1. Introduction
Membrane filtration systems are conceived to perform species separation. They consist of selecting semi-permeable membranes that retain the targeted species, while some others cross the membranes. The retained species then accumulate in a mass boundary layer that develops along the membrane inner surface, giving rise to the so-called polarization of concentration. Such an increase in concentration at the membrane, which results from the competition between advection towards the membrane and diffusion back to the bulk, may induce two types of hindrance to permeation: osmotic (counter-)effects and membrane fouling. This is why particular efforts have been paid to reduce the polarization of concentration through special devices as obstacles or vortices that can prevent the development of a mass boundary layer over a long distance.
On the one hand, the polarization of concentration increases the osmotic pressure between both sides of the membrane. This can result in a severe reduction of the effective operating pressure. This effect, typical of reverse osmosis and nanofiltration, has been the object of a previous theoretical study [
1], the result of which showed that osmosis causes an inflection of logarithmic type in permeation versus pressure [
1]. In the present article, which is our first contribution on fouling, we choose parameters that minimize the effects of osmotic (counter-)pressure. The focus on fouling may help to discriminate fouling from osmotic effects—which are often intertwined by pointing out a behaviour specific to fouling. In this way, the present paper will confirm that limiting flux is caused by fouling, while logarithmic growth is due to osmosis.
On the other hand, membrane fouling is known to affect most of the filtration devices. In particular, fouling corresponds to the most dangerous hindrance that can affect ultrafiltration (although some analyses showed that fouling and osmotic effects may, however, appear as deeply interconnected [
2]). It consists of material deposition due to various possible phenomena: the solution precipitates, a gel develops on the membrane, the over-saturation induces the growth of a solid layer along the membrane, the solute is substantially adsorbed within the membrane pores, etc. This additional material results in an increase in membrane resistance leading to the limiting flux and, finally, in operational cost due to a larger energy demand, an additional effort for cleaning and a shorter membrane life.
In the experimental practice, two kinds of fouling are mainly considered. Fouling is conceived as reversible if the membrane properties are recovered after its cleaning by the solvent, while it is said irreversible when fouling remains after cleaning. Numerous investigations have been devoted to point out and characterize the nature of the fouling. An important literature endeavours to describe the plausible mechanisms and their principal factors, as well as supplying elements for modelling. Of particular interest, the critical flux concept has been established in the mid 1990s [
3,
4,
5] to describe the flux below for which fouling remains insignificant. Further developments on critical flux which concern theory, experimental measurements and applications are summarised in the review paper [
6]. Worthy of note is the experimental method that permits the differentiation between reversible and irreversible fouling [
7]. It is evidently desirable to characterize the spatial dependence of fouling. Thus, experimentalists have carried out time-dependent local measurements of fouling occurrence and growth rate, which have resorted to various methods: X-ray techniques [
8], optical investigation techniques [
9], or nuclear magnetic resonance [
10].
Because solute is carried towards the membrane inner surface by the flow, the understanding of the concentration polarization phenomenon requires to characterize the hydrodynamics related to membrane cross-flow filtration. In that domain, the art started early with the seminal papers [
11,
12,
13,
14] which supposed that solute transport did not affect the flow. The current state in analytical theories [
15,
16,
17,
18,
19] focus on the possible couplings between filtration and pressure variation along the inner side of the membrane.
On the other side, for given particular filtration flows, concentration polarization has benefited from numerous theoretical investigations. The most popular flows are the transverse or quasi-transverse flows, which sustain the various theories linked to the “1D film model”. Among the earliest contributions is the popular “gel layer” polarization model [
20]. Further analytical contributions have extended the earliest approaches to more realistic concentration boundary layers [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30]. Note that these models suppose the existence of a thin film wherein polarization occurs, the axis-evolution of this film being potentially envisaged in the most recent ones.
Although the general coupled problem seems out of the reach of the current analytical methods, it has however been possible to find particular operating domains capable of reducing the strength of the coupling between hydrodynamics and solute transfer, and leading the theoretical approach to analytical expressions. This is the case of the “HP-LR limit” (for High Pressure and Low Recovery), which allowed us to derive an exact expression for the overall solute transport coupled with its related Berman flow [
1]. This solution clearly exhibited the phenomenon of concentration polarization, which was combined with osmotic (counter-)effects to predict the actual permeation. In its domain of validity, such an exact approach moreover exhibits the so-called “inflected flux” phenomenon (i.e., some logarithmic behaviour observed in the experiments as operating pressure increases). Note additionally that certain analytical studies searched after similarity solutions (see, e.g., [
31,
32]).
To end with the theoretical approach, the numerical approach has largely been used to model fouling. Facing the difficulties for the analytical approach to cope with the overall coupling between mass transport and flow, one usually resorts to numerics. In particular, the numerical approach is seemingly indispensable for taking the solute axial variations into account [
33]. As a result, numerous numerical models consider a solute boundary layer growing along the channel [
34,
35,
36,
37,
38,
39,
40]. These numerical models generally solve the Navier–Stokes equations. However, when no stiff variations are present, it can be demonstrated that the framework of the Prandtl parabolic approximation is valid for modelling standard membrane filtration in the cross-flow configuration. As a result, Prandtl equations offer a simplified model which allows us to reduce the computational cost and to easily enforce the nonlinear coupling between filtration and concentration polarization at the membrane surface [
41].
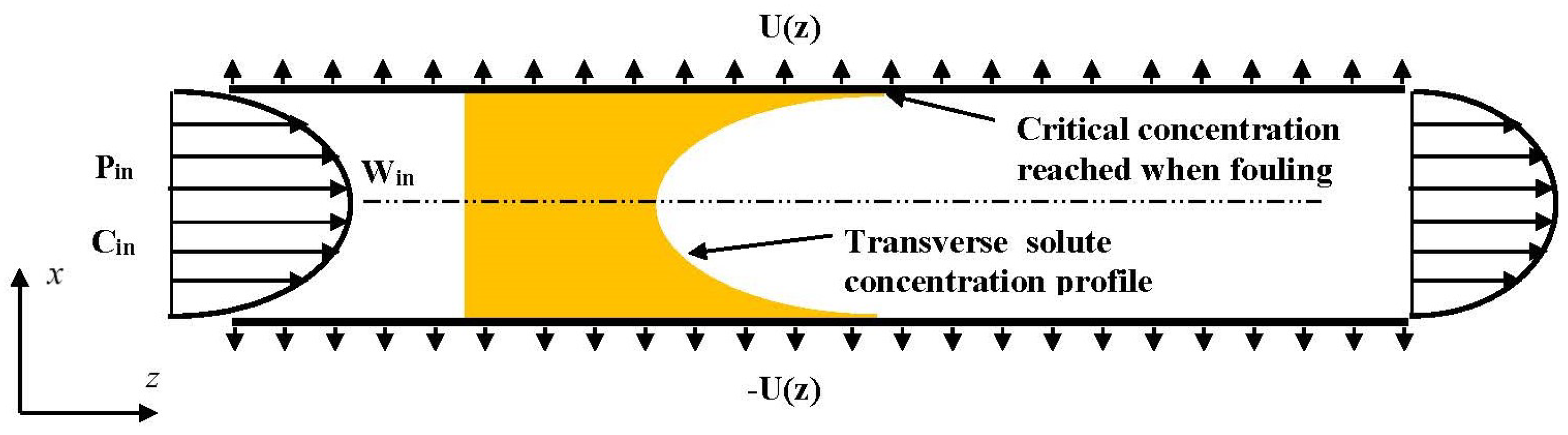
In this way, the present paper numerically investigates the reversible fouling within the framework of a 2D channel flow. Two semi-permeable parallel walls compose the 2D channel as schematically shown in
Figure 1. The channel is of length L and spacing 2d. To reduce the computational cost, and thus to conduct a nearly exhaustive study of all the relevant parameters, the steady conservation laws (continuity, Navier–Stokes and solute convective-diffusive equations) are considered within the framework of the Prandtl approximation [
41]: pressure only depends on the axial coordinate (z) and all phenomena of diffusion along the axis are neglected. For the sake of simplifying the discussion, the present work considers only one species, whereas the membrane selectivity is perfect (the species is fully rejected). Furthermore, as soon as the polarization of concentration reaches a critical value, called concentration of deposit and hereinafter denoted
, any additional solute molecule immediately settles on the membrane inner surface, or in the membrane texture itself. In other words,
is the concentration of equilibrium between solution and deposit. This is therefore the maximum concentration that can be met in the solution. Such an assumption, which considers the kinetics of deposit and dissolution as infinitely fast, is generally admitted for a large set of membrane filtration systems (see, for instance, [
42,
43]). Lastly, to focus on the features specifically related to fouling, pressure drop and osmotic (counter-)effects are supposed very low.
The article is organized as follows.
Section 2 presents the physical model and explains the role of the independent parameters.
Section 3 exposes the mathematical model and discusses the basic assumptions. In
Section 4, the numerical method is described focusing on the specific iterative procedure when fouling occurs.
Section 5 is devoted to the numerical results, their classification with respect to the fouling number
as defined below. The fit with an analytical expression, and the final expression of all the results is investigated in
Section 6.
Section 7 deals with the dimensional reconstruction of the permeate flux. The role played by the main physical parameters is investigated. Conclusions and perspectives concern
Section 8.
3. The Prandtl System
In a previous contribution [
41], it has been demonstrated that the Prandtl approximation may apply in the standard configurations of filtration. An incompressible Newtonian fluid flow of velocity
, pressure
and concentration
, is considered within the open domain
. To write the Prandtl system in non-dimensional form, we define the following set of non-dimensional unknowns and variables:
where the superscript (
) distinguishes the dimensional form of the concerned unknowns. In this way, the computational domain becomes
.
The derivation of the Prandtl set of equations uses the following arguments [
41]. As long as feed axial velocity is large in comparison with permeation velocity, the transverse variation of pressure can be neglected in all filtration systems. Pressure is hence reduced to a single-variable quantity, a function of the axial position
z, only. In the same vein, all axial diffusion terms can be cancelled in the conservation laws, except when a mathematical discontinuity happens (as discussed later). The resulting set of equations then reads
together with appropriate axial boundary conditions and with the following transverse boundary conditions at the wall (
,
):
Note that boundary conditions (18) are often referred to as Starling–Darcy boundary conditions. The above differential system calls for the following remarks.
Remark 1. Equations (15) and (16) are clearly of a parabolic type, the pressure being in every section the Lagrange multiplier that permits constraint (13). The forthcoming numerical method will exploit these mathematical features. Furthermore, a parabolic system admits computational methods of “time marching” type, which only requires “initial” conditions at inlet. Hence, “the appropriate boundary conditions” mentioned above are reduced to entrance conditions. In other words, suitable entrance profiles on C and w are only required at . Remark 2. If the above inlet data at are symmetrical, the laminar solution of the system will develop symmetrically with respect to , the line of symmetry. Therefore, assuming symmetrical boundary conditions at allows us to save half of the computational effort, the computational domain being reduced to .
Remark 3. Conservation laws are nonlinear by nature. In filtration problems, their nonlinearity is increased by boundary condition (17) at the membrane surface, which nonlinearly combines permeation velocity and solute concentration. This coupling is tremendously important because it governs polarization before the occurrence of fouling. Therefore, this nonlinearity will be iteratively enforced in the numerical scheme, as described below. To summarize the present section, Prandtl approximations, constant physical properties, reversible fouling with infinitely fast kinetics and assumption of total solute rejection allow us to construct a minimal model, the validation of which will lie in its capability of predicting the general experimental trends. In terms of computational cost, the Prandtl approximation represents a sizeable simplification. As for the studied range of parameters, this has some price to pay, as will be discussed below.
5. Numerical Investigation on Reversible Fouling
Since the overall problem depends on seven independent numbers, the present numerical experiments consist of fixing certain numbers and varying the others. As previously discussed, the membrane number has been set to zero very early in our analysis. Because we want to focus on fouling, we have decided to neglect pressure drop and osmotic (counter)-effects. This is why number is now set to a small value, say . As for the osmotic number , we decide to also select a small value (say ) that will only serve as a marker of high polarization. In other words, in the absence of extreme polarization, both assumptions on and allow us to consider that the net operating pressure is nearly constant.
