Nanomaterials: Solutions to Water-Concomitant Challenges
Abstract
1. Introduction
2. The Role of Nanomaterials for Effective Pollutant Removal—Heavy Metals, Dyes, and Antibacterial Activity
2.1. Nanofiber Membranes as Pollutant Removal Media
2.1.1. Heavy Metal Removal
2.1.2. Antibacterial Activity
2.1.3. Dye Removal
2.2. Metal Oxides as Pollutant Removal Media
2.2.1. Heavy Metal Removal
2.2.2. Dye Removal using Metal Oxide as a Sorbent
2.2.3. Metal Oxide Nanomaterials with Antibacterial Activity
2.3. Carbonaceous Materials as Pollutant Removal Media
2.3.1. Heavy Metal Removal
2.3.2. Dye Removal
2.3.3. Anti-Bacterial Activity
3. Oil-Water Separation and Removal using Nanofiber Membranes and Carbonaceous Materials
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- UN Water- Coordinating the UN’s Work on Water and Sanitation. Available online: http://www.unwater.org/statistics/en/ (accessed on 14 March 2019).
- Li, X.; Qi, Y.; Li, Y.; Zhang, Y.; He, X.; Wang, Y. Novel magnetic beads based on sodium alginate gel crosslinked by zirconium(IV) and their effective removal for Pb2+ in aqueous solutions by using a batch and continuous systems. Bioresour. Technol. 2013, 142, 611–619. [Google Scholar] [CrossRef]
- Culp, S.J.; Blankenship, L.R.; Kusewitt, D.F.; Doerge, D.R.; Mulligan, L.T.; Beland, F.A. Toxicity and metabolism of malachite green and leucomalachite green during short-term feeding to Fischer 344 rats and B6C3F1 mice. Chem.-Biol. Interact. 1999, 122, 153–170. [Google Scholar] [CrossRef]
- Ngwuluka, N.C.; Ochekpe, N.A.; Odumosu, P.O. An assessment of pharmaceutical waste management in some Nigerian pharmaceutical industries. Afr. J. Biotechnol. 2011, 10, 11259–11264. [Google Scholar] [CrossRef]
- Tania, D. Nanotechnology for Water Purification; Brown Walker Press: Boca Raton, FL, USA, 2012; Volume 1, pp. 1–29. [Google Scholar]
- Madadrang, C.J.; Kim, H.Y.; Gao, G.; Wang, N.; Zhu, J.; Feng, H.; Gorring, M.; Kasner, M.L.; Hou, S. Adsorption Behavior of EDTA-Graphene Oxide for Pb (II) Removal. ACS Appl. Mater. Interface 2012, 4, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, S.H.; Shehata, A.M.A.; El-Shahat, M.F. Removal of lead ions from industrial waste water by different types of natural materials. Water Res. 2003, 37, 1678–1683. [Google Scholar] [CrossRef]
- Xu, M.; Hadi, P.; Chen, G.; McKay, G. Removal of cadmium ions from wastewater using innovative electronic waste-derived material. J. Hazard. Mater. 2014, 273, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Chen, L.; Huang, X.; Liu, J. Preparation and evaluation of magnetic nanoparticles impregnated chitosan beads for arsenic removal from water. Chem. Eng. J. 2014, 251, 25–34. [Google Scholar] [CrossRef]
- Awual, M.R. A novel facial composite adsorbent for enhanced copper (II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375. [Google Scholar] [CrossRef]
- Ravindra, K.G.; Pavan, K.G.; Sushmita, B.; Shivani, S.; Sanjeev, K.S.; Mahesh, C.C. Removal of Ni(II) by magnetic nanoparticles. J. Mol. Liq. 2015, 204, 60–69. [Google Scholar]
- Sneha, L.; Shripal, S.; Amalendu, S. Magnetic iron oxide (Fe3O4) nanoparticles from tea waste for arsenic removal. J. Magn. Magn. Mater. 2014, 356, 21–31. [Google Scholar]
- Mark, A.B.; Stephen, C.D.V. Predicting azo dye toxicity. Crit. Rev. Environ. Sci. Technol. 1993, 23, 249–324. [Google Scholar]
- Nafarethinam, K.; Mariappan, M.S. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dyes Pigments 2001, 51, 25–40. [Google Scholar]
- Badr, Y.; Abd El-Wahed, M.G.; Mahmoud, M.A. Photocatalytic degradation of methyl red dye by silica nanoparticles. J. Hazard. Mater. 2008, 154, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, B.E.; Hunt, L.R.; Hildenbrand, Z.L.; Carlton, D.D., Jr.; Oka, H.; Walton, J.L.; Hopkins, D.; Osorio, A.; Bjorndal, B.; Hu, Q.H.; et al. An evaluation of water quality in private drinking water wells near natural gas extraction sites in the Barnett shale formation. Environ. Sci. Technol. 2013, 47, 10032–10040. [Google Scholar] [CrossRef]
- Stephen, G.O.; Avner, V.; Nathaniel, R.W.; Robert, B.J. Methane contamination of drinking water accompanying gas-well drilling and hydraulic fracturing. Proc. Natl. Acad. Sci. USA 2011, 108, 8172–8176. [Google Scholar]
- Marisa, P.; Anbarasan, A.; Rosa, A.B.; Britt, M.S.; Maria, J.; Bo, M. Degradation of a textile azo dye using biological treatment followed by photo-Fenton oxidation: Evaluation of toxicity and microbial community structure. Chem. Eng. J. 2015, 270, 290–299. [Google Scholar]
- Dong, Y.; Su, Y.; Chen, W.; Peng, J.; Zhang, Y.; Jang, Z. Ultrafiltration Enhanced with Activated Carbon Adsorption for Efficient Dye Removal from Aqueous Solution. Chin. J. Chem. Eng. 2011, 19, 863–869. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kim, E.J.; Yang, J.W.; Shin, H.J. Removal of malachite green by adsorption and precipitation using amino propyl functionalized magnesium phyllosilicate. J. Hazard. Mater. 2011, 192, 62–70. [Google Scholar]
- Anca, D.; Maria, V. Simultaneous removal of two industrial dyes by adsorption and photocatalysis on a fly-ash–TiO2 composite. J. Photochem. Photobiol. A Chem. 2015, 306, 21–30. [Google Scholar]
- Harold, F.U. The Deepwater Horizon Oil Spill and the Gulf of Mexico Fishing Industry. 2011. Available online: https://fas.org/sgp/crs/misc/R41640.pdf (accessed on 14 March 2019).
