Bioelectrochemical Properties of Enzyme-Containing Multilayer Polyelectrolyte Microcapsules Modified with Multiwalled Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Formation of a Prussian Blue-Based Chemical Sensor
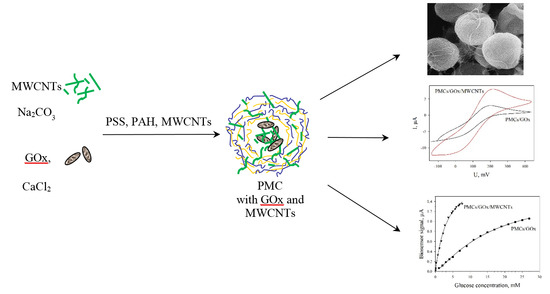
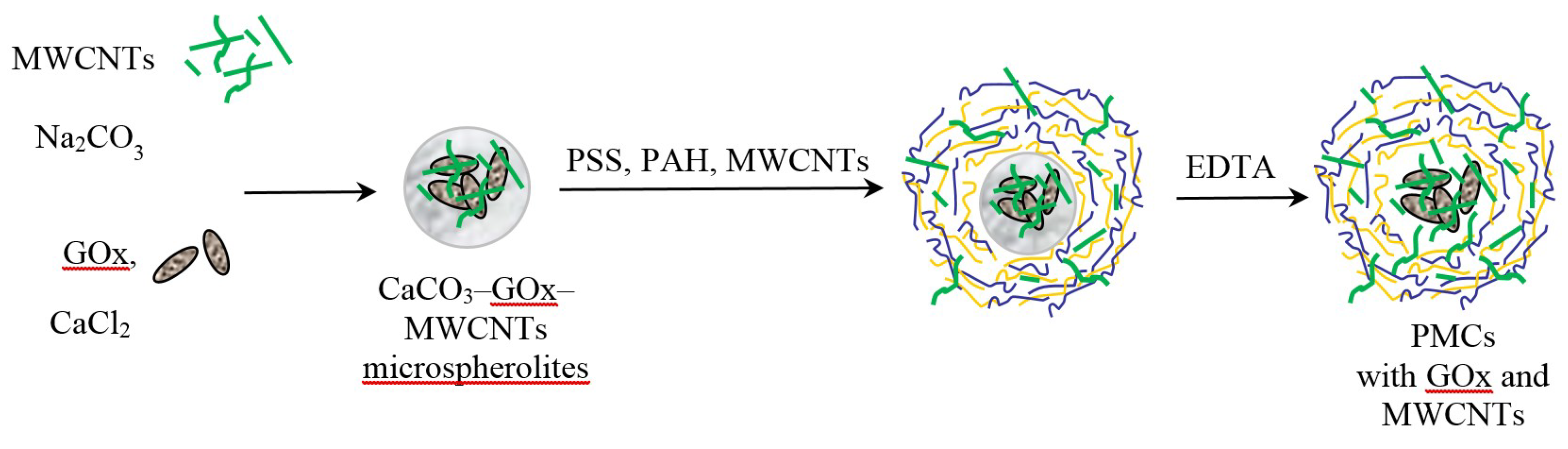
2.3. Formation of CaCO3-Protein Microspherolites
2.4. Preparation of PMCs
2.5. Formation of Glucose Biosensors
2.6. Optical Microscopy
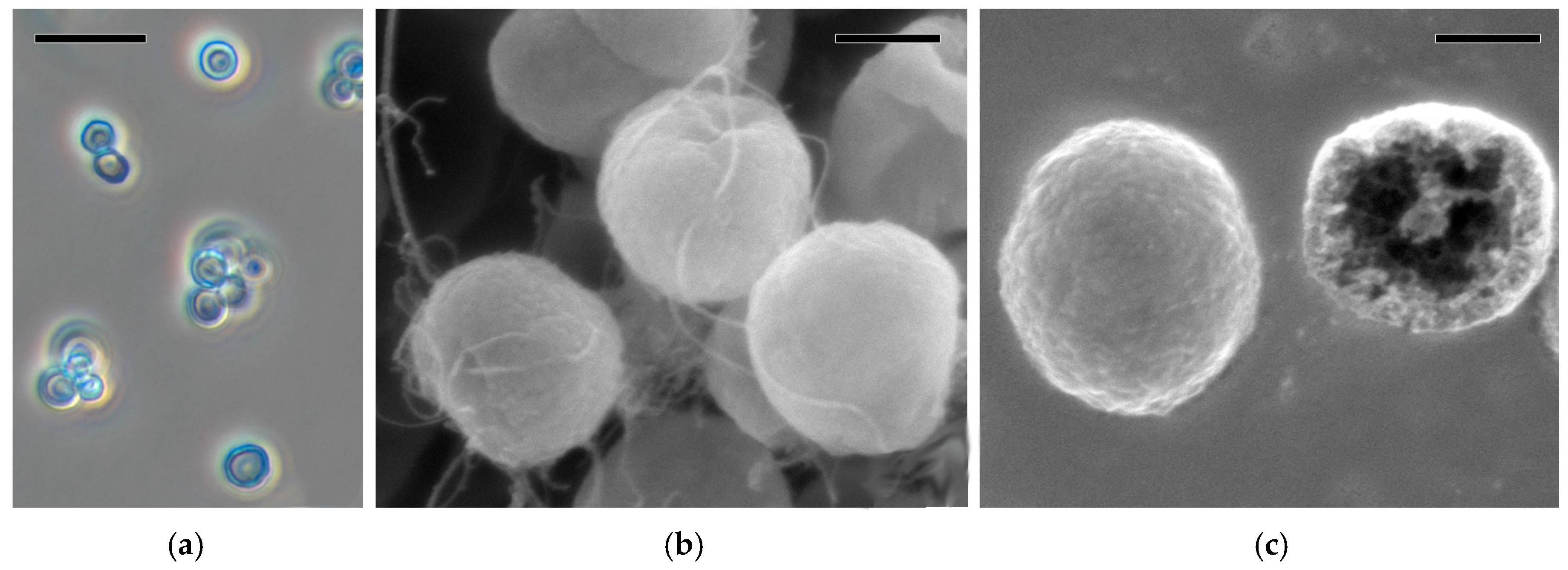
2.7. Scanning Electron Microscopy
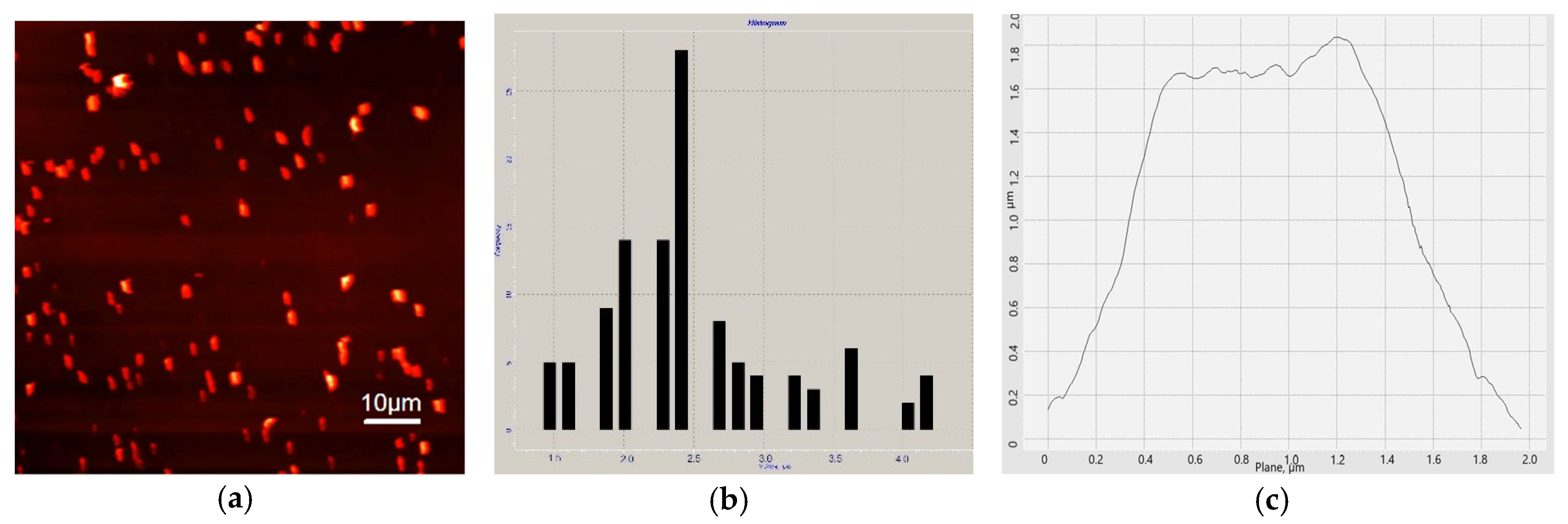
2.8. Atomic Force Microscopy
3. Results
3.1. Characterization of PMCs by AFM and SEM
3.2. Impedance Characteristics of Various Types of Electrodes
3.3. Cyclic Voltammograms of Modified Electrodes
3.4. Determination of Kinetic Parameters of PMC/GOx and PMC/GOx/MWCNTs Biosensors
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strawski, M.; Granicka, L.H.; Szklarczyk, M. Redox properties of polyelectrolyte multilayer modified electrodes: A significant effect of the interactions between the polyelectrolyte layers in the films. Electrochim. Acta 2017, 226, 121–131. [Google Scholar] [CrossRef]
- Lalonde, J.; Margolin, A. Immobilization of enzymes. In Enzyme Catalysis in Organic Synthesis, 2nd ed.; Drauz, K., Waldmann, H., Eds.; WileyVCH Verlag GmbH: Weinheim, Germany, 2002; pp. 163–184. ISBN 3-527-29949-1. [Google Scholar]
- Gemeiner, P. Enzyme Engineering: Immobilized Biosystems; Ellis Horwood, Ltd.: Chichester, UK, 1992. [Google Scholar]
- Peyratout, C.S.; Dähne, L. Tailor-made polyelectrolyte microcapsules: From multilayers to smart containers. Angew. Chem. Int. Ed. 2004, 43, 3762–3783. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Mateos-Maroto, A.; Ruano, M.; Ortega, F.; Rubio, R.G. Layer-by-layer polyelectrolyte assemblies for encapsulation and release of active compounds. Adv. Colloid Interface Sci. 2017, 249, 290–307. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Charaabi, S.; Picton, L.; Le Cerf, D.; Carbonnier, B. Glucose-sensitive polyelectrolyte microcapsules based on (alginate/chitosan) pair. Carbohydr. Polym. 2018, 184, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Decher, G.; Hong, J.D. Buildup of ultrathin multilayer films by a self-assembly process, 1: Consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Brett, C.M.A. Perspectives and challenges for self-assembled layer-by-layer biosensor and biomaterial architectures. Curr. Opin. Electrochem. 2018, 12, 21–26. [Google Scholar] [CrossRef]
- Ivanov, A.; Davletshina, R.; Sharafieva, I.; Evtugyn, G. Electrochemical biosensor based on polyelectrolyte complexes for the determination of reversible inhibitors of acetylcholinesterase. Talanta 2019, 194, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Eremenko, A.V.; Varfolomeev, S.D.; Yaroslavov, A.A.; Pergushov, D.V.; Papkov, V.S.; Sigolaeva, L.V.; Kurochkin, I.N.; Zezin, A.B.; Tur, D.R.; Dubacheva, G.V.; et al. Nanostructured polyelectrolyte films for engineering highly sensitive tyrosinase biosensors: Specifics of enzyme-polyelectrolyte structures. Nanotechnol. Russ. 2008, 3, 221–227. [Google Scholar] [CrossRef]
- Lin, C.; Chen, Q.; Yi, S.; Wang, M.; Regen, S.L. Polyelectrolyte multilayers on PTMSP as asymmetric membranes for gas separations: Langmuir–Blodgett versus self-assembly methods of anchoring. Langmuir 2014, 30, 687–691. [Google Scholar] [CrossRef]
- Gribova, V.; Auzely-Velty, R.; Picart, C. Polyelectrolyte multilayer assemblies on materials surfaces: From cell adhesion to tissue engineering. Chem. Mater. 2012, 24, 854–869. [Google Scholar] [CrossRef]
- Illergård, J.; Römling, U.; Wågberg, L.; Ek, M. Biointeractive antibacterial fibres using polyelectrolyte multilayer modification. Cellulose 2012, 19, 1731–1741. [Google Scholar] [CrossRef]