Now, in the absence of fouling, both assumptions have a simple consequence: permeation is more or less constant. Thus, as in a nearly “clean water” experiment, axial flow exhaustion will be found at about (or at about in non-dimensional form). Therefore, in the possible presence of fouling (which will delay axial flow exhaustion), it is meaningful to at least study the problem in the parameter range (and obviously for ). At the channel entrance, the feed flow profile is supposed to be a Hagen–Poiseuille type and the concentration profile is uniformly set to , or 1 in non-dimensional form.
5.1. Description of the Fouling Onset
Within the framework of the Berman theory, which treats of channel flow with uniform leakage, any pressure change along the axis is found to be on the order of the quantity
, where
is the so-called Berman constant. Since
has a limited range of variation for the standard filtration configurations (say
), any variation of
will have a negligible effect on pressure as long as
. In other words, at steady state, hydraulic effects are expected to be small in comparison with mass transfer effects. This analysis is corroborated by
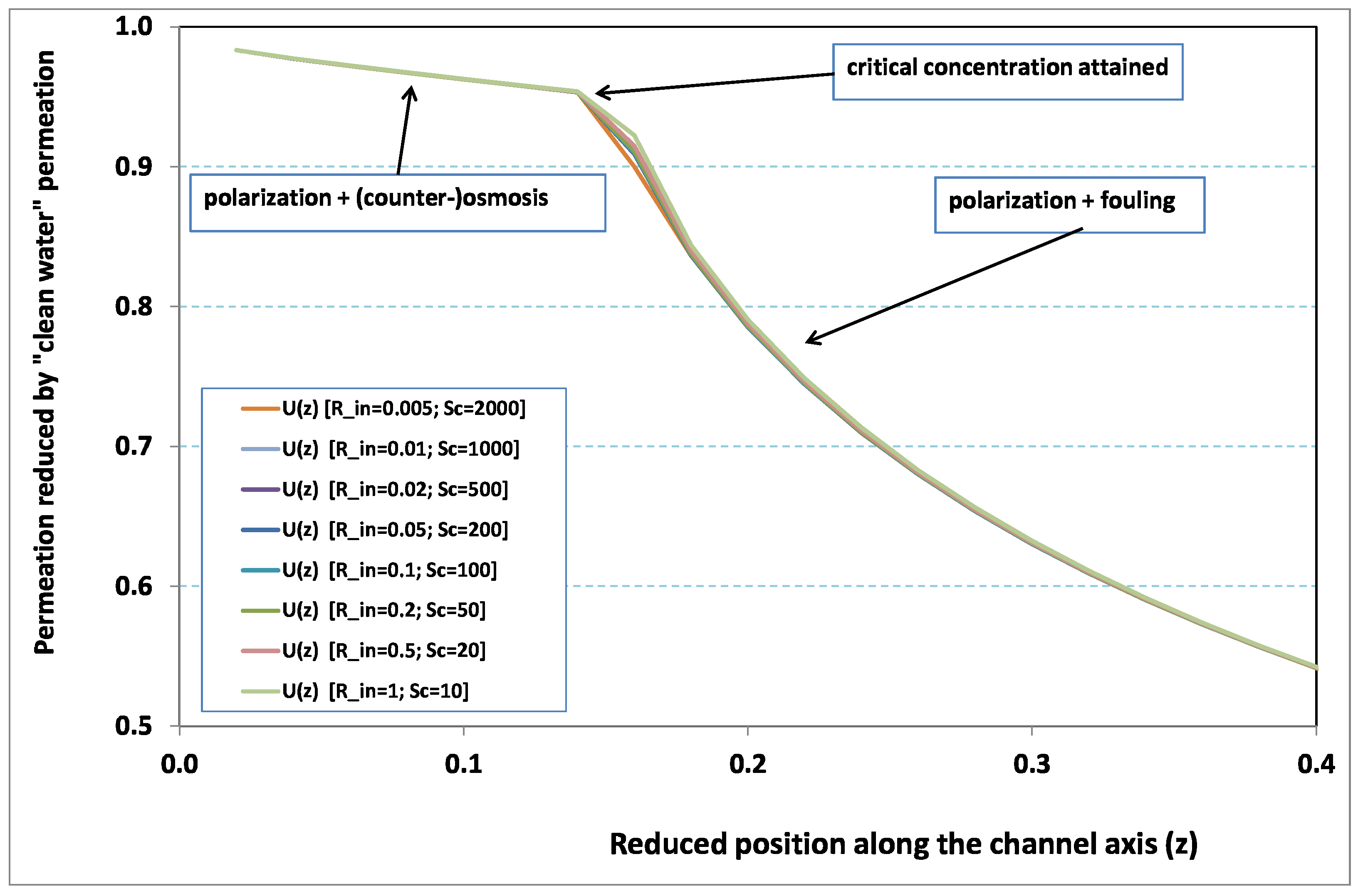
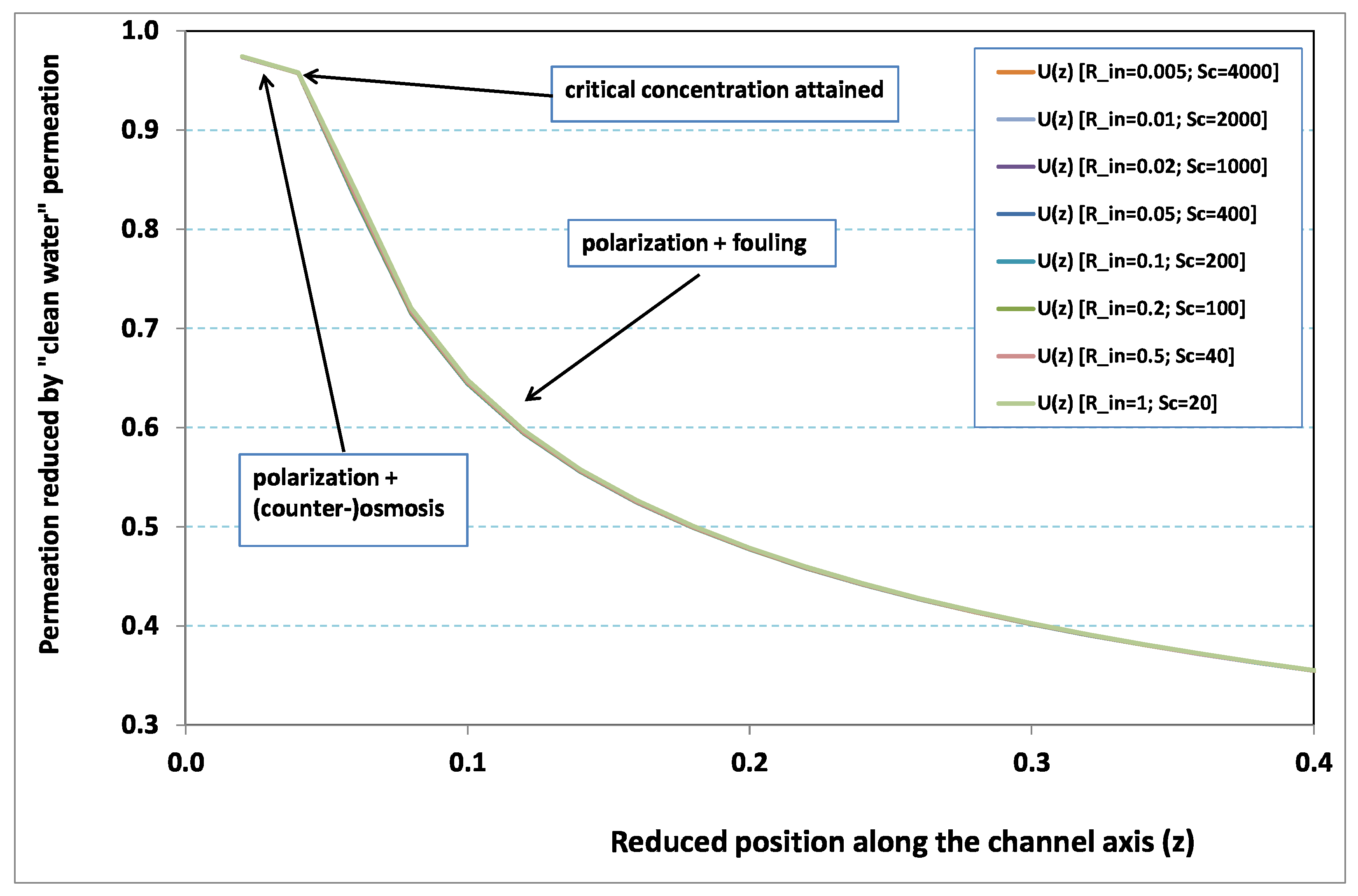
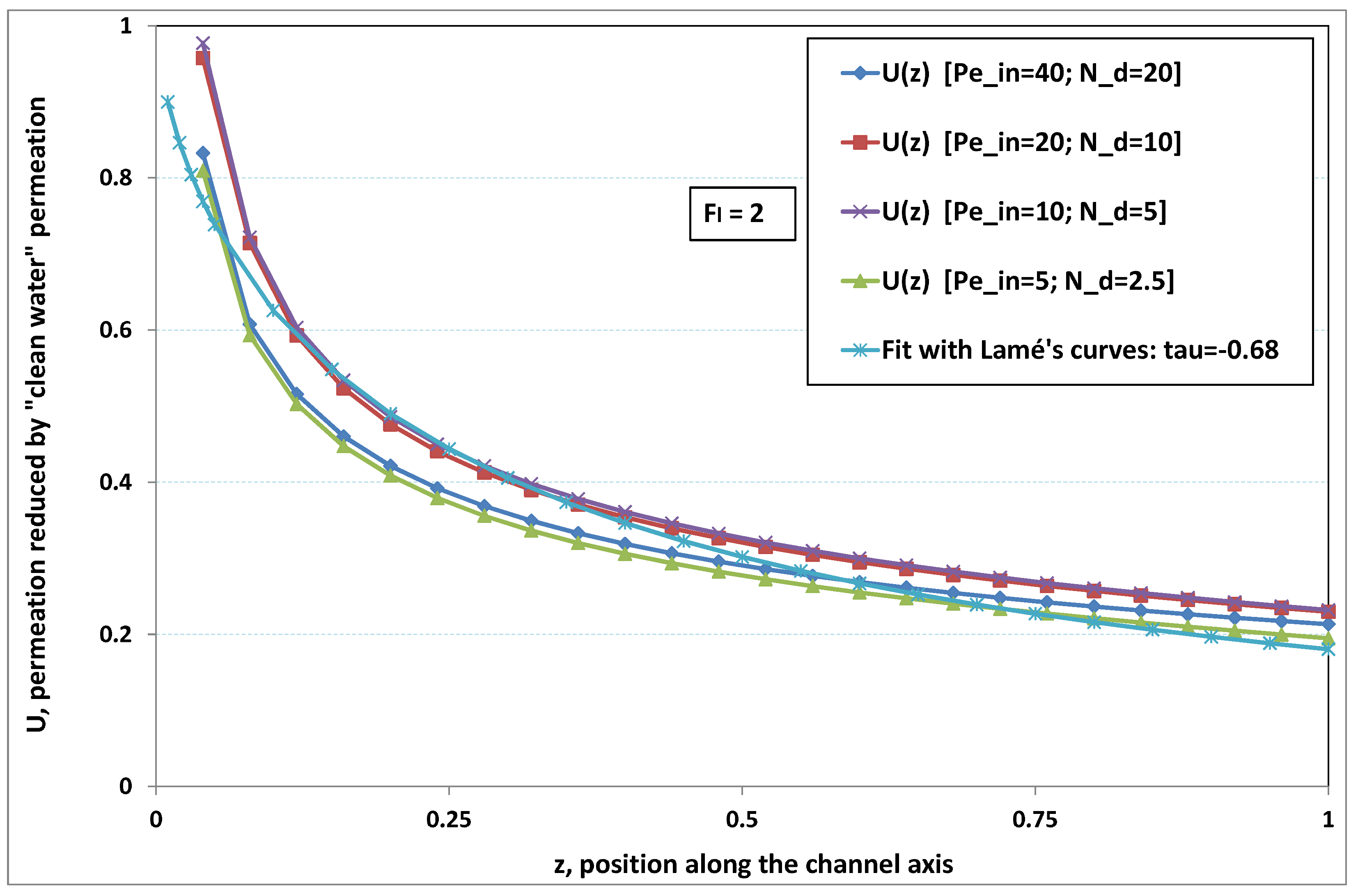
Figure 2 and
Figure 3, where local permeation is drawn against the axial position in the channel. Note that permeation is normalized by the “clear water” permeation
, whereas axial position is reduced by the “clear water” exhaustion length
. In both figures, any variation of
does not modify the curves, provided that both parameters
and
are varied in such a manner that their product, the transverse Péclet number
, remains identical for all curves.
