. 2009, 342, 291–299. [Google Scholar] [CrossRef]
- Ghanbari, H.; Cousins, B.G.; Seifalian, A.M. A nanocage for nanomedicine: Polyhedral oligomeric silsesquioxane (POSS). Macromol. Rapid Commun. 2011, 32, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, J.; Zhou, J.; Jin, K.; Fang, Q. Fluorinated and Thermo-Cross-Linked Polyhedral Oligomeric Silsesquioxanes: New Organic-Inorganic Hybrid Materials for High-Performance Dielectric Application. ACS Appl. Mater. Interfaces 2017, 9, 12782–12790. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Tanaka, K.; Chujo, Y. Rational design of polyhedral oligomeric silsesquioxane fillers for simultaneous improvements of thermomechanical properties and lowering refractive indices of polymer films. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3583–3589. [Google Scholar] [CrossRef]
- Yoshimatsu, M.; Komori, K.; Ohnagamitsu, Y.; Sueyoshi, N.; Kawashima, N.; Chinen, S.; Murakami, Y.; Izumi, J.; Inoki, D.; Sakai, K.; et al. Necklace-shaped Dimethylsiloxane Polymers Bearing a Polyhedral Oligomeric Silsesquioxane Cage Prepared by Polycondensation and Ring-opening Polymerization. Chem. Lett. 2012, 41, 622–624. [Google Scholar] [CrossRef]
- Kunitake, M. Necklace-Shaped Dimethylsiloxane Polymers Bearing Polyhedral Oligomeric Silsesquioxane Cages as a New Type of Organic—Inorganic Hybrid. In New Polymeric Materials Based on Element-Blocks; Springer: Berlin/Heidelberg, Germany, 2019; pp. 139–151. ISBN 9789811328893. [Google Scholar]
- Katsuta, N.; Yoshimatsu, M.; Komori, K.; Natsuaki, T.; Suwa, K.; Sakai, K.; Matsuo, T.; Ohba, T.; Uemura, S.; Watanabe, S.; et al. Necklace-shaped dimethylsiloxane polymers bearing polyhedral oligomeric silsesquioxane cages with alternating length chains. Polymer (Guildf.) 2017, 127, 8–14. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Neumann, S.; Bolmer, S.; Khan, M.M.; Abetz, V. PEBAX® with PEG functionalized POSS as nanocomposite membranes for CO2 separation. J. Memb. Sci. 2013, 437, 286–297. [Google Scholar] [CrossRef]
- Raaijmakers, M.J.T.; Hempenius, M.A.; Schön, P.M.; Vancso, G.J.; Nijmeijer, A.; Wessling, M.; Benes, N.E. Sieving of hot gases by hyper-cross-linked nanoscale-hybrid membranes. J. Am. Chem. Soc. 2014, 136, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, M.J.T.; Wessling, M.; Nijmeijer, A.; Benes, N.E. Hybrid polyhedral oligomeric silsesquioxanes-imides with tailored intercage spacing for sieving of hot gases. Chem. Mater. 2014, 26, 3660–3664. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Y.; Zhang, H.; Zhou, Y.; Huang, C.; Xiong, C. Influence of Polyhedral Oligomeric Silsesquioxanes (POSS) on Thermal and Mechanical Properties of Polydimethylsiloxane (PDMS) Composites Filled with Fumed Silica. J. Inorg. Organomet. Polym. 2013, 23, 1375–1382. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Shi, Y.; Huang, G. Effect of polyhedral oligomeric silsesquioxanes (POSS) on crystallization behaviors of POSS/polydimethylsiloxane rubber nanocomposites. RSC Adv. 2014, 4, 6275–6283. [Google Scholar] [CrossRef]
- Madhavan, K.; Gnanasekaran, D.; Reddy, B.S.R. Poly(dimethylsiloxane-urethane) membranes: Effect of linear siloxane chain and caged silsesquioxane on gas transport properties. J. Polym. Res. 2011, 18, 1851–1861. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Vatani, A.; Mohammadi, T. Synthesis and gas transport properties of crosslinked poly(dimethylsiloxane) nanocomposite membranes using octatrimethylsiloxy POSS nanoparticles. J. Nat. Gas Sci. Eng. 2016, 30, 10–18. [Google Scholar] [CrossRef]
- Thornton, A.; Robeson, L.; Freeman, B.; Uhlmann, D. Polymer Gas Separation Membrane Database. Available online: https://membrane-australasia.org/msa-activities/polymer-gas-separation-membrane-database/ (accessed on 28 March 2019).