- Peng, H.; Tremblay, A.Y.; Veinot, D.E. The use of back flushed coalescing microfiltration as a pretreatment for the ultrafiltration of bilge water. Desalination 2005, 181, 109–120. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Ramakrishna, S. New Directions in Nanofiltration Applications—Are Nanofibers the Right Materials as Membranes in Desalination? Desalination 2013, 308, 198–208. [Google Scholar]
- Ramalingam, B.; Subramanian, S.; Seeram, R. Recent Trends in Nanofibrous Membranes and Their Suitability for Air and Water Filtrations. Membranes 2011, 1, 232–248. [Google Scholar]
- Shaik, A.A.N.N.; Subramanian, S.; Syed, A.S.N.; Ramalingam, B.; Seeram, R. Advancement in Electrospun Nanofibrous Membranes Modification and Their Application in Water Treatment. Membranes 2013, 3, 266–284. [Google Scholar]
- Renuga, G.; Satinderpal, K.; Chao, Y.F.; Casey, C.; Seeram, R.; Shahram, T.; Takeshi, M. Electrospun nanofibrous polysulfone membranes as pre-filters: Particulate removal. J. Membr. Sci. 2007, 289, 210–219. [Google Scholar]
- Liu, Y.; Wang, R.; Ma, H.; Hsiao, B.S.; Chu, B. High-flux microfiltration filters based on electrospun poly vinyl alcohol nanofibrous membranes. Polymer 2013, 54, 548–556. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Ultra-fine cellulose nanofibers: New nano-scale materials for water purification. J. Mater. Chem. 2011, 21, 7507–7510. [Google Scholar] [CrossRef]
- Shaik, A.A.N.N.; Subramanian, S.; Syed, A.S.N.; Ramalingam, B.; Seeram, R. In situ polymerization of PVDF-HEMA polymers: Electrospun membranes with improved flux and antifouling properties for water filtration. Polym. J. 2014, 46, 167–174. [Google Scholar]
- Li, M.; Xue, X.; Wang, D.; Lu, Y.; Wu, Z.; Zou, H. High performance filtration nanofibrous membranes based on hydrophilic poly (vinyl alcohol-co-ethylene) copolymer. Desalination 2013, 329, 50–56. [Google Scholar] [CrossRef]
- Sang, Y.; Gu, Q.; Sun, T.; Li, F.; Liang, C. Filtration by a novel nanofiber membrane and alumina adsorption to remove copper (II) from groundwater. J. Hazard. Mater. 2008, 153, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, C.; Qi, G.; Pan, K.; Cao, B. Mechanism study of selective heavy metal ion removal with polypyrrole-functionalized polyacrylonitrile nanofiber mats. Appl. Surf. Sci. 2014, 316, 245–250. [Google Scholar] [CrossRef]
- He, X.; Cheng, L.; Wang, Y.; Zhao, J.; Zhang, W.; Lu, C. Aerogels from quaternary ammonium functionalized cellulose nanofibers for rapid removal of Cr(VI) from water. Carbohydr. Polym. 2014, 111, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Wu, D.; Zhao, Y.; Wei, A.; Wei, Q.; Fong, H. Electrospun AOPAN/RC blend nanofiber membrane for efficient removal of heavy metal ions from water. J. Hazard. Mater. 2018, 344, 819–828. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, H.; Wang, J.; Ling, Z.; Qiu, J. In situ synthesis of chemically active ZIF coordinated with electrospun fibrous film for heavy metal removal with a high flux. Sep. Purif. Technol. 2017, 177, 257–262. [Google Scholar] [CrossRef]
- Wang, X.; Yeh, T.M.; Wang, Z.; Yang, R.; Wang, R.; Ma, H.; Hsiao, B.S.; Chu, B. Nanofiltration membranes prepared by interfacial polymerization on thin-film nanofibrous composite scaffold. Polymer 2014, 55, 1358–1366. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. High flux nanofiltration membranes based on interfacially polymerized polyamide barrier layer on polyacrylonitrile nanofibrous scaffolds. J. Membr. Sci. 2009, 326, 484–492. [Google Scholar] [CrossRef]
- Cheng, H.L.; Chang, L.C.; Shih, J.L. Electrospun nanofibrous rhodanine/polymethylmethacrylate membranes for the removal of heavy metal ions. Sep. Purif. Technol. 2013, 118, 737–743. [Google Scholar]
- Iman, Y.S.; Sagar, T.; Kennedy, O.; Ola, A.M.; Adam, K.W. Polymeric nanofibers for the removal of Cr (III) from tannery waste water. J. Environ. Manag. 2013, 129, 410–413. [Google Scholar]
- Arash, T.; Mohammad, K.P.; Munir, A.; Zohreh, P. New efficient inorganic-organic nanofibers electrospun membrane for fluorescence detection and removal of mercury (II) ions. J. Mol. Struct. 2019, 1179, 242–251. [Google Scholar]