Figure 2 [resp.
Figure 3] concerns
[resp.
]. In each figure, all curves superimpose nearly perfectly. In other words, the relevant parameter is
, and not the pair
. This remark induces a significant gain for the present analysis, since the remaining parameters to be investigated are now reduced to
.
In
Figure 2 and
Figure 3, permeation starts with a value close to 1 (i.e., nearly the permeation of “clean water”, because polarization of concentration and the subsequent osmotic (counter-)effects are low). Then, the solute boundary layer develops along the membrane inner surface and polarization arises. The increasing polarization along the downstream direction is indicated by the osmotic effects that lower the permeation (slightly, since the osmotic number has been chosen feeble). Farther along the axis, polarization of concentration reaches the critical concentration at the membrane, fouling occurs, and permeation hindrance becomes significant (provoking a rapid change in the permeation curve slope). We additionally observe that increasing
enhances polarization and the onset of fouling occurs earlier in the channel. To summarize, the present numerical experiments corroborate the fact that
does not play the leading part in such a permeation system (steady laminar flow and
, provided). By contrast,
, the transverse Péclet number, controls the locus where fouling starts to take place, as well as the permeation under fouling.
5.2. Mass Transfer Controls Permeation
As a consequence of the previous paragraph, only the variation of
will be considered hereinafter, whereas
and
will no longer be seen as independent parameters.
Figure 4 and
Figure 5 investigate the role played by
on permeation for two different critical concentrations characterized by the deposit numbers
and
, respectively.
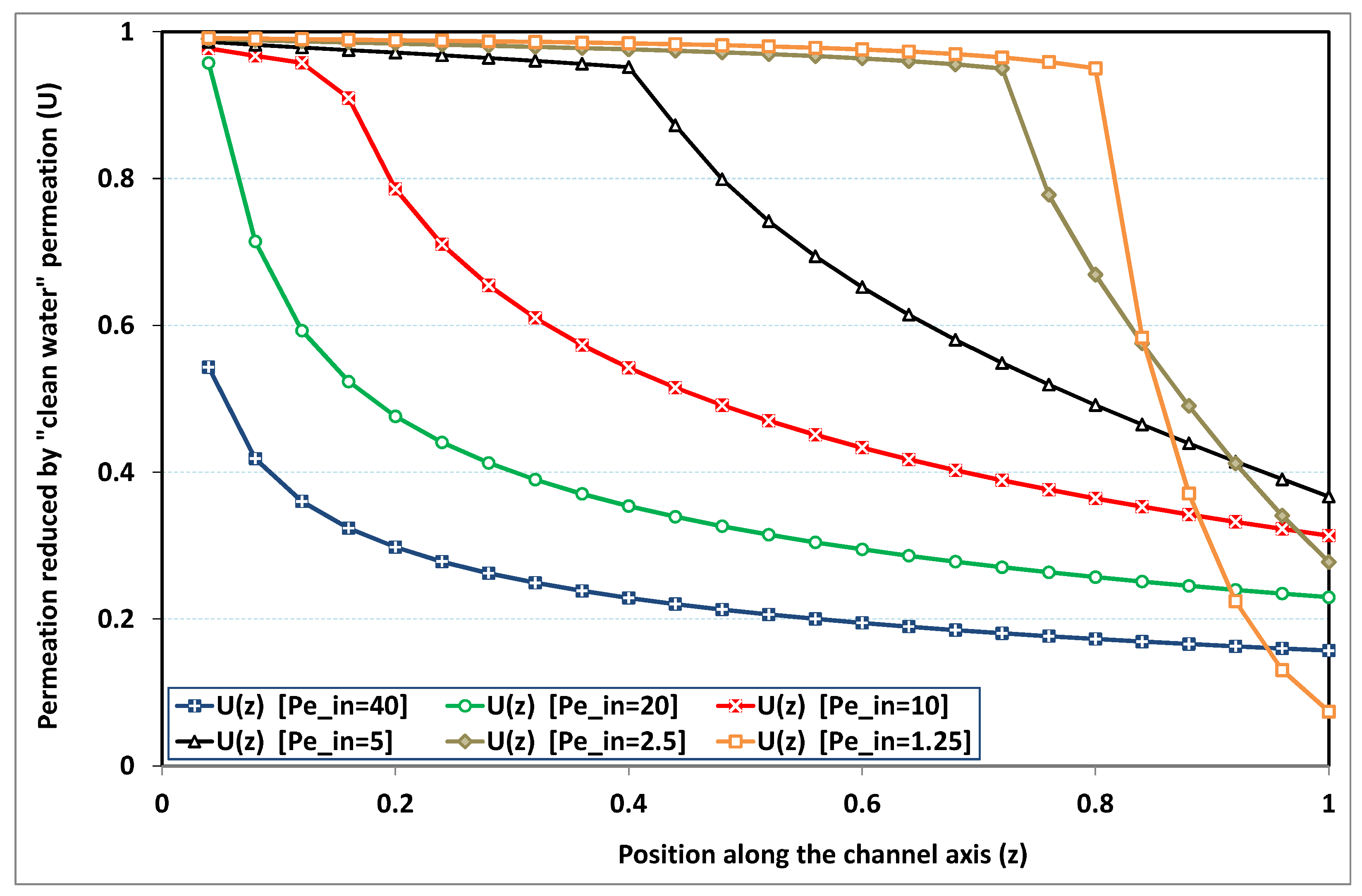
In
Figure 4, permeation is plotted for
, which is a standard value for the deposit number: it particularly corresponds to sea water desalination, where salt concentration is around 35 kg/m
, while salt maximum solubility in water is about 350 kg/m
at standard conditions. At high transverse Péclet number, a high level of polarization is rapidly obtained at the channel entrance, so that the permeation strength of clean water never occurs. On the other hand, if the transverse Péclet number is low, permeation remains close to that of clean water in the largest part of the channel. However, as the bulk solute concentration increases (due to solvent leakage) when approaching the dead-end length, the deposit (or saturation) concentration is reached and the phenomenon of fouling makes the permeation rapidly diminishing (in the absence of fouling and osmotic effects, we recall that permeation would stop at
). For intermediate Péclet numbers, fouling arises earlier in the channel. There is a transitional value of
, for which fouling occurs immediately at the start of the channel (say here for
).
Note that the case of high polarization obtained for clearly stands beyond the validity domain of the Prandtl approximation, since a strong discontinuity occurs at the channel entrance. Evidently, axial diffusion cannot be neglected at this point. A crude use of the present analysis for would hence underestimate permeation.
When
is chosen higher, the previous general trend is maintained. Comparing
Figure 4 and
Figure 5, we note that the upper four curves remains strictly identical as long as no fouling appears. The difference lies in the fact that the fouling events of
Figure 4 occurred sooner in the channel. As an illustration, the highest curve in
Figure 5 (obtained for the lowest
) indicates that fouling here arises farther, close to the dead end length (of clean water permeation). In the same manner, all the curves of
Figure 5 show a better permeation due to a delayed occurrence of fouling. By contrast with the previous value of
(i.e.,
), the case with
exhibits results that are situated in the validity domain of the Prandtl approximation, except the case
which nevertheless presents a rather limited discontinuity at entrance.
To sum up, two intuitive features have been confirmed in this paragraph:
- -
increasing , the transverse Péclet number, enhances polarization of concentration, making the occurrence of fouling earlier and reducing the overall permeation.
- -
increasing , the deposit (or saturation) number, delays the occurrence of fouling, and enhances the overall permeation.
To account for both opposite trends, the idea followed in the next paragraph consists of introducing a new number (hereafter called fouling number), built as the ratio of the transverse Péclet number to the deposit number. More precisely, the new number reads:
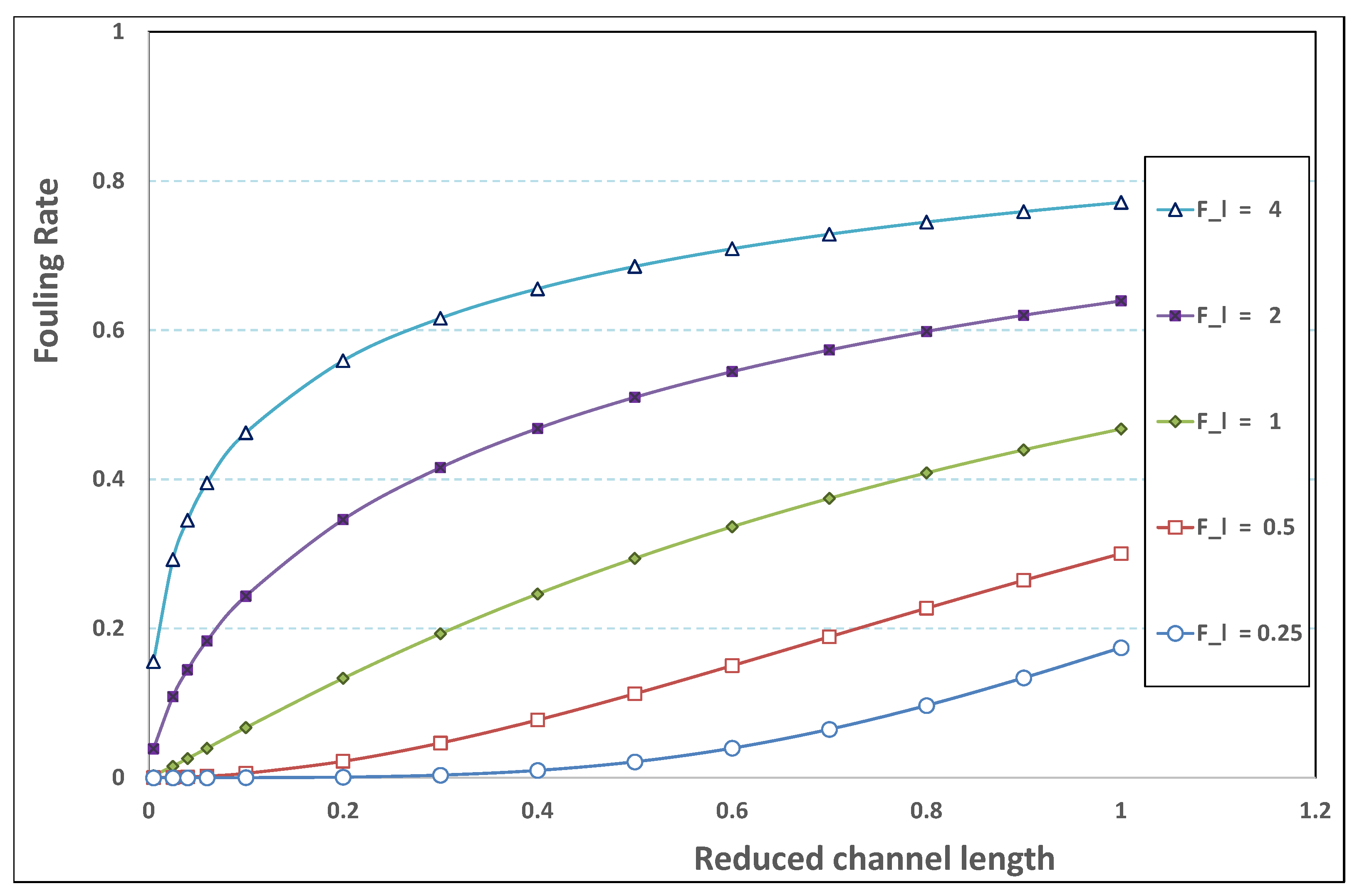
What follows consists of performing a new sorting of the various permeation computations (as those described previously) for various pairs . The new classification will next affirm the major role played by .