- Lichter, J.A.; Van Vlietpa, K.J.; Rubner, M.F. Design of antibacterial surfaces and interfaces: Polyelectrolyte multilayers as a multifunctional platform. Macromolecules 2009, 42, 8573–8586. [Google Scholar] [CrossRef]
- Larrañaga, A.; Lomora, M.; Sarasua, J.R.; Palivan, C.G.; Pandit, A. Polymer capsules as micro-/nanoreactors for therapeutic applications: Current strategies to control membrane permeability. Prog. Mater. Sci. 2017, 90, 325–357. [Google Scholar] [CrossRef]
- Cerqueira, M.Â.; Pinheiro, A.C.; Ramos, O.L.; Silva, H.; Bourbon, A.I.; Vicente, A.A. Advances in Food Nanotechnology. In Emerging Nanotechnologies in Food Science; Elsevier: Boston, MA, USA, 2017; pp. 11–38. ISBN 9780323429801. [Google Scholar]
- Montazer, M.; Harifi, T. Nanoencapsulation techniques for textile finishing. In Nanofinishing of Textile Materials; Woodhead Publishing: Sawston, UK, 2018; pp. 295–310. ISBN 9780081012505. [Google Scholar]
- Balkundi, S.S.; Veerabadran, N.G.; Eby, D.M.; Johnson, G.R.; Lvov, Y.M. Encapsulation of bacterial spores in nanoorganized polyelectrolyte shells. Langmuir 2009, 25, 14011–14016. [Google Scholar] [CrossRef]
- Ternovskij, V.I.; Chernohvostov, Y.u.V.; Fomkina, M.G.; Montrel, M.M. Electrometric sensor on the basis of the urease immobilized in polyelectrolytic microcapsules. Biofizika 2007, 52, 825–829. [Google Scholar]
- Montrel, M.M.; Ternovskij, V.I.; Fomkina, M.G.; Petrov, A.I. Ultrathin Polymeric Covering, Way of His Production and Enzymatic Biosensor on Its Basis. Patent No. 2333231 2008. (In Russian). [Google Scholar]
- Reshetilov, A.N.; Plekhanova, Y.V.; Tikhonenko, S.A.; Dubrovskii, A.V. Polyelectrolyte microcapsules with urease and paramagnetic particles as a basis for a potentiometric biosensor for determining urea. J. Anal. Chem. 2015, 70, 1368–1372. [Google Scholar] [CrossRef]