- Rathinam, K.; Sankaran, M. Removal of Pb (II) and Cd (II) ions from aqueous solution usingpolyaniline grafted chitosan. Chem. Eng. J. 2015, 63, 168–177. [Google Scholar]
- Yang, D.; Li, L.; Chen, B.; Shi, S.; Nie, J.; Ma, G. Functionalized chitosan electrospun nanofiber membranes for heavy-metal removal. Polymer 2019, 163, 74–85. [Google Scholar] [CrossRef]
- Yoo, H.; Kwak, S.Y. Surface functionalization of PTFE membranes with hyperbranched poly (amidoamine) for the removal of Cu2+ ions from aqueous solution. J. Membr. Sci. 2013, 448, 125–134. [Google Scholar] [CrossRef]
- Abdouss, M.; Mousavi Shoushtari, A.; Majidi Simakani, A.; Akbari, S.; Haji, A. Citric acid-modified acrylicmicro and nanofibers for removal of heavy metal ions from aqueous media. Desalination Water Treat. 2014, 52, 7133–7142. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, Y.; Li, Z.; Tian, D.; Chen, L.; Chen, P. Chitin nanofibrils for rapid and efficient removal of metal ions from water system. Carbohydr. Polym. 2013, 98, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Rad, L.R.; Momeni, A.; Ghazani, B.F.; Irani, M.; Mahmoudi, M.; Noghreh, B. Removal of Ni2+ and Cd2+ ions from aqueous solutions using electrospun PVA/zeolite nanofibrous adsorbent. Chem. Eng. J. 2014, 256, 119–127. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Shen, M.; Tan, X.; Ding, Y.; Jiang, Z.; Wang, C. Preparation of phosphorylated polyacrylonitrile-based nanofiber mat and its application for heavy metal ion removal. Chem. Eng. J. 2015, 268, 290–299. [Google Scholar] [CrossRef]
- Zhou, W.; He, J.; Cui, S.; Gao, W. Preparation of electrospun silk fibroin/Cellulose Acetate blend nanofibers and their applications to heavy metal ions adsorption. Fibers Polym. 2011, 12, 431–437. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Zhang, J.; Kim, E.J. Capture of toxic radioactive and heavy metal ions from water by using titanate nanofibers. J. Alloys Compd. 2014, 614, 389–393. [Google Scholar] [CrossRef]
- Mahapatra, A.; Mishra, B.G.; Hota, G. Electrospun Fe2O3–Al2O3 nanocomposite fibers as efficient adsorbent for removal of heavy metal ions from aqueous solution. J. Hazard. Mater. 2013, 258–259, 116–123. [Google Scholar] [CrossRef]
- Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Adsorptive removal of heavy metals from water using sodium titanate nanofibers loaded onto GAC in fixed-bed columns. J. Hazard. Mater. 2015, 287, 306–316. [Google Scholar] [CrossRef]
- Ma, H.; Hsiao, B.S.; Chu, B. Functionalized electrospun nanofibrous microfiltration membranes for removal of bacteria and viruses. J. Membr. Sci. 2014, 452, 446–452. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Liu, J.; Zhang, H.; Wang, K. Preparation and antibacterial property of Polyethersulfone ultrafiltration hybrid membrane containing halloysite nanotubes loaded with copper ions. Chem. Eng. J. 2012, 210, 298–308. [Google Scholar] [CrossRef]
- Almajhdi, F.N.; Fouad, H.; Khalil, K.A.; Awad, H.M.; Mohamed, S.H.S.; Elsarnagawy, T.; Albarrag, A.M.; Al-Jassir, F.F.; Abdo, H.S. In-vitro anticancer and antimicrobial activities of PLGA/silver nanofiber composites prepared by electrospinning. J. Mater. Sci. 2014, 25, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.R.; Pandeya, D.R.; Nam, K.T.; Baek, W.I.; Hong, S.T.; Kim, H.Y. Photocatalytic and antibacterial properties of a TiO2/nylon-6 electrospun nanocomposite mat containing silver nanoparticles. J. Hazard. Mater. 2011, 189, 465–471. [Google Scholar] [CrossRef]
- Gilchrist, S.E.; Lange, D.; Letchford, K.; Bach, H.; Fazli, L.; Burt, H.M. Fusidic acid and rifampicin co-loaded PLGA nanofibers for the prevention of orthopedic implant associated infections. J. Control. Release 2013, 170, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Daels, N.; De Vrieze, S.; Sampers, I.; Decostere, B.; Westbroek, P.; Dumoulin, A.; Dejans, P.; De Clerck, K.; Van Hulle, S.W.H. Potential of a functionalized nanofiber microfiltration membrane as an antibacterial water filter. Desalination 2011, 275, 285–290. [Google Scholar] [CrossRef]