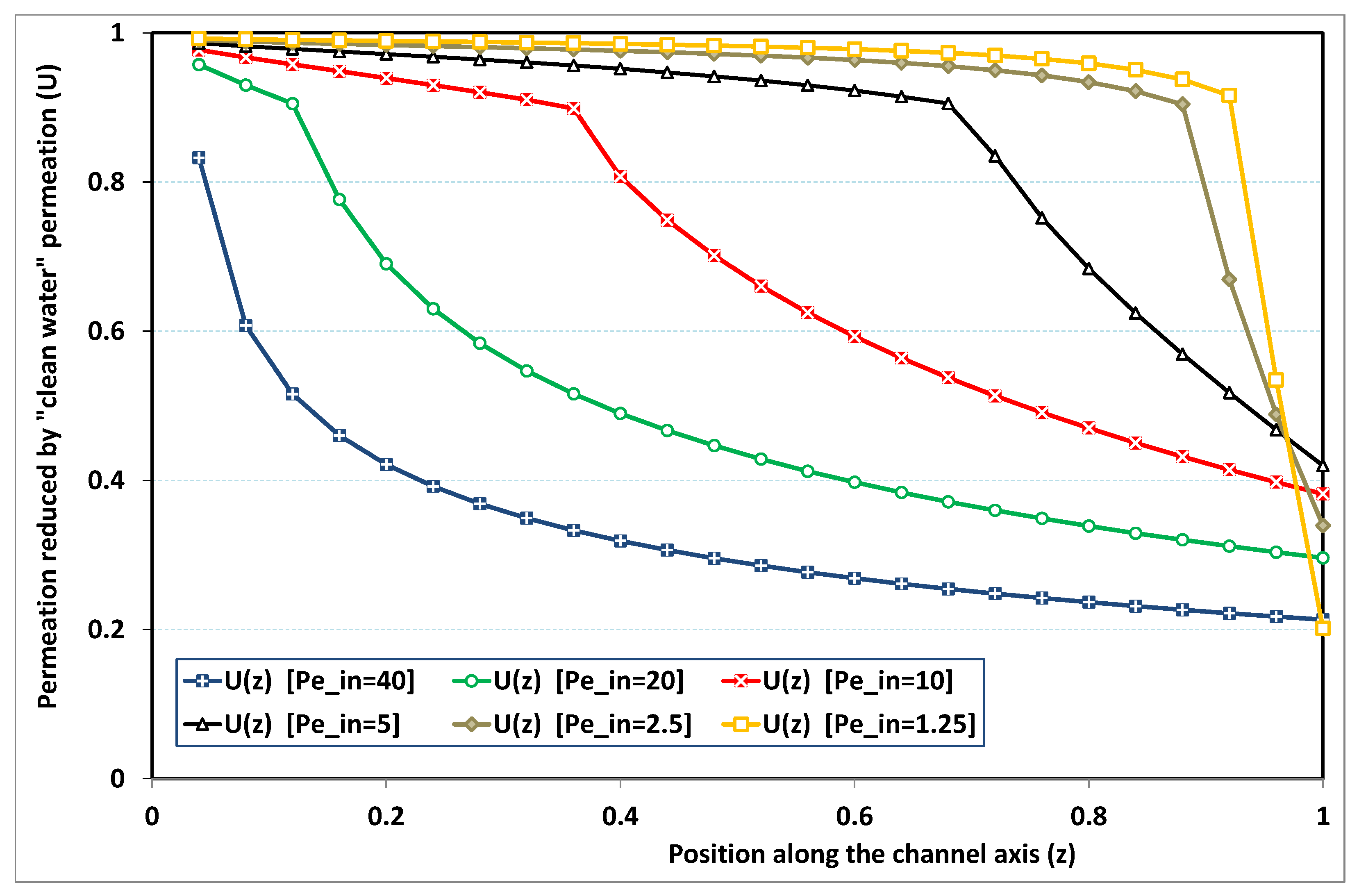
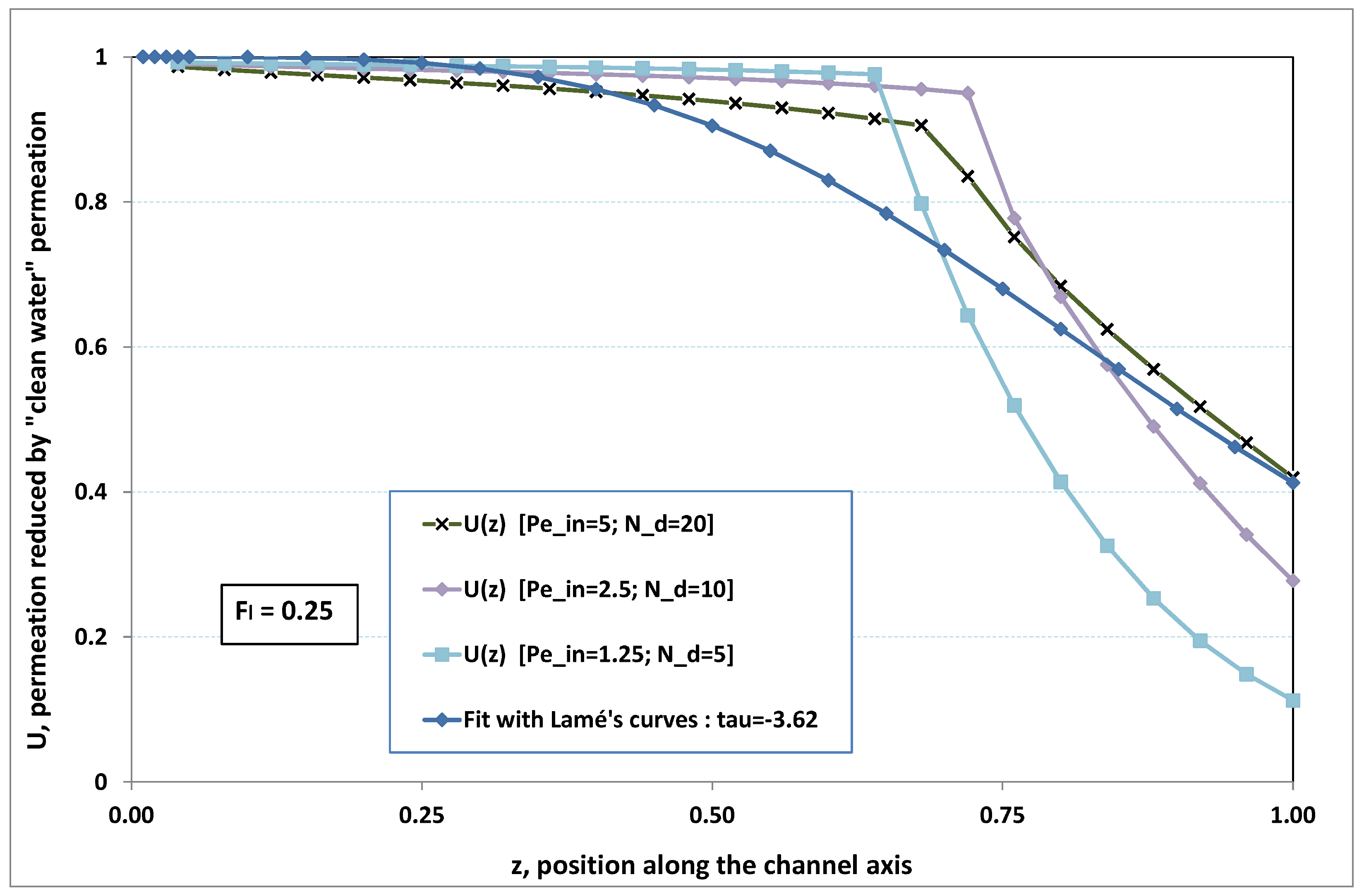
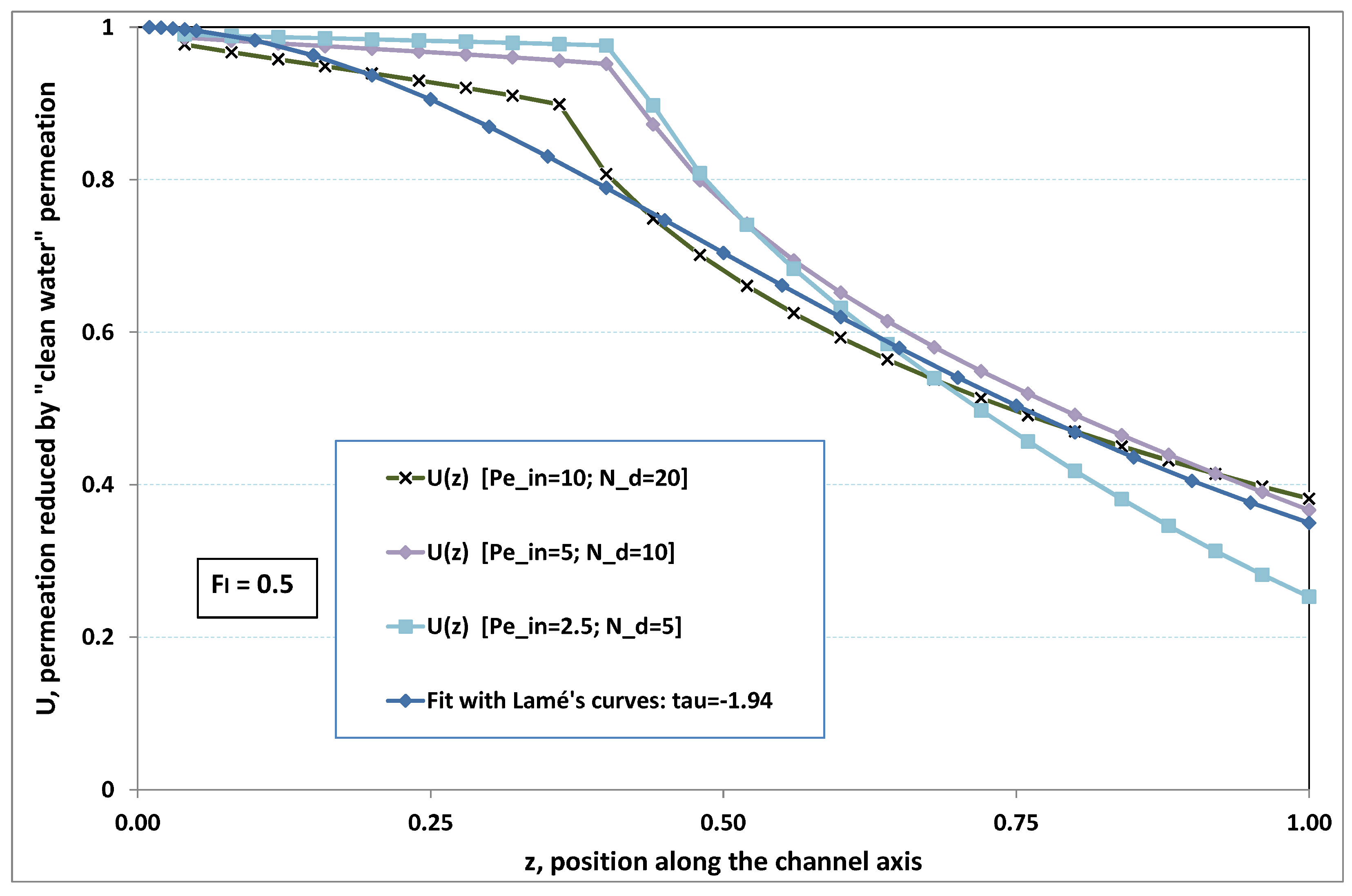
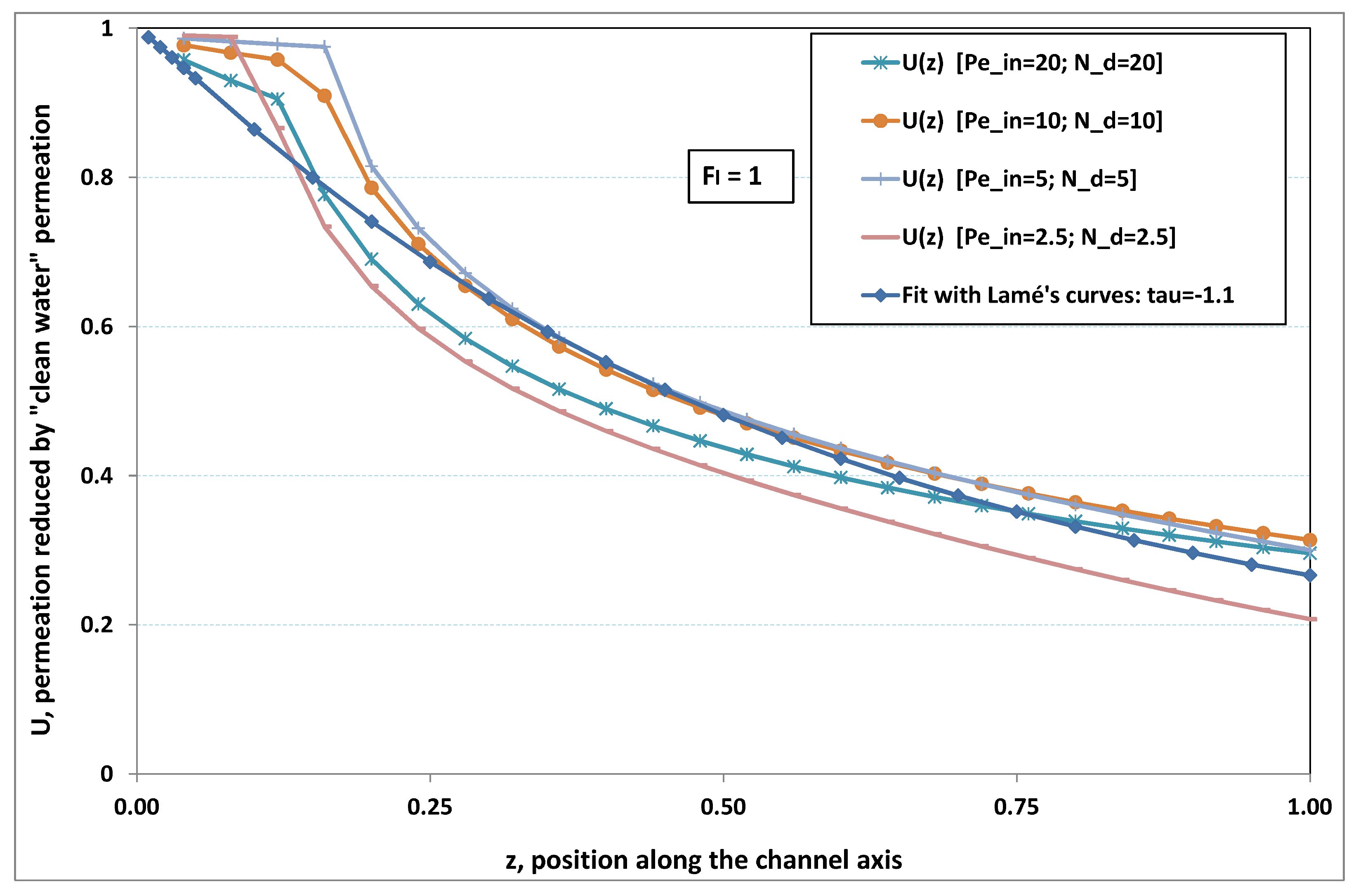
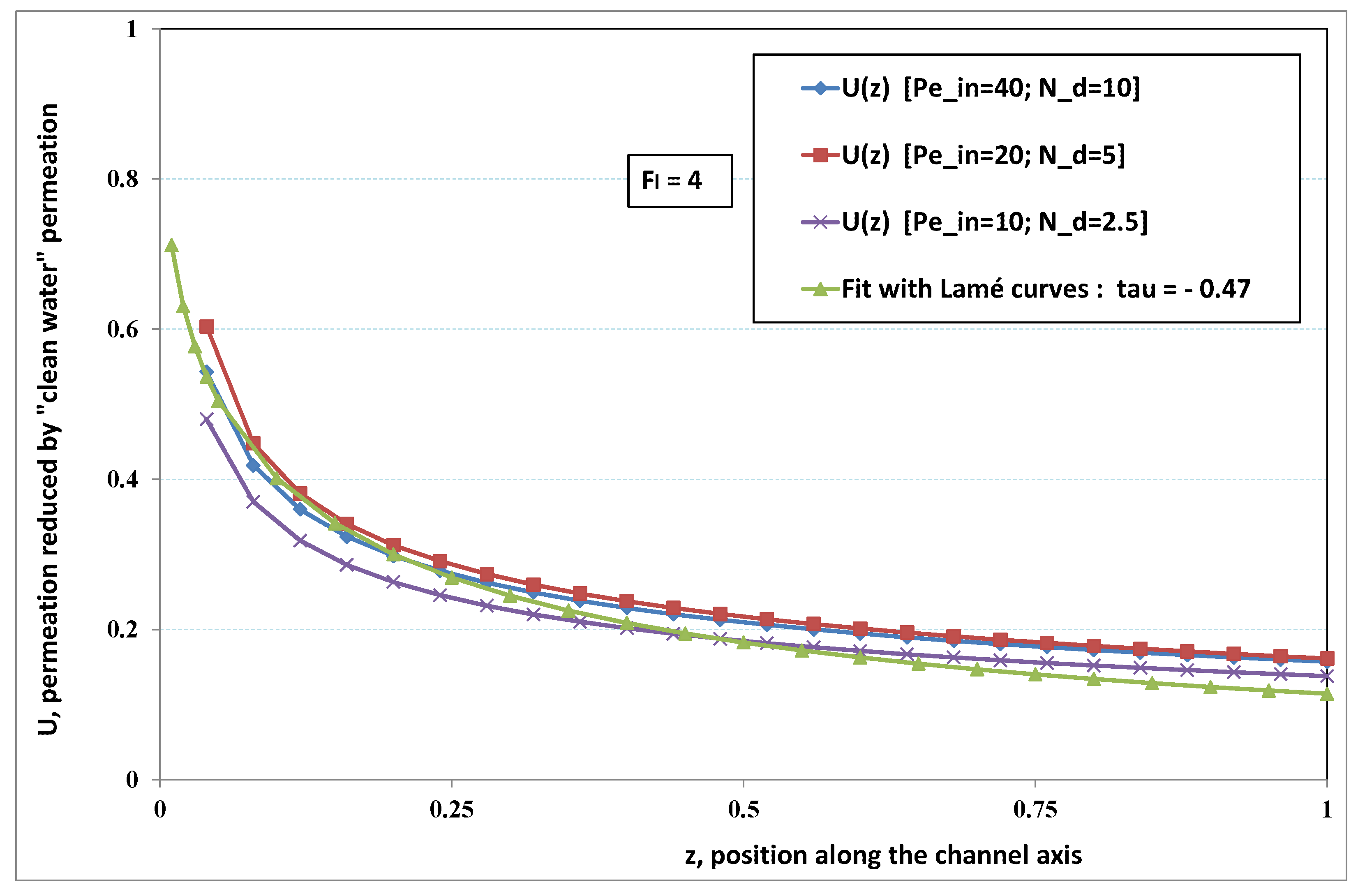
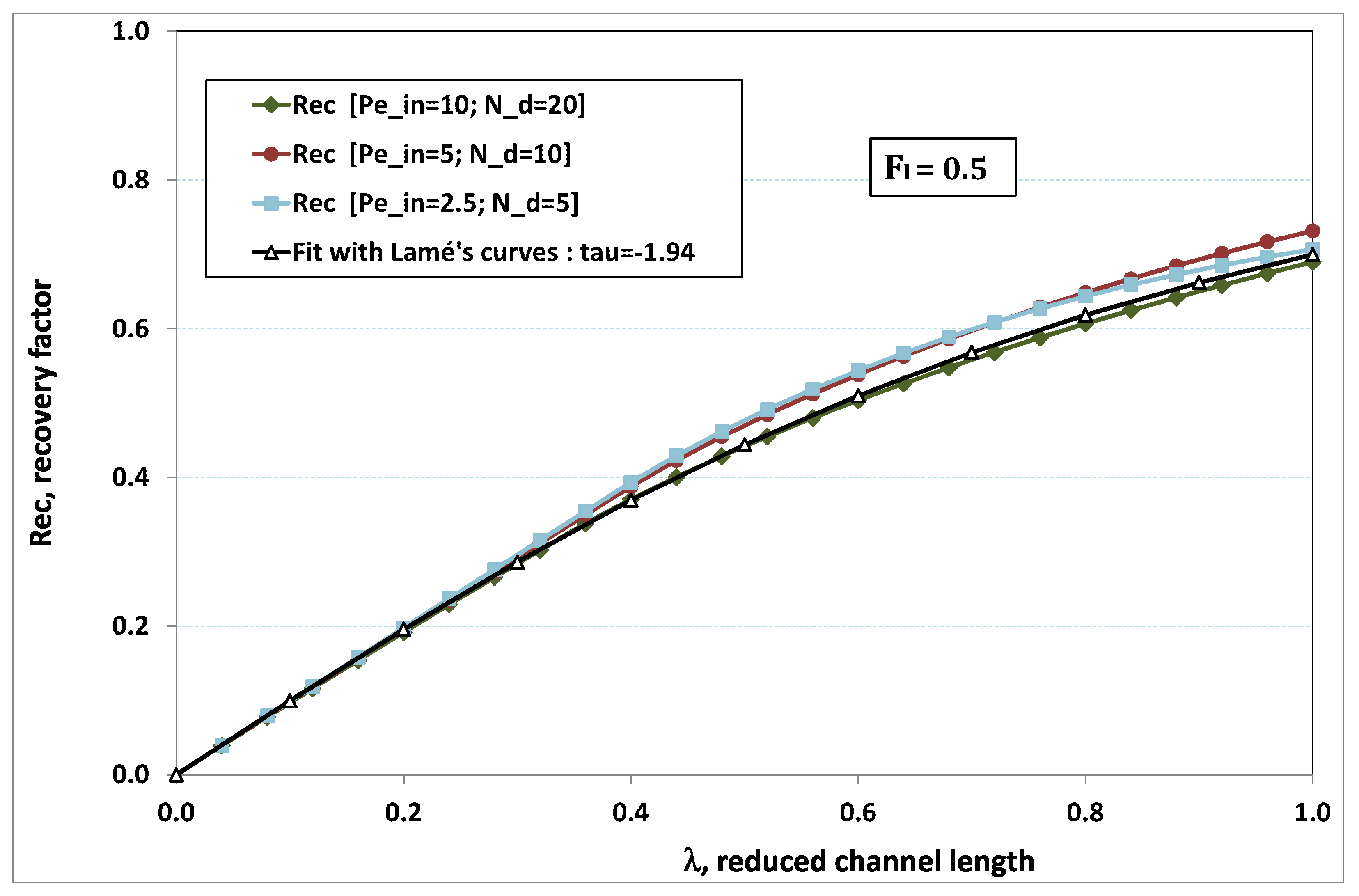
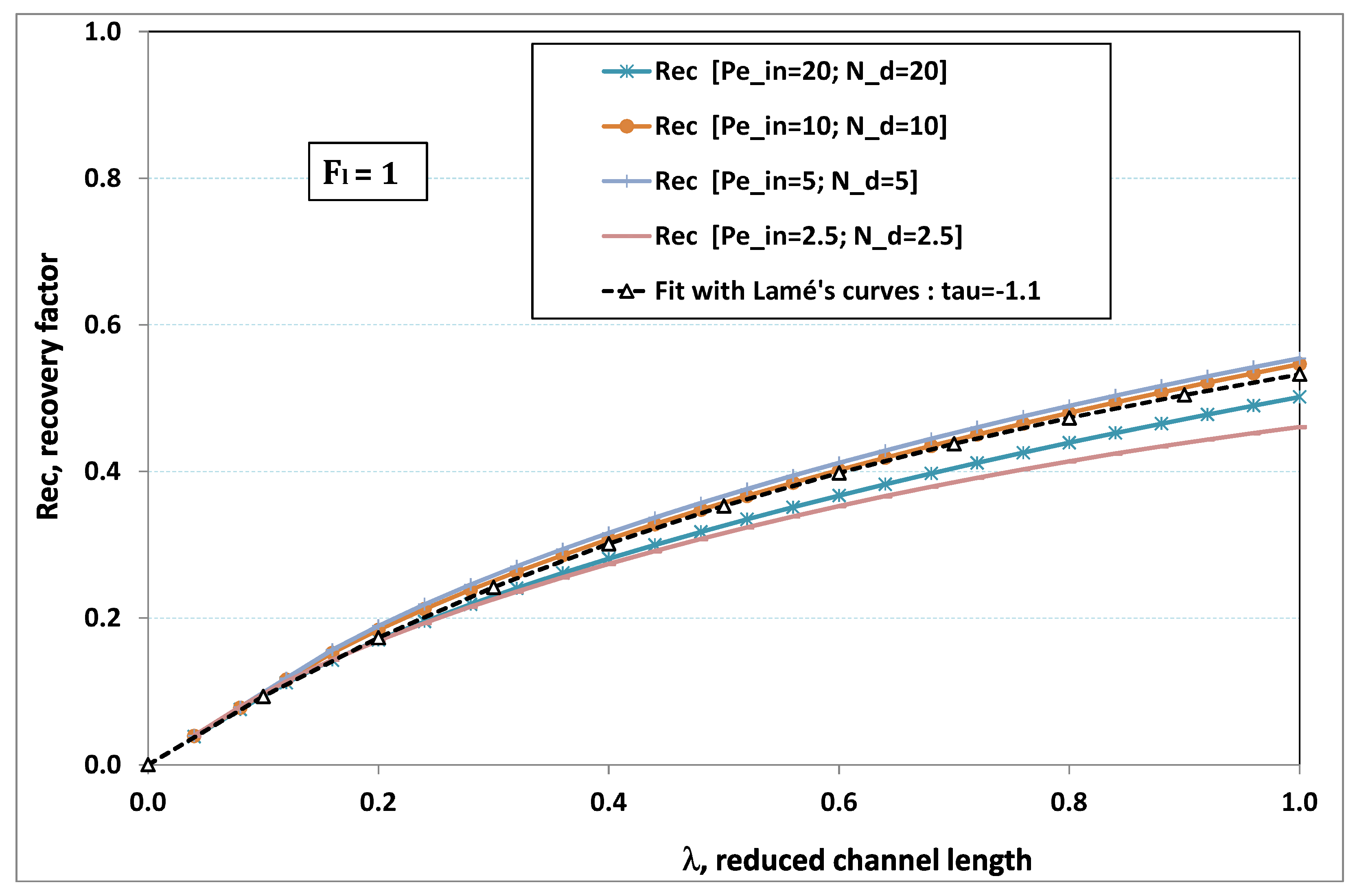
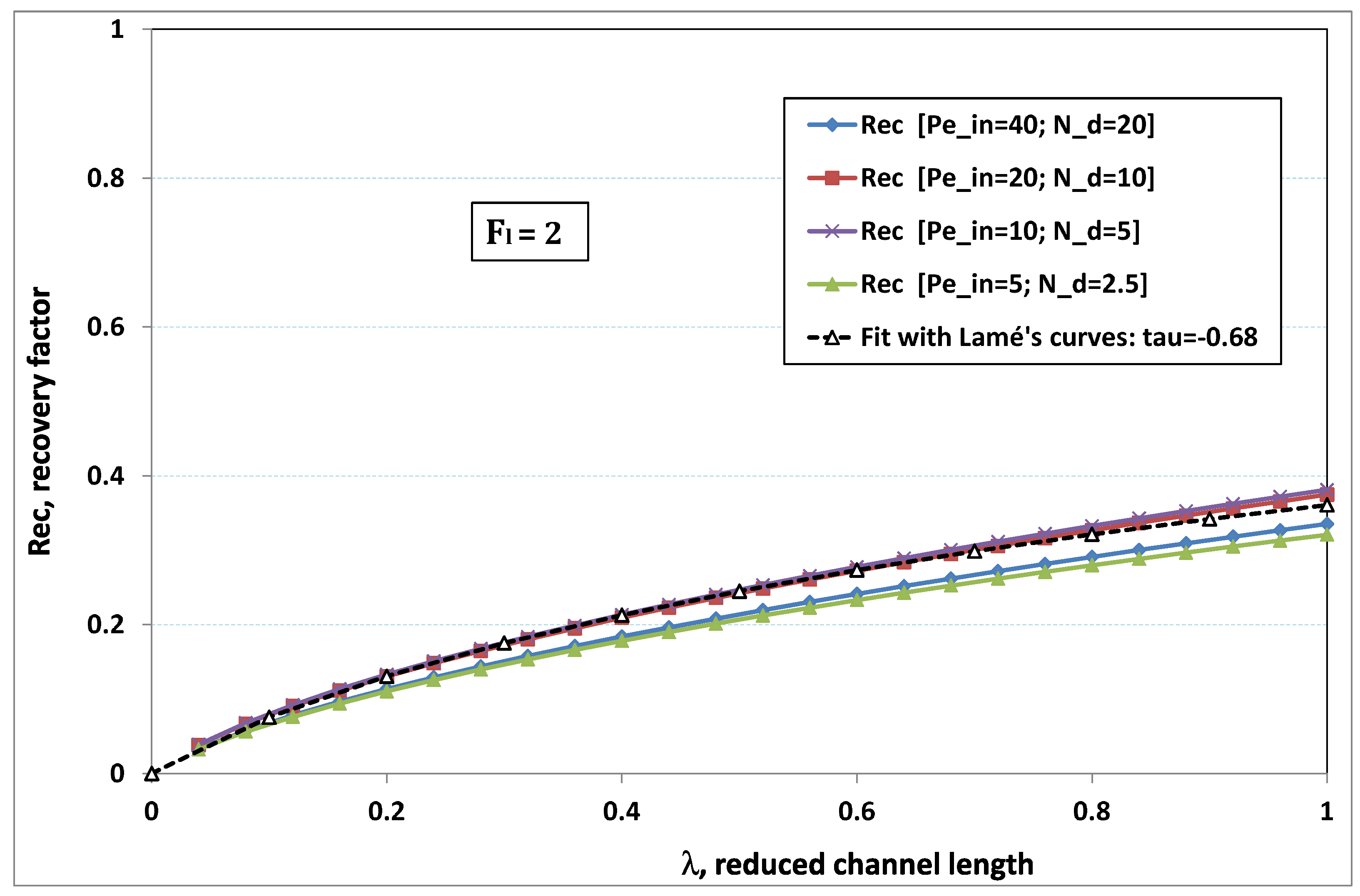
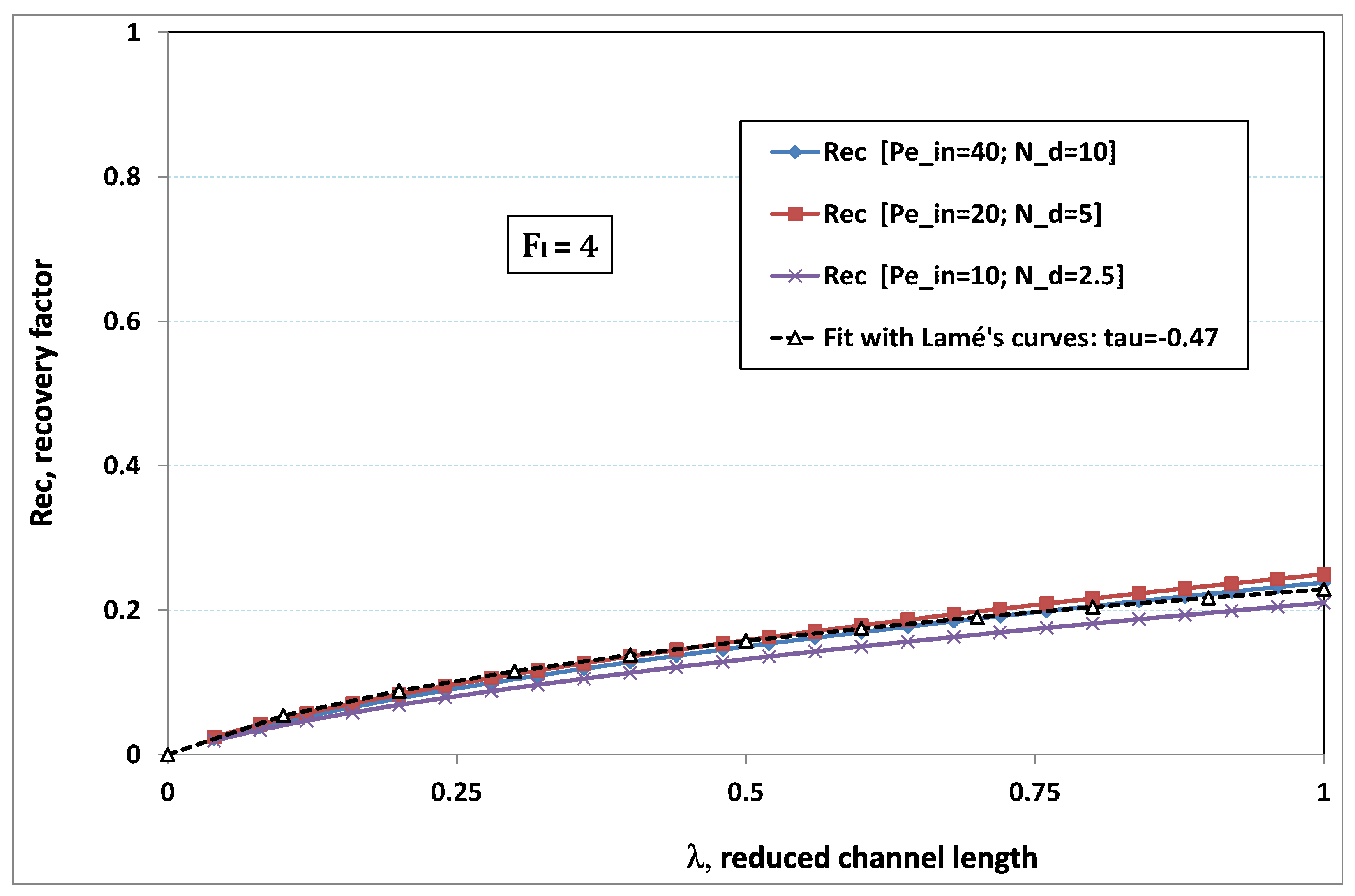
5.3. Role of Fouling Number
The point developed in the present paragraph concerns the claim that the single number
plays the leading role on permeation. To demonstrate this point, we consider all our numerical results on local permeation, and in the same figure we gather the curves that correspond to a given
. We obtain the
Figure 6,
Figure 7,
Figure 8,
Figure 9 and
Figure 10. The classification of these figures follows an increasing sorting of
, namely
. In every figure, the curves are plotted for various pairs
, the ratio of which gives the same value of
. We observe that, in a given figure, all curves follow an identical general trend. Note that an additional curve is plotted in each figure: it corresponds to an attempt for fitting the numerical results by a particular family of analytical curves; this is the aim of
Section 6.