- Yagodina, L.O.; Chernokhvostov, Yu.V. Immobilizatsiya acetilkholisterazi inkapsulatsiei v polimernix nano- i mikrokapsulah. Vestn. Kazan Technol. Univ. 2013, 16, 117–119. [Google Scholar]
- Kazakova, L.I.; Sirota, N.P.; Sirota, T.V.; Shabarchina, L.I. The study of a fluorescent biosensor based on polyelectrolyte microcapsules with encapsulated glucose oxidase. Russ. J. Phys. Chem. A 2017, 91, 1828–1832. [Google Scholar] [CrossRef]
- Šefčovičová, J.; Tkac, J. Application of nanomaterials in microbial-cell biosensor constructions. Chem. Pap. 2015, 69, 42–53. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Hassan, S.H.A.; Oh, S.E.; Ismail, M.; Rahimnejad, M.; Jahim, J.M. Nano-structured carbon as electrode material in microbial fuel cells: A comprehensive review. J. Alloys Compd. 2013, 580, 245–255. [Google Scholar] [CrossRef]
- Cosnier, S.; Holzinger, M.; Le Goff, A. Recent advances in carbon nanotube-based enzymatic fuel cells. Front. Bioeng. Biotechnol. 2014, 2. [Google Scholar] [CrossRef]
- Karimi, A.; Othman, A.; Uzunoglu, A.; Stanciu, L.; Andreescu, S. Graphene based enzymatic bioelectrodes and biofuel cells. Nanoscale 2015, 7, 6909–6923. [Google Scholar] [CrossRef]
- Trifonov, A.; Herkendell, K.; Tel-Vered, R.; Yehezkeli, O.; Woerner, M.; Willner, I. Enzyme-capped relay-functionalized mesoporous carbon nanoparticles: Effective bioelectrocatalytic matrices for sensing and biofuel cell applications. ACS Nano 2013, 7, 11358–11368. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Kitazumi, Y.; Shirai, O.; Kano, K. Effects of mesoporous structures on direct electron transfer-type bioelectrocatalysis: Facts and simulation on a three-dimensional model of random orientation of enzymes. Electrochemistry 2017, 85, 82–87. [Google Scholar] [CrossRef]