- Yao, C.; Li, X.; Neoh, K.G.; Shi, Z.; Kang, E.T. Surface modification and antibacterial activity of electrospun polyurethane fibrous membranes with quaternary ammonium moieties. J. Membr. Sci. 2008, 320, 259–267. [Google Scholar] [CrossRef]
- Mei, Y.; Yao, C.; Fan, K.; Li, X. Surface modification of polyacrylonitrile nanofibrous membranes with superior antibacterial and easy-cleaning properties through hydrophilic flexible spacers. J. Membr. Sci. 2012, 417–418, 20–27. [Google Scholar] [CrossRef]
- Heinz, L. Color Chemistry: Synthesis, Properties and Applications of Organic Dyes and Pigmens; VCH Publishes: New York, NY, USA, 1987; pp. 92–100. [Google Scholar]
- Patel, S.; Hota, G. Adsorptive Removal of Malachite Green Dye by Functionalized Electrospun PAN Nanofibers Membrane. Fibers Polym. 2014, 15, 2272–2282. [Google Scholar] [CrossRef]
- Almasian, A.; Olya, M.E.; Mahmoodi, N.M. Synthesis of polyacrylonitrile/polyamidoamine composite nanofibers using electrospinning technique and their dye removal capacity. J. Taiwan Inst. Chem. Eng. 2015, 49, 119–128. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, S.; Ye, Z. Preparation and characterization of new foam adsorbents of poly (vinyl alcohol)/chitosan composites and their removal for dye and heavy metal from aqueous solution. Chem. Eng. J. 2012, 183, 88–97. [Google Scholar] [CrossRef]
- Basiri, F.; Abdolkarim, H.R.S.; Feiz, M.; Moheb, A. Recycling of Direct Dyes Wastewater by Nylon-6 Nanofibrous Membrane. Curr. Nanosci. 2011, 7, 633–639. [Google Scholar] [CrossRef]
- Fu, G.; Su, Z.; Jiang, X.; Yin, J. Photo cross-linked nanofibers of poly (ether amine) (PEA) for the ultrafast separation of dyes through molecular filtration. Polym. Chem. 2014, 5, 2027–2034. [Google Scholar] [CrossRef]
- Mozhdeh, G.; Ali Akbar, G.; Mokhtar, A.; Negar, T.; Babak, R. Fabrication of Electrospun Polyamide-6/Chitosan Nanofibrous Membrane toward Anionic Dyes Removal. J. Nanotech. 2014, 278418. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Bai, H.; Sun, D.D. Concurrent filtration and solar photocatalytic disinfection/degradation using high-performance Ag/TiO2 nanofiber membrane. Water Res. 2012, 46, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, D.; Gao, C.; Wei, Q.; Cai, Y.; Xu, J.; Liu, X.; Xu, Y. Removal of a Cationic Dye by Adsorption/Photo degradation Using Electrospun PAN/O-MMT Composite Nanofibrous Membranes Coated with TiO2. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
- Aluigi, A.; Rombaldoni, F.; Tonetti, C.; Jannoke, L. Study of Methylene Blue adsorption on keratin nanofibrous membranes. J. Hazard. Mater. 2014, 268, 156–165. [Google Scholar] [CrossRef]
- Xu, Y.; Bao, J.; Zhang, X.; Li, W.; Xie, Y.; Sun, S.; Zhao, W.; Zhao, C. Functionalized polyethersulfone nanofibrous membranes with ultra-high adsorption capacity for organic dyes by one-step electrospinning. J. Colloid Interface Sci. 2019, 533, 526–538. [Google Scholar] [CrossRef]
- Bai, L.; Jia, L.; Yan, Z.; Liu, Z.; Liu, Y. Plasma-etched electrospun nanofiber membrane as adsorbent for dye removal. Chem. Eng. Res. Des. 2018, 132, 445–451. [Google Scholar] [CrossRef]
- Bekir, S.; Peter, M.B.; Tamer, U. Systematic hydrolysis of PIM-1 and electrospinning of hydrolyzed PIM-1 ultrafine fibers for an efficient removal of dye from water. React. Funct. Polym. 2017, 121, 67–75. [Google Scholar]
- Chaúque, E.F.; Dlamini, L.N.; Adelodun, A.A.; Greyling, C.J.; Ngila, J.C. Electrospun polyacrylonitrile nanofibers functionalized with EDTA for adsorption of ionic dyes. Phys. Chem. Earth Parts A/B/C 2017, 100, 201–211. [Google Scholar]
- Zhang, J.; Zhai, S.; Li, S.; Xiao, Z.; Song, Y.; An, Q.; Tian, G. Pb (II) removal of Fe3O4@SiO2–NH2 core–shell nanomaterials prepared via controllable sol–gel process. Chem. Eng. J. 2013, 215–216, 461–471. [Google Scholar] [CrossRef]
- Madhu, K.; Charles, U.P.; Dinesh, M. Heavy metals [chromium (VI) and lead (II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J Colloid Interface Sci. 2015, 442, 120–132. [Google Scholar]
- Ali, A.; Shahriar, H.; Ahmad, R.M.; Emaeel, D.; Houshang, A.H. Optimization of heavy metal removal from aqueous solutions by maghemite (γ-Fe2O3) nanoparticles using response surface methodology. J. Geochem. Explor. 2014, 147, 151–158. [Google Scholar]