- Robeson, L.M. The upper bound revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Nakagawa, T.; Nishimura, T.; Higuchi, A. Morphology and gas permeability in copolyimides containing polydimethylsiloxane block. J. Memb. Sci. 2002, 206, 149–163. [Google Scholar] [CrossRef]
- Merkel, T.C.; Bondar, V.I.; Nagai, K.; Freeman, B.D.; Pinnau, I. Gas sorption, diffusion, and permeation in poly(dimethylsiloxane). J. Polym. Sci. Part B Polym. Phys. 2000, 38, 415–434. [Google Scholar] [CrossRef]
- Berean, K.; Ou, J.Z.; Nour, M.; Latham, K.; McSweeney, C.; Paull, D.; Halim, A.; Kentish, S.; Doherty, C.M.; Hill, A.J.; et al. The effect of crosslinking temperature on the permeability of PDMS membranes: Evidence of extraordinary CO2 and CH4 gas permeation. Sep. Purif. Technol. 2014, 122, 96–104. [Google Scholar] [CrossRef]
- Bhandarkar, M.; Shelekhin, A.B.; Dixon, A.G.; Ma, Y.H. Adsorption, permeation, and diffusion of gases in microporous membranes. I. Adsorption of gases on microporous glass membranes. J. Memb. Sci. 1992, 75, 221–231. [Google Scholar] [CrossRef]
- Komatsuka, T.; Nagai, K. Temperature Dependence on Gas Permeability and Permselectivity of Poly(lactic acid) Blend Membranes. Polym. J. 2009, 41, 455–458. [Google Scholar] [CrossRef]
- Guerrero, G.; Hägg, M.B.; Simon, C.; Peters, T.; Rival, N.; Denonville, C. CO2 separation in nanocomposite membranes by the addition of amidine and lactamide functionalized POSS® nanoparticles into a PVA layer. Membranes 2018, 8, 3390. [Google Scholar] [CrossRef]