- Gross, A.J.; Holzinger, M.; Cosnier, S. Buckypaper bioelectrodes: Emerging materials for implantable and wearable biofuel cells. Energy Environ. Sci. 2018, 11, 1670–1687. [Google Scholar] [CrossRef]
- Barsan, M.M.; Brett, C.M.A. Graphene and carbon nanotube nanomaterials in layer-by-layer structured electrochemical enzymatic biosensors: A review. Stud. Univ. Babes Bolyai Chem. 2015, 60, 31–52. [Google Scholar]
- Gao, Q.; Guo, Y.; Zhang, W.; Qi, H.; Zhang, C. An amperometric glucose biosensor based on layer-by-layer GOx–SWCNT conjugate/redox polymer multilayer on a screen-printed carbon electrode. Sens. Actuators B Chem. 2011, 153, 219–225. [Google Scholar] [CrossRef]
- Gao, Q.; Guo, Y.; Liu, J.; Yuan, X.; Qi, H.; Zhang, C. A biosensor prepared by co-entrapment of a glucose oxidase and a carbon nanotube within an electrochemically deposited redox polymer multilayer. Bioelectrochemistry 2011, 81, 109–113. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Gitelmacher, O.V.; Karyakina, E.E. A high-sensitive glucose amperometric biosensor based on prussian blue modified electrodes. Anal. Lett. 1994, 27, 2861–2869. [Google Scholar] [CrossRef]
- Petrov, A.I.; Volodkin, D.V.; Sukhorukov, G.B. Protein–calcium carbonate coprecipitation: A tool for protein encapsulation. Biotechnol. Prog. 2005, 21, 918–925. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Dubrovskii, A.V.; Moshkov, D.A.; Shabarchina, L.I.; Sukhorukov, B.I. An electron microscopy study of the structure of polyelectrolyte microcapsules containing protein and containing no protein. Biofizika 2007, 52, 850–854. [Google Scholar]
- Fiorito, P.A.; Brett, C.M.A.; Córdoba De Torresi, S.I. Polypyrrole/copper hexacyanoferrate hybrid as redox mediator for glucose biosensors. Talanta 2006, 69, 403–408. [Google Scholar] [CrossRef]
- Li, J.; Qiu, J.D.; Xu, J.J.; Chen, H.Y.; Xia, X.H. The synergistic effect of prussian-blue-grafted carbon nanotube/poly(4-vinylpyridine) composites for amperometric sensing. Adv. Funct. Mater. 2007, 17, 1574–1580. [Google Scholar] [CrossRef]