- Negin, G.; Sayed, S.M.; Parisa, D.; Hamid, R.; Sirus, Z.; Abdolhamid, A.; Rouhollah, H.; Mojtaba, B.; Sohrab, G. Polyethersulfone membrane enhanced with iron oxide nanoparticles for copper removal from water: Application of new functionalized Fe3O4 nanoparticles. Chem. Eng. J. 2015, 263, 101–112. [Google Scholar]
- Cai, X.; Gao, Y.; Sun, Q.; Chen, Z.; Megharaj, M.; Naidu, R. Removal of co-contaminants Cu (II) and nitrate from aqueous solution using kaolin-Fe/Ni nanoparticles. Chem. Eng. J. 2014, 244, 19–26. [Google Scholar] [CrossRef]
- Phuengprasop, T.; Sittiwong, J.; Unob, F. Removal of heavy metal ions by iron oxide coated sewage sludge. J. Hazard. Mater. 2011, 186, 502–507. [Google Scholar] [CrossRef]
- Yoann, G.; Ahmad, B.A.; Jose, G.; Eghe, O.; Chirangano, M.; Claire, G.; Stephen, J.A.; Gavin, M.W. Adsorption study using optimized 3D organized mesoporous silica coated with Fe and Al oxides for specific As(III) and As(V) removal from contaminated synthetic groundwater. Microporous Mesoporous Mater. 2014, 198, 101–114. [Google Scholar]
- Dadfarnia, S.; Shabani, A.M.H.; Moradi, S.E.; Emami, S. Methyl red removal from water by iron based metal-organic frameworks loaded onto iron oxide nanoparticle adsorbent. Appl. Surf. Sci. 2015, 330, 85–93. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, J.; Wang, Q.; Li, S.; Qi, Q.; Zhu, R. Synthesis of 3D hierarchical porous iron oxides for adsorption of Congo red from dye wastewater. J. Alloys Compd. 2015, 622, 587–595. [Google Scholar] [CrossRef]
- Mahapatra, A.; Mishra, B.G.; Hota, G. Adsorptive removal of Congo red dye from waste water by mixed iron oxide- alumina nanocomposites. Ceram. Int. 2013, 39, 5443–5451. [Google Scholar] [CrossRef]
- Kumar, K.Y.; Muralidhara, H.B.; Nayaka, YA.; Balasubramanyam, J.; Hanumanthappa, H. Lowcost synthesis of metal oxide nanoparticles and their application in adsorption of commercial dye and heavy metal ion in aqueous solution. Powder Technol. 2013, 246, 125–136. [Google Scholar] [CrossRef]
- Al-Nakib, C.; Abdur, R.; Yusuf, J.F.; Md.Shafiul, A.; Mufazzal, H.M. Cobalt–nickel mixed oxide surface: A promising adsorbent for the removal of PR dye from water. Appl. Surf. Sci. 2010, 256, 3718–3724. [Google Scholar]
- Gupta, V.K.; Agarwa, S.; Pathania, D.; Kothiyal, N.C.; Sharma, G. Use of pectin–thorium (IV) tungstomolybdate nanocomposite for photocatalytic degradation of methylene blue. Carbohydr. Polym. 2013, 96, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tang, W.; Gao, J.; Zhou, L.; He, Y. Immobilization of horseradish peroxidase in phospholipid-templated Titania and its applications in phenolic compounds and dye removal. Enzym. Microb. Technol. 2014, 55, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sarika, S.; Barick, K.C.; Bahadur, D. Fe3O4 embedded ZnO nanocomposites for the removal of toxic metal ions, organic dyes and bacterial pathogens. J. Mater. Chem. A 2013, 1, 3325–3333. [Google Scholar]
- Chen, D.; Li, Y.; Zhang, J.; Li, W.; Zhou, J.; Shao, L.; Qian, G. Efficient removal of dyes by a novel magnetic Fe3O4/ZnCr-layered double hydroxide adsorbent from heavy metal wastewater. J. Hazard. Mater. 2012, 243, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.J.; Lin, R.D. BiFeO3/α-Fe2O3 core/shell composite particles for fast and selective removal of methyl orange dye in water. J. Colloid Interface Sci. 2014, 428, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Hye, J.H.; Jungmin, K.; Jung, S.Y.; Eun, J.K.; Jin, W.Y. Removal of Anionic and Cationic Dyes from Water by Fe-Al Binary Oxide. Sep. Sci. Technol. 2012, 47, 2218–2224. [Google Scholar]
- Chen, L.; Song, Z.; Wang, X.; Prikhodko, S.V.; Hu, J.; Kodambaka, S.; Richards, R. Three-Dimensional Morphology Control during Wet Chemical Synthesis of Porous Chromium Oxide Spheres. ACS Appl. Mater. Interfaces 2009, 1, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J.; The Healthcare Infection Control Practices Advisory Committee (HICPAC). Guide for Disinfection and Sterilization in Health Care Facilities; CDC: Atlanta, GA, USA, 2008.