On the one hand, when increases from to 1, fouling conditions are attained earlier and earlier in the channel. In the first channel part, polarization grows downstream of the channel, and produces a slight drop in permeation owing to the non-zero osmotic number. This decline nevertheless remains insignificant, since the osmotic number has been chosen to be very low (as well as pressure drop). When the deposit conditions are attained at the channel wall, fouling occurs. The occurrence of fouling implies a rapid change in the slope of the permeation curves. This change is all the more rapid that fouling arises lately in the channel (because the bulk concentration is already high). More precisely, for , fouling hardly occurs and the permeation curves remain slowly decreasing straight lines up to the vicinity of the dead-end length. At such a point (which is situated close to the locus of exhaustion for the “clear water” flow), the concentration in the retentate is, however, very high, since most of the solvent has permeated. It is clear that our model with constant solute diffusion locally here again leaves its validity domain, i.e., when the solution’s concentration is too high.
On the other hand, for large values of
(say,
, see
Figure 8,
Figure 9 and
Figure 10), we are faced with a series of curves, again very close to each other in every figure. The curvature of these curves is more or less everywhere positive. Fouling appears in a region close to the channel entrance, since polarization of concentration becomes rapidly high, or the critical concentration is easy to reach. Hence, the concentration for deposit is very early attained in the channel. From a quantitative point of view, the results of
Figure 10 must certainly be considered as inaccurate, since the Prandtl model fails to treat the channel very entrance, where a discontinuity in concentration is computed. Hence, the results for
cannot be considered as consistent with the Prandtl assumptions.
7. Dimensional Interpretation
For a given experimental situation, the standard parameters that are generally varied are , the transmembrane pressure, , the feed concentration, and , the mean inlet velocity of the feed flow. Since both and enter in the definition of , we need to return to a dimensional interpretation for separating the role of the different parameters. Before leaving the dimensionless forms, let us stress on the merits of Dimensional Analysis, which here allowed us to drastically reduce the number of the relevant parameters and conduct a nearly exhaustive analysis.
7.1. Dimensional Expression of Permeation Flux
Let us now express
, the recovery ratio given in expression (
36), in terms of physical quantities. This gives
In the “clear water” limit case,
tends to
. When
, we easily retrieve that the limit of expression (
39) is
Let us now denote by
, the (overall) flux of permeation which is obtained for a given filtration system. In the case of a “clear water” flow, the permeation flux per surface unit of membrane (denoted
) is simply provided by
With the use of
, the fouling rate given by expression (
37), we obtain the permeation flux per unit of membrane surface as
Formula (
42) gathers all previous numerical results (
and
provided) and gives them in dimensional form. This expression can be directly compared with the experiments. From expression (
42), interesting general trends observed in the experiments can be retrieved, as described in the following paragraph.
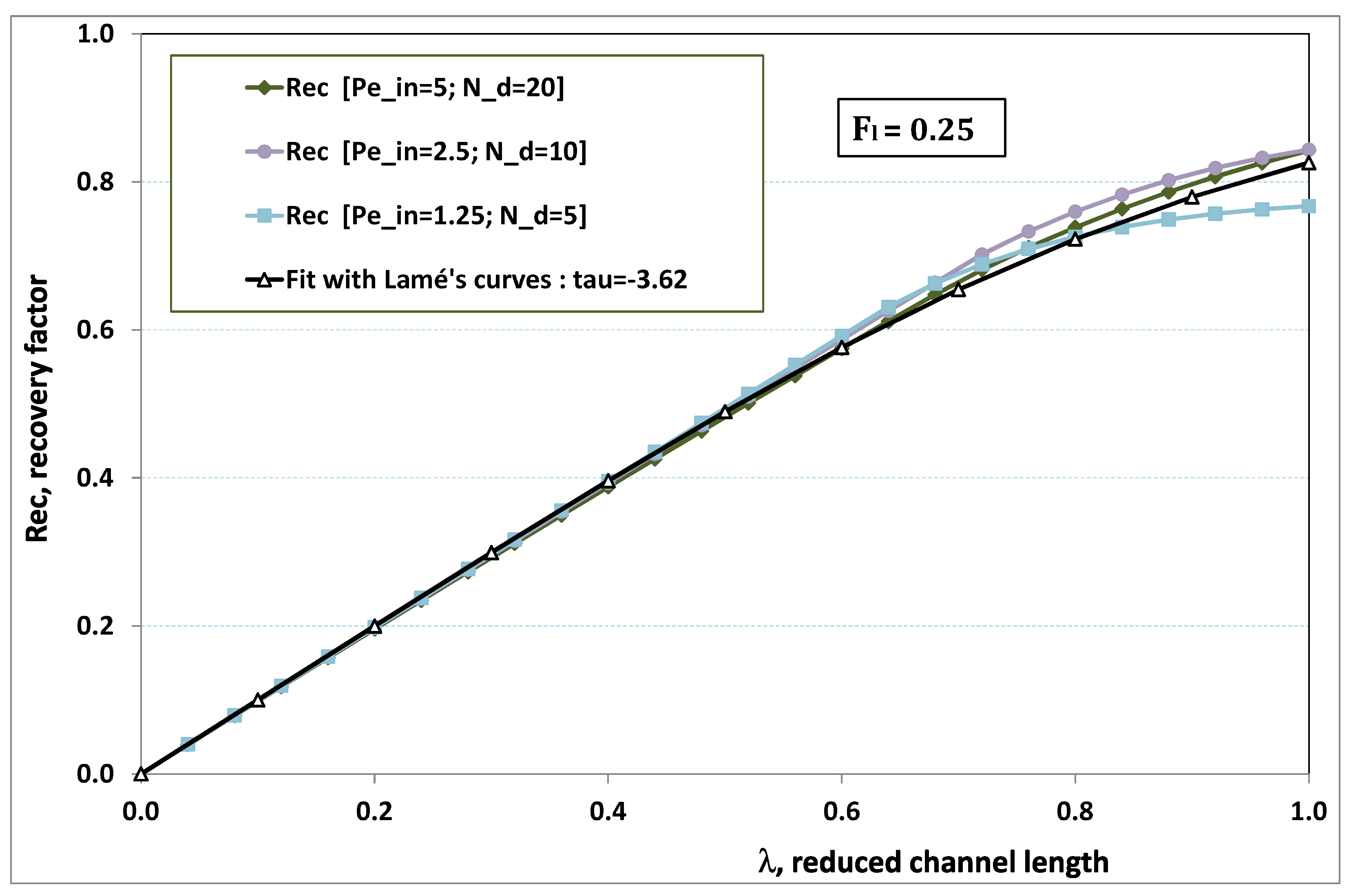
Regarding the permeate flux, there are several experimental measurements often provided in the literature: (a) the filtration flux against to exhibit the role of pressure on polarization and subsequent fouling: (a1) for various fixed values of , or (a2) for different . Measurement (a1) are devoted to illustrate the part played by the “shear at the membrane surface”, while cases (a2) search for the role of the feed initial concentration on permeation. Another frequent measurement is (b) the filtration flux against to point out the role of the feed concentration level on fouling. Therefore, the purpose of the next paragraph consists of varying the following three parameters , and drawing the curves related to cases (a1), (a2) and (b).
7.2. Discussing the Roles of the Main Parameters
First of all, varying consists of fixing the other parameters. To keep the highest level of generality, let us imagine an experiment of reference, carried out in a range of parameters that does not lead to fouling. This experiment of “clear water” type has both pressure and concentration low enough that the fouling number is low, say . On the other hand, a moderate value has to be chosen for because the exhaustion length must remain much larger than the actual channel length. Let us choose such that . We additionally denote the permeation flux that results from this experiment by , which is nothing but .
From this experiment of reference, we now use the set of units, namely
, to reduce the three main parameters and the permeation flux. Therefore, from Equation (
42), we write the normalized permeation flux, as follows:
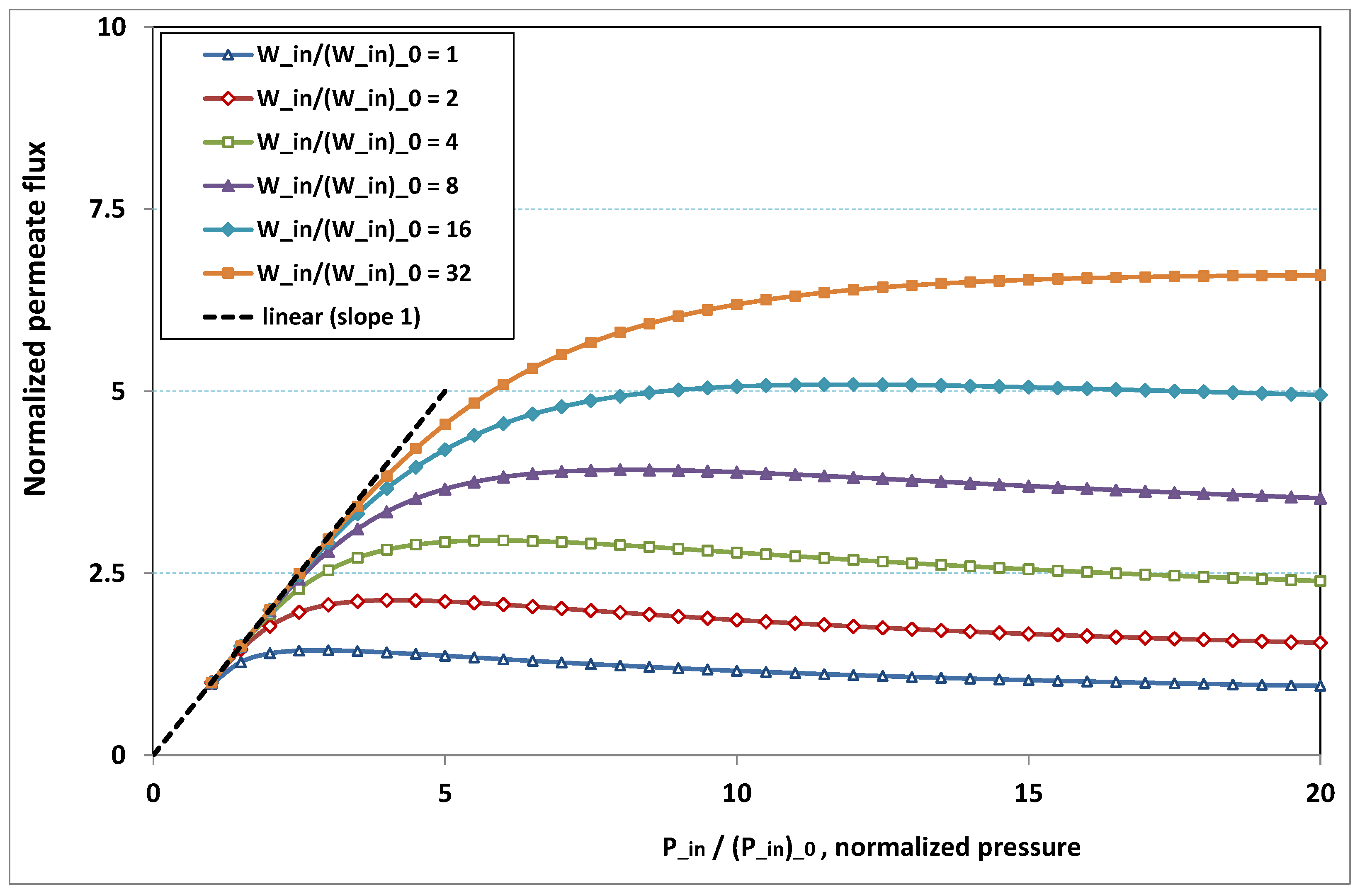
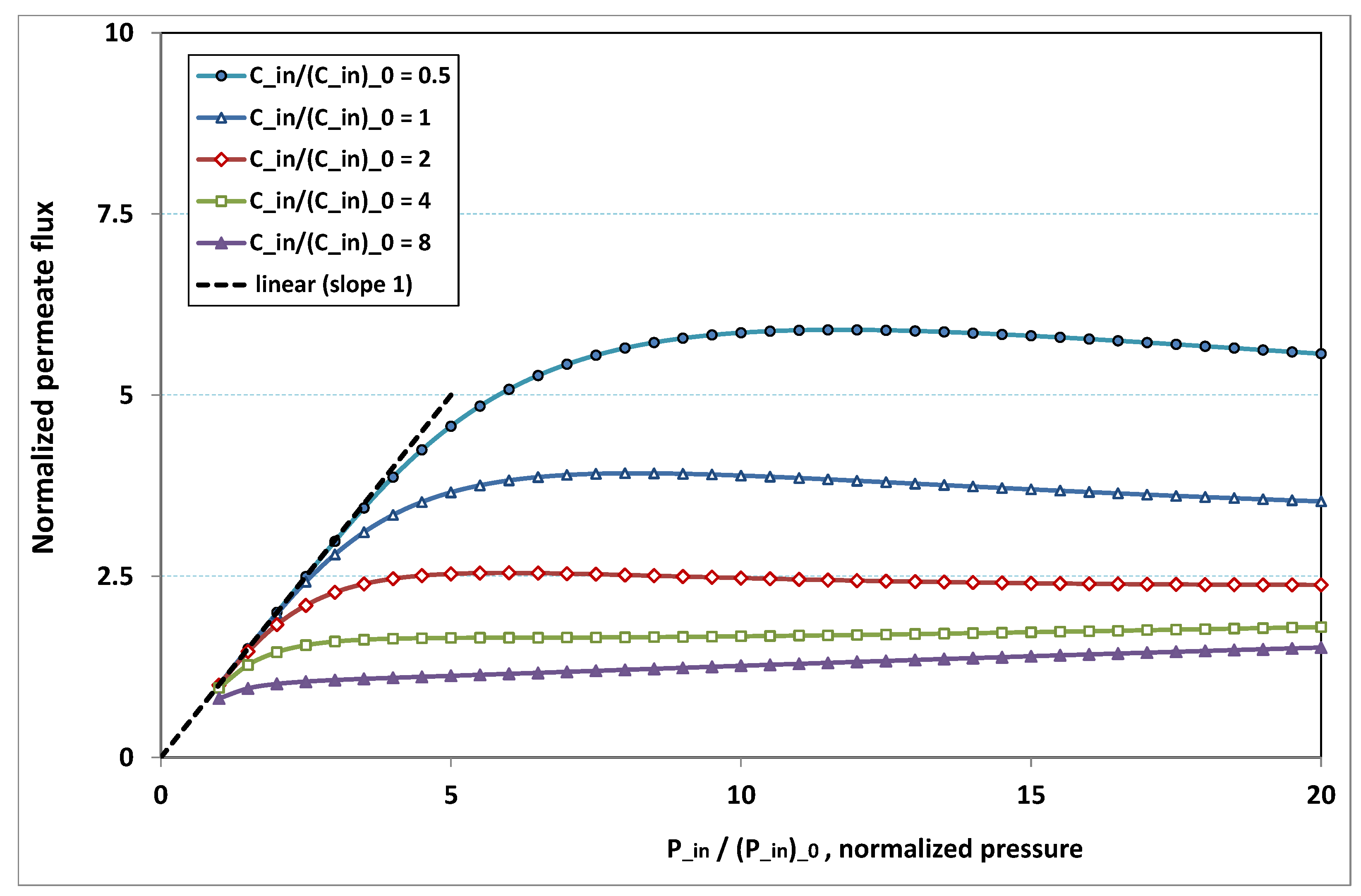
The plot of
, the normalized permeation flux, against
, the normalized pressure, is drawn in
Figure 18 for various normalized feed flow
, with the normalized concentration
being set to 1. On the other side, the same quantities are again plotted in
Figure 19, but, for various normalized feed normalized concentration
, while the normalized feed flow
is now set to 8. Note that the data of the curve for
in
Figure 18 are the same as the data of the curve for
in
Figure 19.