- Ridhuan, N.S.; Abdul Razak, K.; Lockman, Z. Fabrication and Characterization of Glucose Biosensors by Using Hydrothermally Grown ZnO Nanorods. Sci. Rep. 2018, 8, 13722. [Google Scholar] [CrossRef]
- Rassas, I.; Braiek, M.; Bonhomme, A.; Bessueille, F.; Raffin, G.; Majdoub, H.; Jaffrezic-Renault, N. Highly sensitive voltammetric glucose biosensor based on glucose oxidase encapsulated in a chitosan/kappa-carrageenan/gold nanoparticle bionanocomposite. Sensors 2019, 19, 154. [Google Scholar] [CrossRef]
- Çetin, M.Z.; Camurlu, P. An amperometric glucose biosensor based on PEDOT nanofibers. RSC Adv. 2018, 8, 19724–19731. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Hernandez-Rangel, A.; Pal, U.; Ceballos-Sanchez, O.; Flores-Ruiz, F.J.; Prokhorov, E.; Arias De Fuentes, O.; Esparza, R.; Meyyappan, M. Surface functionalized halloysite nanotubes decorated with silver nanoparticles for enzyme immobilization and biosensing. J. Mater. Chem. B 2016, 4, 2553–2560. [Google Scholar] [CrossRef]
- Hou, C.; He, F.; Liu, M.; Wang, Y.; Li, X.; Zhang, Y. Preparation of graphene nano-sheet bonded PDA/MOF microcapsules with immobilized glucose oxidase as a mimetic multi-enzyme system for electrochemical sensing of glucose. J. Mater. Chem. B 2016, 4, 3695–3702. [Google Scholar]
- Hervás Pérez, J.P.; López-Ruiz, B.; López-Cabarcos, E. Synthesis and characterization of microparticles based on poly-methacrylic acid with glucose oxidase for biosensor applications. Talanta 2016, 149, 310–318. [Google Scholar] [CrossRef]
- Chapel, J.P.; Berret, J.F. Versatile electrostatic assembly of nanoparticles and polyelectrolytes: Coating, clustering and layer-by-layer processes. Curr. Opin. Colloid Interface Sci. 2012, 17, 97–105. [Google Scholar] [CrossRef]