- Tamar, G.; Benny, P.; Ofir, H.; Israel, F.; Ehud, B.; Shlomo, M. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloid Surf. A Physicochem. Eng. Asp. 2011, 374, 1–8. [Google Scholar]
- Antony, A.; Subramanian, D.; Moon, S.H.; Young, S.M. Copper oxide nanomaterials: Synthesis, characterization and structure-specific antibacterial performance. Chem. Eng. J. 2015, 262, 179–188. [Google Scholar]
- Amin, Y.B.; Rong, W.; Rong, X. Simple method of deposition of CuO nanoparticles on a cellulose paper and its antibacterial activity. Chem. Eng. J. 2015, 262, 999–1008. [Google Scholar]
- Jasna, H.; Jelena, M.; Nina, D.; Renata, M.K.; Nevenka, R. Antimicrobial activity of metal oxide nanoparticles supported onto natural clinoptilolite. Chemosphere 2012, 88, 1103–1107. [Google Scholar]
- Zhang, L.; Pei, Y.T.; Chee, L.C.; Chiew, K.L.; Ooi, K.T.; Man, S.T.; Chun, C.S. Antibacterial activities of mechanochemically synthesized perovskite strontium titanate ferrite metal oxide. Colloid Surf. A Physicochem. Eng. Asp. 2014, 456, 169–175. [Google Scholar] [CrossRef]
- Cui, L.; Guo, X.; Wei, Q.; Wang, Y.; Gao, L.; Yan, L.; Yan, T.; Du, B. Removal of mercury and methylene blue from aqueous solution by xanthate functionalized magnetic graphene oxide: Sorption kinetic and uptake mechanism. J. Colloid Interface Sci. 2015, 439, 112–120. [Google Scholar] [CrossRef]
- Sreeprasad, T.S.; Shihabudheen, M.M.; Lisha, K.P.; Pradeep, T. Reduced graphene oxide–metal/metal oxide composites: Facile synthesis and application in water purification. J. Hazard. Mater. 2011, 186, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, M.; Ahmadpour, A.; Asl, A.H. Removal of mercury from water by carbonaceous sorbents derived from walnut shell. J. Hazard. Mater. 2009, 67, 230–236. [Google Scholar] [CrossRef]
- Jiu, H.D.; Xiu, R.Z.; Guang, M.Z.; Ji, L.G.; Qiu, Y.N.; Jie, L. Simultaneous removal of Cd(II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chem. Eng. J. 2013, 226, 189–200. [Google Scholar]
- Fu, Y.; Wang, J.; Liu, Q.; Zeng, H. Water-dispersible magnetic nanoparticle–graphene oxide composites for selenium removal. Carbon 2014, 77, 710–721. [Google Scholar] [CrossRef]
- Mohamed, E.M.; Maher, M.O.; Somia, B.A.; Tarek, M.A. Improved adsorptive removal of cadmium from water by hybrid chemically and biologically carbonaceous sorbents. Chem. Eng. J. 2011, 175, 84–94. [Google Scholar]
- Mona, K.; Ahmad, K.; Hanafy, H.; Zakia, O. Heavy Metals Removal Using Activated Carbon, Silica and Silica Activated Carbon Composite. Energy Procedia 2014, 50, 113–120. [Google Scholar]
- Ashish, K.M.; Ramaprabhu, S. Functionalized graphene sheets for arsenic removal and desalination of sea water. Desalination 2011, 282, 39–45. [Google Scholar]