- Selyanchyn, R.; Ariyoshi, M.; Fujikawa, S. Thickness Effect on CO2/N2 Separation in Double Layer Pebax-1657®/PDMS Membranes. Membranes 2018, 8, 121. [Google Scholar] [CrossRef]
- Selyanchyn, R.; Fujikawa, S. Membrane thinning for efficient CO2 capture. Sci. Technol. Adv. Mater. 2017, 18, 816–827. [Google Scholar] [CrossRef]
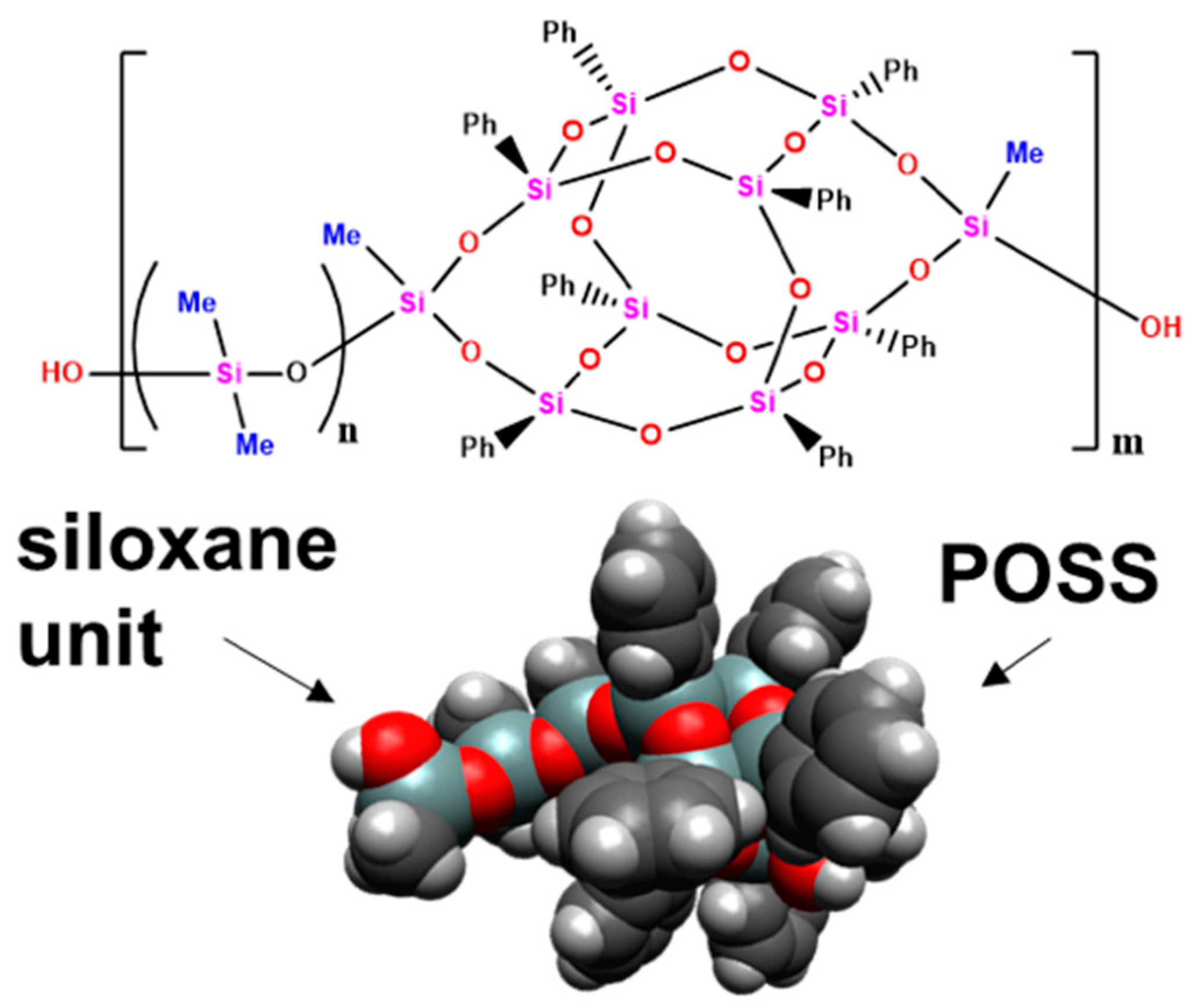


| Material Code | Average DMS Length, n | MW | MN | Crosslinking Ratio, MS-51:DMS@POSS | Siloxane Mass Content, % |
|---|---|---|---|---|---|
| 4.4 | 315,000 | 146,000 | 1:15 | 22.5 | |
| 4.0 | 110,000 | 81,500 | 1:15 | 20.9 | |
| 3.4 | 110,000 | 64,900 | 1:5 | 18.3 | |
| 3.4 | 110,000 | 64,900 | 1:15 | 18.3 | |
| 3.4 | 110,000 | 64,900 | 1:30 | 18.3 | |
| 2.0 * | 76,700 | 40,100 | 1:15 | 11.7 |
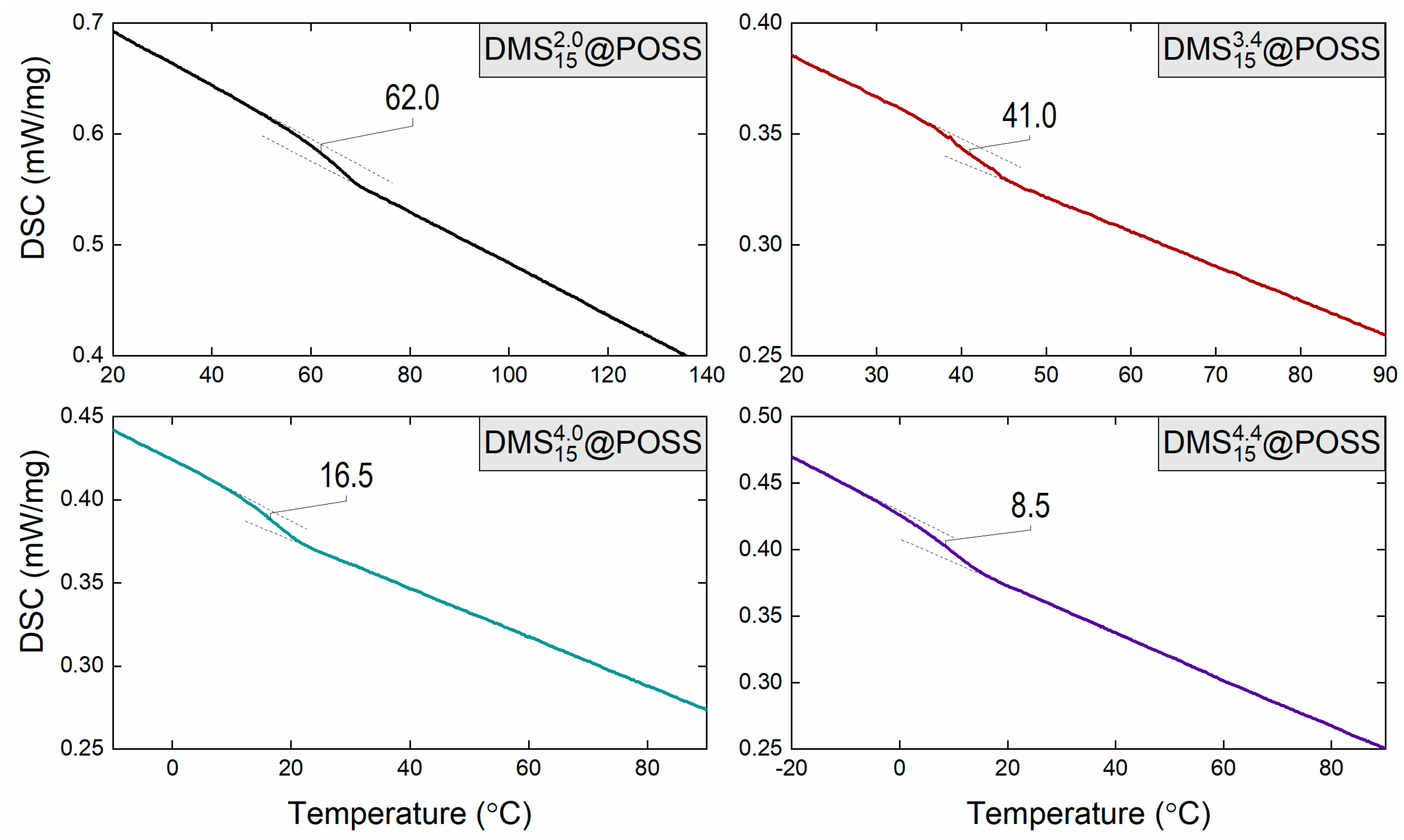
| Material | Tg, °C by TMA | Tg, °C by DSC |
|---|---|---|
| ~10 | 8.5 | |
| 18.6 | 16.5 | |
| 36.1 | – | |
| 36.1 | 41.0 | |
| 36.1 | – | |
| ~100 | 62.0 |
| Material | Average DMS Length, n | Permeability, Barrer | Siloxane Mass Content, % | |||
|---|---|---|---|---|---|---|
| H2 | CO2 | O2 | N2 | |||
| 2.0 * | 87 | 121 | 16 | 4.8 | 11.7 | |
| 3.4 | 101 | 169 | 26 | 6.9 | 18.3 | |
| 3.4 | 99 | 201 | 27 | 7.6 | 18.3 | |
| 3.4 | 113 | 198 | 25 | 8.1 | 18.3 | |
| 4.0 | 143 | 374 | 50 | 15.3 | 20.9 | |