- Ma, L.; Lu, W.; Wen, J. Encapsulation of lactate dehydrogenase in carbon nanotube doped alginate-chitosan capsules. J. Mol. Catal. B Enzym. 2009, 56, 102–107. [Google Scholar] [CrossRef]
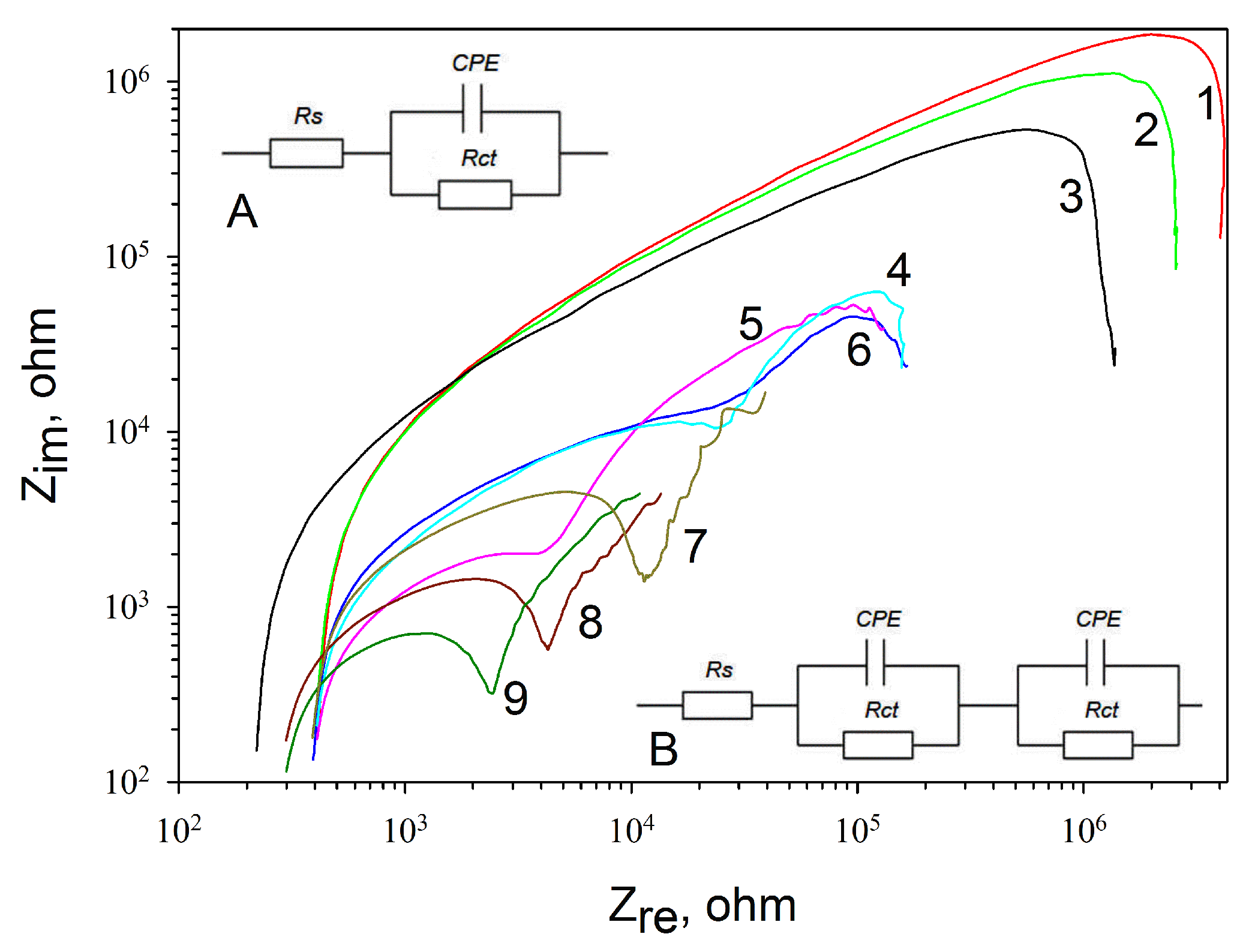
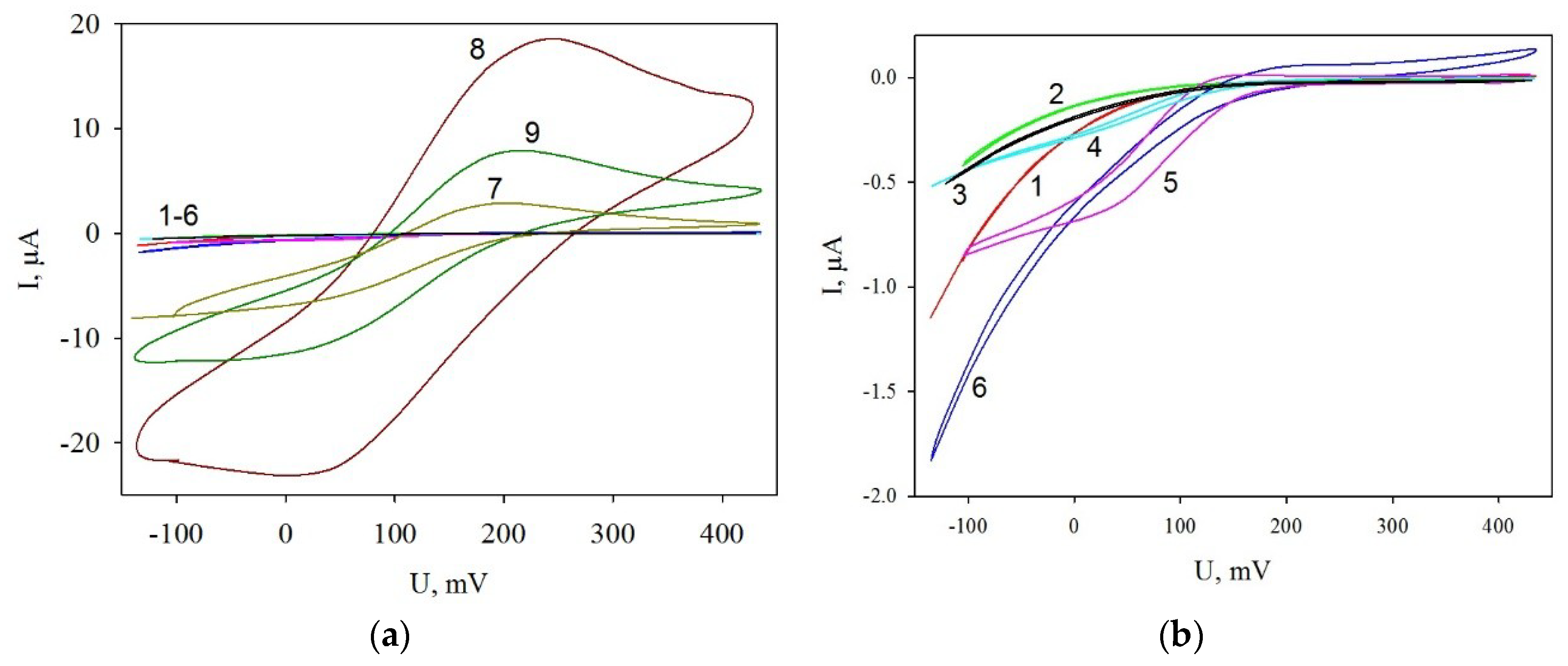
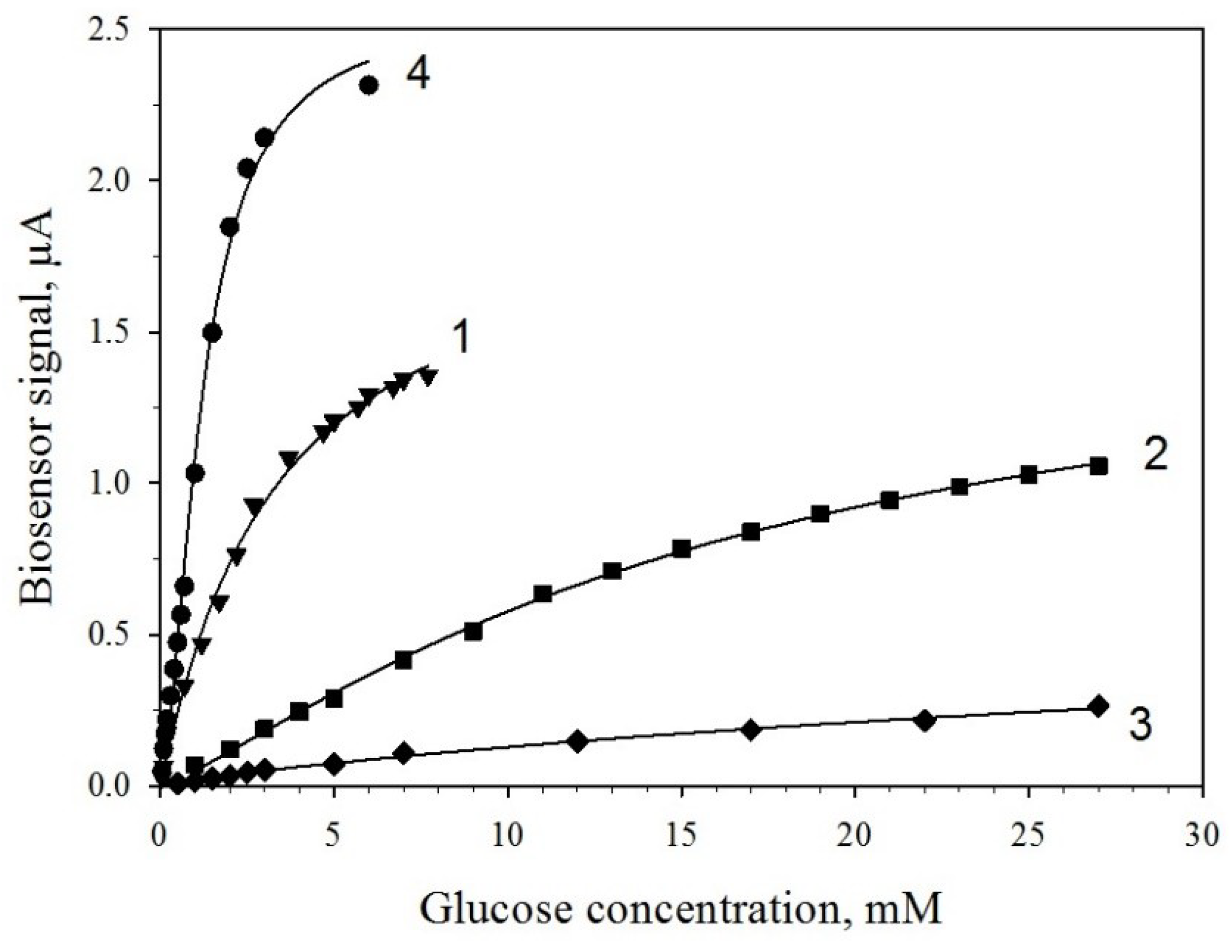
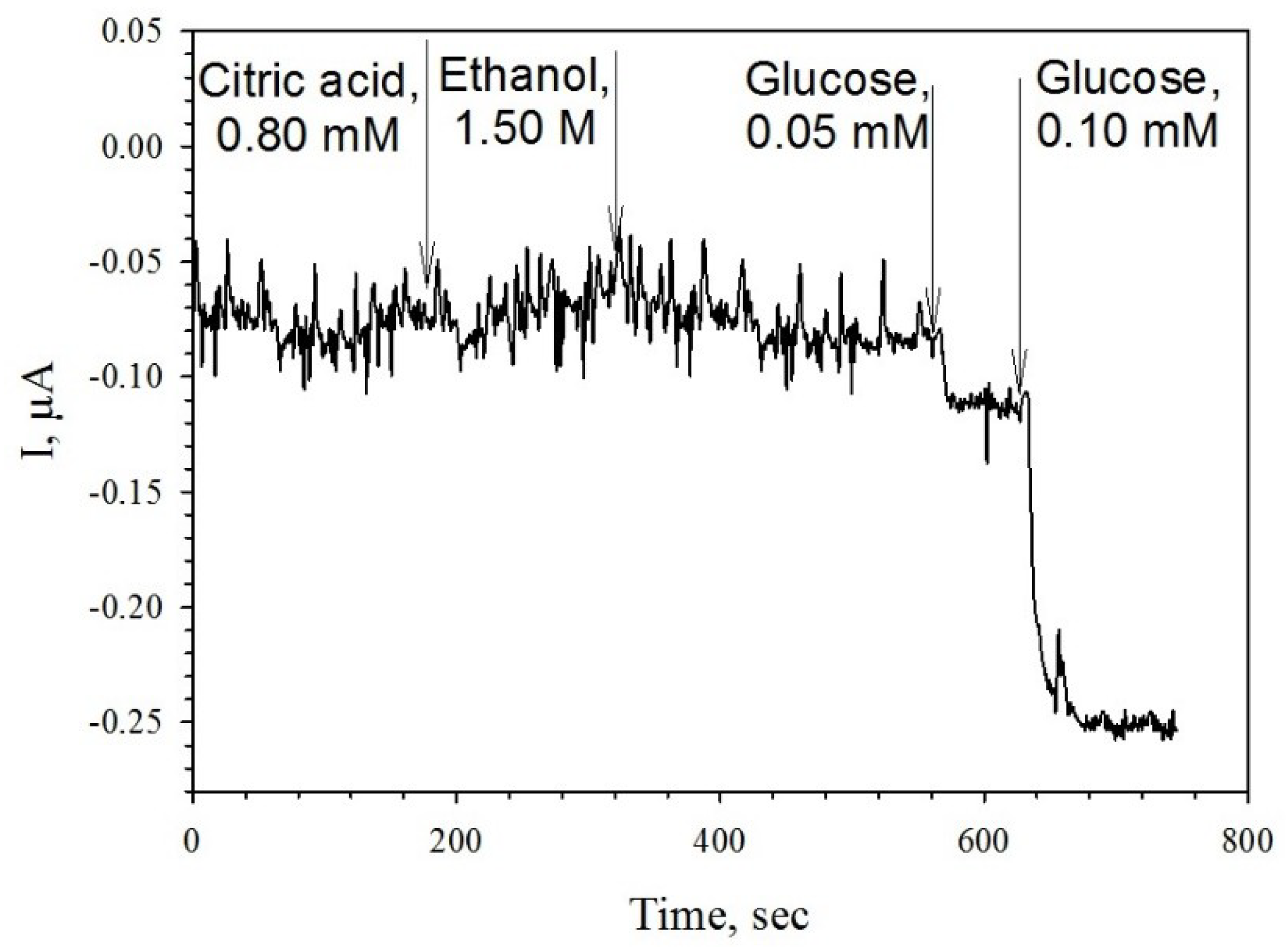
| Graphite Electrodes Modified with Various Components | Total Resistance, kOhm |
|---|---|
| Nonmodified screen-printed electrodes | 4200 ± 200 |
| PMCs | 2560 ± 170 |
| PMCs/MWCNTs in the hollow | 172 ± 8 |
| PMCs/MWCNTs between layers of polyelectrolytes | 181 ± 6 |
| PMCs/MWCNTs in the hollow and between layers of polyelectrolytes | 162 ± 6 |
| GOx | 1200 ± 30 |
| MWCNTs | 34 ± 1 |
| PMCs with GOx | 120 ± 4 |
| PMCs with GOx and MWCNTs in the hollow and between layers of polyelectrolytes | 25 ± 1 |
| Composition of Biocatalyst | PMCs/GOx/MWCNTs * | PMCs/GOx/MWCNTs | PMCs/GOx | GOx | |
|---|---|---|---|---|---|
| Parameter | |||||
| Equation describing the calibration dependence | |||||
| Parameter values of the calibration dependence | Vmax = 2.560; h = 1.667; Km= 1.203; R2 = 0.99 | Vmax = 1.938; h = 1.050; Km = 3.194; R2 = 0.99 | Vmax = 1.639; h = 1.238; Km = 16.393; R2 = 0.99 | Vmax = 0.959; h = 0.865; Km = 86.692; R2 = 0.99 | |
| Linear range of detection, mM | 0.05–2 | 0.2–2.7 | 1–15 | 0.5–7 | |
| Regression equation for the linear segment, correlation coefficient R2 | y = 0.9443x + 0.0193, R2 = 0.99 | y = 0.2966x − 0.1161, R2 = 0.99 | y = 0.0524x + 0.0293, R2 = 0.99 | y = 0.0149x + 0.0029, R2 = 0.99 | |
| Sensitivity coefficient, μA/mM | 0.94 | 0.30 | 0.05 | 0.01 | |
| Minimal range of detection, mM | 0.05 | 0.05 | 1 | 0.5 | |
| Detection range, mM | 0.05–3 | 0.05–6 | 1–25 | 0.5–25 | |
| Biosensor Composition | Limit of Detection, μM | Sensivity | References |
|---|---|---|---|
| Nafion/GOx/ZnO/ITO | 50 | 3.87 µA/mM·cm2 | [40] |