- Wen, Y.; Ma, J.; Chen, J.; Shen, C.; Li, H.; Liu, W. Carbonaceous sulfur-containing chitosan–Fe (III): A novel adsorbent for efficient removal of copper (II) from water. Chem. Eng. J. 2015, 259, 372–380. [Google Scholar] [CrossRef]
- Guo, X.; Du, B.; Wei, Q.; Yang, J.; Hu, L.; Yan, L.; Xu, W. Synthesis of amino functionalized magnetic graphenes composite material and its application to remove Cr(VI), Pb(II), Hg(II), Cd(II) and Ni(II) from contaminated water. J. Hazard. Mater. 2014, 278, 211–220. [Google Scholar] [CrossRef]
- Renu, D.; Baipai, J.; Baipa, A.K. Green synthesis of graphene sand composite (GSC) as novel adsorbent for efficient removal of Cr (VI) ions from aqueous solution. J. Water Process Eng. 2015, 5, 83–94. [Google Scholar]
- Qle, F.; Baoliang, C. Self-assembly of graphene oxide aerogels by layered double hydroxides cross-linking and their application in water purification. J. Mater. Chem. A 2014, 2, 8941–8951. [Google Scholar]
- Luo, S.; Xu, X.; Zhou, G.; Liu, C.; Tang, Y.; Liu, Y. Amino siloxane oligomer-linked graphene oxide as an efficient adsorbent for removal of Pb(II) from wastewater. J. Hazard. Mater. 2014, 274, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, F.; Luo, Y.; Zhang, L. Synthesis of three-dimensional graphene oxide foam for the removal of heavy metal ions. Chem. Phys./Lett. 2014, 593, 122–127. [Google Scholar] [CrossRef]
- Nikolina, A.T.; George, Z.K.; Nikolaos, K.L.; Eleni, A.D. Graphite oxide/chitosan composite for reactive dye removal. Chem. Eng. J. 2013, 217, 256–265. [Google Scholar]
- Wu, Z.; Zhong, H.; Yuan, X.; Wang, H.; Wang, L.; Chen, X.; Zeng, G.; Wu, Y. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from Wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.; Anming, H.; Melisa, H.F.; Norman, Z. Fundamentals on Adsorption, Membrane Filtration, and Advanced Oxidation Processes for Water Treatment. Nanotechnol. Water Treat. Purif. 2014, 22, 1–45. [Google Scholar]
- Andreia, F.F.; Diego, S.T.M.; Stela, M.M.M.; Ana, C.M.M.; Adriano, B.; Antonio, G.S.F.; Oswaldo, L.A. Anti-adhesion and antibacterial activity of silver nanoparticles supported on grapheme oxide sheets. Colloids Surf. B Biointerfaces 2014, 113, 115–124. [Google Scholar]
- Fernandez-Ibanez, P.; Polo-Lopez, M.I.; Malato, S.; Wadhwa, S.; Hamilton, J.W.J.; Dunlop, P.S.M.; D’Sa, R.; Magee, E.; Shea, K.O.; Dionysiou, D.D. Solar photocatalytic disinfection of water using titanium dioxide graphene composites. Chem. Eng. J. 2015, 261, 36–44. [Google Scholar] [CrossRef]