| 4.4 | 173 | 441 | 54 | 20.1 | 22.5 | |
| PDMS [22,18] | n/a | 573 | 2850 | 533 | 250 | n/a * |
| Siloxane rubber | n/a | 1013 | 4343 | 828 | 387.2 | n/a * |
| Material | P0, N2 | EP (KJ/MOL), N2 | P0, CO2 | EP (KJ/MOL), CO2 |
|---|---|---|---|---|
| 1.41 × 106 | 30.3 | 1.19 × 105 | 16.6 | |
| 1.17 × 106 | 28.3 | 1.06 × 105 | 14.8 | |
| 1.44 × 106 | 30.2 | 1.95 × 105 | 17.2 | |
| PDMS [18,22] | 3.46 × 104 | 12.2 | 4.81 × 103 | 1.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selyanchyn, R.; Fujikawa, S.; Katsuta, N.; Suwa, K.; Kunitake, M. Study of Gases Permeation in Necklace-Shaped Dimethylsiloxane Polymers Bearing POSS Cages. Membranes 2019, 9, 54. https://doi.org/10.3390/membranes9040054
Selyanchyn R, Fujikawa S, Katsuta N, Suwa K, Kunitake M. Study of Gases Permeation in Necklace-Shaped Dimethylsiloxane Polymers Bearing POSS Cages. Membranes. 2019; 9(4):54. https://doi.org/10.3390/membranes9040054
Chicago/Turabian StyleSelyanchyn, Roman, Shigenori Fujikawa, Naohiro Katsuta, Kazuya Suwa, and Masashi Kunitake. 2019. "Study of Gases Permeation in Necklace-Shaped Dimethylsiloxane Polymers Bearing POSS Cages" Membranes 9, no. 4: 54. https://doi.org/10.3390/membranes9040054
APA StyleSelyanchyn, R., Fujikawa, S., Katsuta, N., Suwa, K., & Kunitake, M. (2019). Study of Gases Permeation in Necklace-Shaped Dimethylsiloxane Polymers Bearing POSS Cages. Membranes, 9(4), 54. https://doi.org/10.3390/membranes9040054