- -
at low pressure, no hindrance due to fouling occurs; the increase in permeation remains in direct ratio to pressure.
- -
the absence of fouling is maintained for higher pressures as feed flow (“the shear”) increases (
Figure 18), or as feed concentration diminishes (
Figure 19).
- -
when fouling arises, the dependence on pressure exhibits a saturation, and even a weakly marked maximum either for fixed feed flow (
Figure 18), or for fixed feed concentration (
Figure 19).
- -
when fouling takes place, this maximum increases either as the feed flow rate is enhanced (
Figure 18) or as the feed concentration diminishes (
Figure 19).
In both
Figure 18 and
Figure 19, the non-monotonous dependence of permeation on pressure is striking. This behaviour has already been observed in numerous experiments. What follows is an attempt at interpreting the occurrence of such a maximum in permeation as pressure increases. In
Figure 19, a careful inspection shows that all the maxima happen for pressures that give a more or less constant value for
, say
. Now, if we look at
Figure 9, we observe that the whole channel is already affected by a serious fouling. Increasing pressure beyond these optimal pressures will impose fouling everywhere in the channel, with the subsequent deposit resistance, which seemingly increases faster than operating pressure. This can result from the fact that, when high polarization (and subsequent fouling) is achieved at the channel very entrance, the boundary layer is enlarged everywhere downstream, so that solute back-diffusion towards the bulk is lowered in the whole channel, then increasing concentration polarization, and therefore reducing permeation.
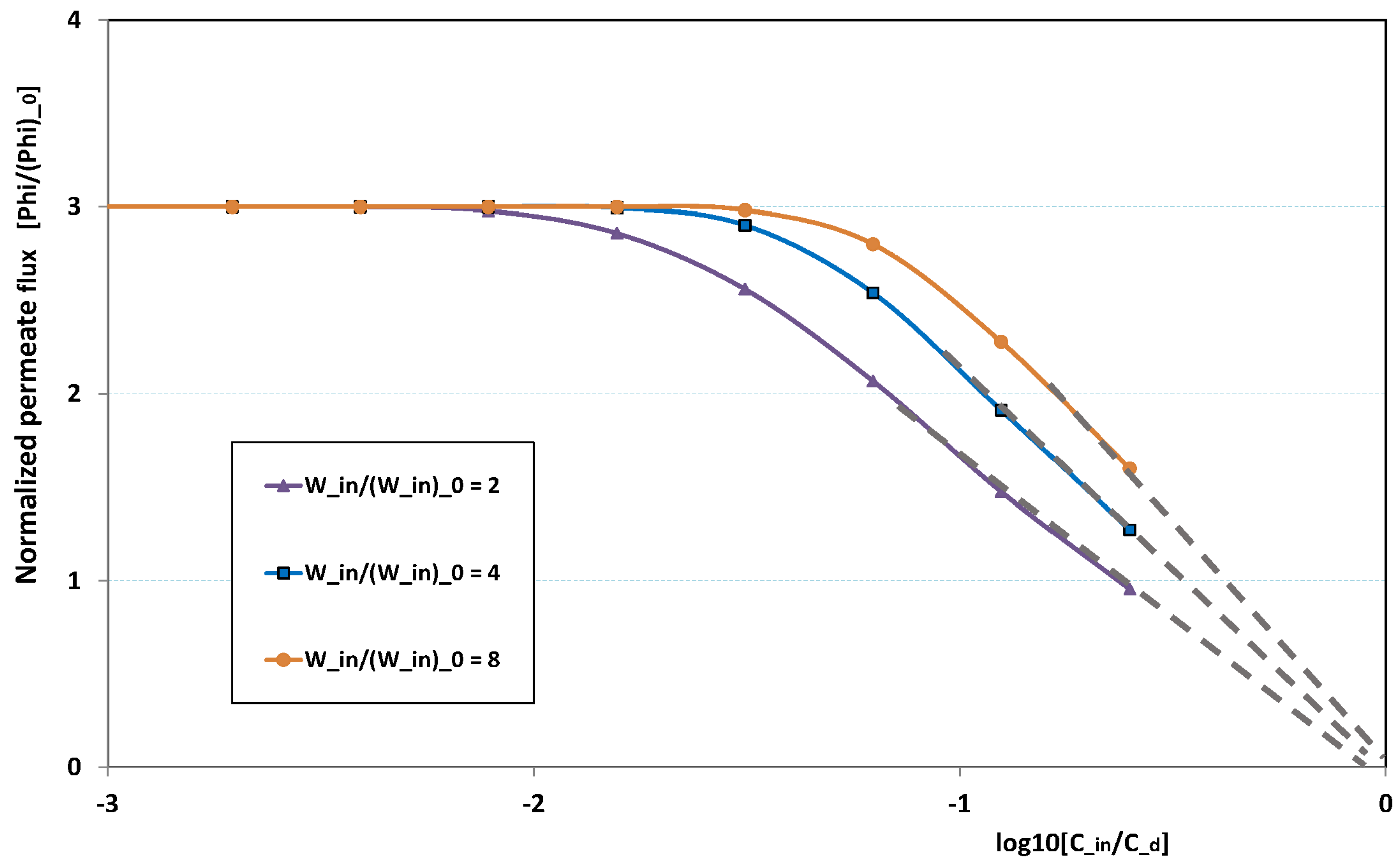
Figure 20 illustrates the role played by the inlet feed concentration for a given pressure. The figure plots the reduced permeation against
for three inlet flow rates. At low
, fouling is non-existent. This is why there is no variation with
, the permeation being determined by the trans-membrane pressure, which is here fixed to three times the pressure of reference. This is why the “clear water” permeation is three times the permeation of reference.
At larger , fouling can arise, depending on the flow rate: at low flow rate, fouling starts for lower feed concentration than for high flow rate. When the feed concentration is high, permeation rapidly vanishes and tends towards zero linearly with , as observed in numerous experiments. As a result, all permeation curves seem to converge to nil for , the critical concentration for deposit. Note these curves are not plotted for very high , since our Prandtl model becomes inaccurate when is of the same order as .
8. Conclusions
A situation of permeation controlled by Darcy’s law in cross-flow filtration has been studied together with the implications of a standard model of reversible fouling, which consists of imposing a material deposit, as long as a critical concentration is reached. Deposit stops—and the related wall resistance reaches a steady state—when the permeation diminishes and causes a polarization of concentration that corresponds to the critical concentration. The present contribution on reversible fouling can be summarized in several stages.
First, the general set of control parameters contains seven numbers: two numbers that control osmotic pressure and pressure drop have been chosen in such a manner that the related phenomena are minimized. As for the membrane number , it is a vanishing quantity that justifies the use of the Prandtl approximation, which has then been studied numerically. The results have shown that mass transfer utterly controls filtration: this led us to discard the role played by , the transverse Reynolds number. Hence, the intensive numerical study consisted in varying , the Péclet transverse number, , the deposit number, and , the reduced channel length. Furthermore, the analysis of the local permeation led us to interpret the results with the use of , the so-called fouling number. Even though some scatter can be observed in this classification, the z-integration, which provides us with the channel overall permeation, markedly improves its quality.
It then turned out that all the numerical results can be gathered accordingly with two parameters: the channel reduced length
and the fouling number
. This allowed us to search for a family of curves with a single parameter to perform the step of fit. This led us to the analytical expression (
42), which provided us with the overall permeate flux in dimensional form as a function of all the parameters in dimensional form. Note that a crude use of expression (
42) for
would neglect axial diffusion and therefore underestimate permeation.
Figure 18,
Figure 19 and
Figure 20 help us to appreciate the quality of the predictions brought by expression (
42). They appear satisfactory, since they qualitatively exhibit all the features related to fouling experiments in ultrafiltration. In particular, the existence of a “critical flux” and of a “limiting flux” has been pointed out, as well as some non-monotonic dependence of permeation vs. pressure. Furthermore, numerous experimental configurations correspond to situations where mass transfer is mainly turbulent, owing to the resort to obstacles or vortices imposed on the feed flow. A straightforward manner to extend the present laminar approach to turbulence consists of introducing a rough modelling of turbulent transfer thanks to the turbulent diffusion coefficient
and in substituting it for
in all the present analytical expressions.
Other regimes of filtration, such as reverse osmosis or nanofiltration, also experience fouling. Their interpretation can resort to the results of the present paper as a milestone. Strictly speaking, this needs to extend the present results to non-vanishing values of
, which will conduct a rather complex discussion with four parameters. Nonetheless, the present approach confirms that the limiting flux phenomenon is intrinsic to fouling, to be compared with the logarithmic growth due to osmotic effects. In other words, the present study combined with Ref. [
1] should help to discriminate both hindrance phenomena.