| PEC/AuNPs/GOx/Au | 5 | 283.9 µA/log[glucose] | [41] |
| Poly(3,4-ethylenedioxythiophene) nanofiber based glucose biosensor | 67.8 | 272.58 μA/mM cm2 | [42] |
| GOx/AgNPs/HNTs | 200 | 5.1 μA/mM cm2 | [43] |
| rGO/PDA/MOF/GOx | 0.3 | 9.6 μA/mM cm2 | [44] |
| p-MAA/Nafion/GOx | 10 | 12.0 μA/mM cm2 | [45] |
| PMCs/MWCNTs/GOx | 50 | 13.4 μA/mM cm2 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reshetilov, A.; Plekhanova, Y.; Tarasov, S.; Tikhonenko, S.; Dubrovsky, A.; Kim, A.; Kashin, V.; Machulin, A.; Wang, G.-J.; Kolesov, V.; et al. Bioelectrochemical Properties of Enzyme-Containing Multilayer Polyelectrolyte Microcapsules Modified with Multiwalled Carbon Nanotubes. Membranes 2019, 9, 53. https://doi.org/10.3390/membranes9040053
Reshetilov A, Plekhanova Y, Tarasov S, Tikhonenko S, Dubrovsky A, Kim A, Kashin V, Machulin A, Wang G-J, Kolesov V, et al. Bioelectrochemical Properties of Enzyme-Containing Multilayer Polyelectrolyte Microcapsules Modified with Multiwalled Carbon Nanotubes. Membranes. 2019; 9(4):53. https://doi.org/10.3390/membranes9040053
Chicago/Turabian StyleReshetilov, Anatoly, Yulia Plekhanova, Sergei Tarasov, Sergei Tikhonenko, Alexey Dubrovsky, Alexander Kim, Vadim Kashin, Andrey Machulin, Gou-Jen Wang, Vladimir Kolesov, and et al. 2019. "Bioelectrochemical Properties of Enzyme-Containing Multilayer Polyelectrolyte Microcapsules Modified with Multiwalled Carbon Nanotubes" Membranes 9, no. 4: 53. https://doi.org/10.3390/membranes9040053
APA StyleReshetilov, A., Plekhanova, Y., Tarasov, S., Tikhonenko, S., Dubrovsky, A., Kim, A., Kashin, V., Machulin, A., Wang, G.-J., Kolesov, V., & Kuznetsova, I. (2019). Bioelectrochemical Properties of Enzyme-Containing Multilayer Polyelectrolyte Microcapsules Modified with Multiwalled Carbon Nanotubes. Membranes, 9(4), 53. https://doi.org/10.3390/membranes9040053