- Bishweshwar, P.; Hem, R.P.; Nasser, AM.B.; Mira, P.; Kyungsoo, J.; Yuri, C.; Hak-yong, K. Carbon nanofibers decorated with binary semiconductor (TiO2/ZnO) nanocomposites for the effective removal of organic pollutants and the enhancement of antibacterial activities. Ceram. Int. 2013, 39, 7029–7035. [Google Scholar]
- Isis, E.M.C.; Joey, D.M.; Hang, N.N.; Rigoberto, C.A.; Debora, F.R. Graphene oxide functionalized with ethylenediamine triacetic acid for heavy metal adsorption and anti-microbial applications. Carbon 2014, 77, 289–301. [Google Scholar]
- Obaid, M.; Nasser, A.M.B.; Fadali, O.A.; Moaaed, M.; Abdulhakim, A.A.; Khalil, A.K. Effective and reusable oil/water separation membranes based on modified polysulfone electrospun nanofiber mats. Chem. Eng. J. 2015, 259, 449–456. [Google Scholar] [CrossRef]
- Zhang, C.; Li, P.; Cao, B. Electrospun Microfibrous Membranes Based on PIM-1/POSS with High Oil Wettability for Separation of Oil–Water Mixtures and Cleanup of Oil Soluble Contaminants. Ind. Eng. Chem. Res. 2015, 54, 8772–8781. [Google Scholar] [CrossRef]
- Zhang, C.; Li, P.; Cao, B. Fabrication of Superhydrophobic–Superoleophilic Fabrics by an Etching and Dip-Coating Two-Step Method for Oil–Water Separation. Ind. Eng. Chem. Res. 2016, 55, 5030–5035. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, F.; Xue, L. Under seawater superoleophobic PVDF membrane inspired by polydopamine for efficient oil/sea water separation. J. Membr. Sci. 2015, 476, 321–329. [Google Scholar] [CrossRef]
- Mehrdad, K.; Omid, P.; Tahmasb, H. The evaluation of thin film composite membrane composed of an electrospun polyacrylonitrile nanofibrous mid-layer for separating oil-water mixture. Desalination 2015, 359, 14–21. [Google Scholar]
- Farah, E.A.; Boor, S.L.; Nidal, H.; Raed, H. Underwater superoleophobic cellulose/electrospun PVDF–HFP membranes for efficient oil/water separation. Desalination 2014, 344, 48–54. [Google Scholar]
- Shervin, K.; Diana, N.H.T.; Tariq, A.; Dusan, L. Outstanding adsorption performance of graphene–carbon nanotube aerogels for continuous oil removal. Carbon 2014, 80, 523–533. [Google Scholar]
- Liu, Y.; Zhou, J.; Zhu, E.; Tang, J.; Liu, X.; Tang, W. Covalently intercalated graphene oxide for oil–water separation. Carbon 2015, 82, 264–272. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Y.; Zhou, J.; Wang, Y.; Liang, J.; Zhang, X.; Chang, Q.; Song, L. The improved oil/water separation performance of graphene oxide modified Al2O3 microfiltration membrane. J. Membr. Sci. 2015, 476, 200–204. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, P.; Chen, J.; Zhang, J.; Huang, Y.; Chen, T. Janus Polymer/Carbon Nanotube Hybrid Membranes for Oil/Water Separation. ACS Appl. Mater. Interfaces 2014, 6, 16204–16209. [Google Scholar] [CrossRef]
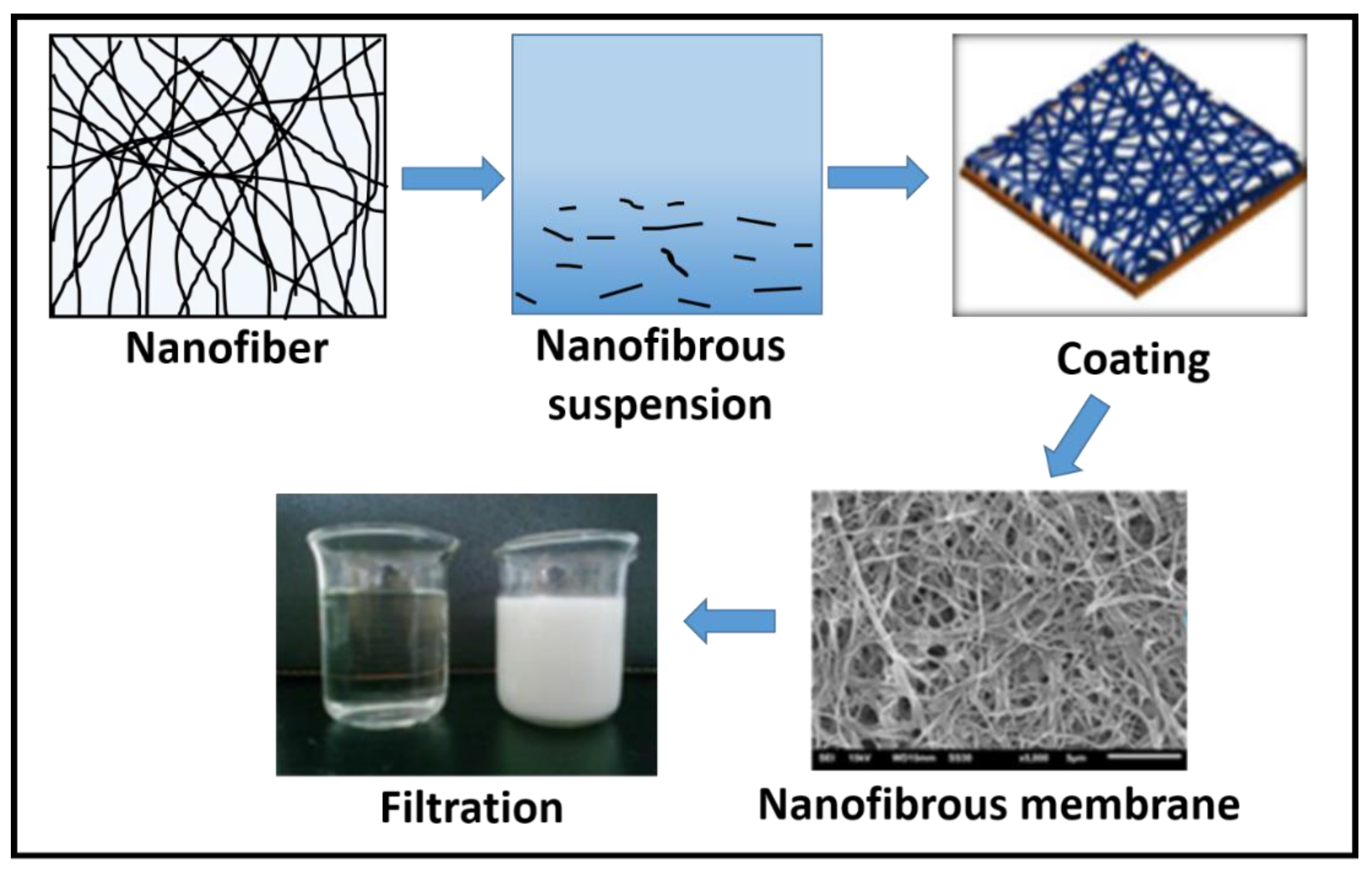

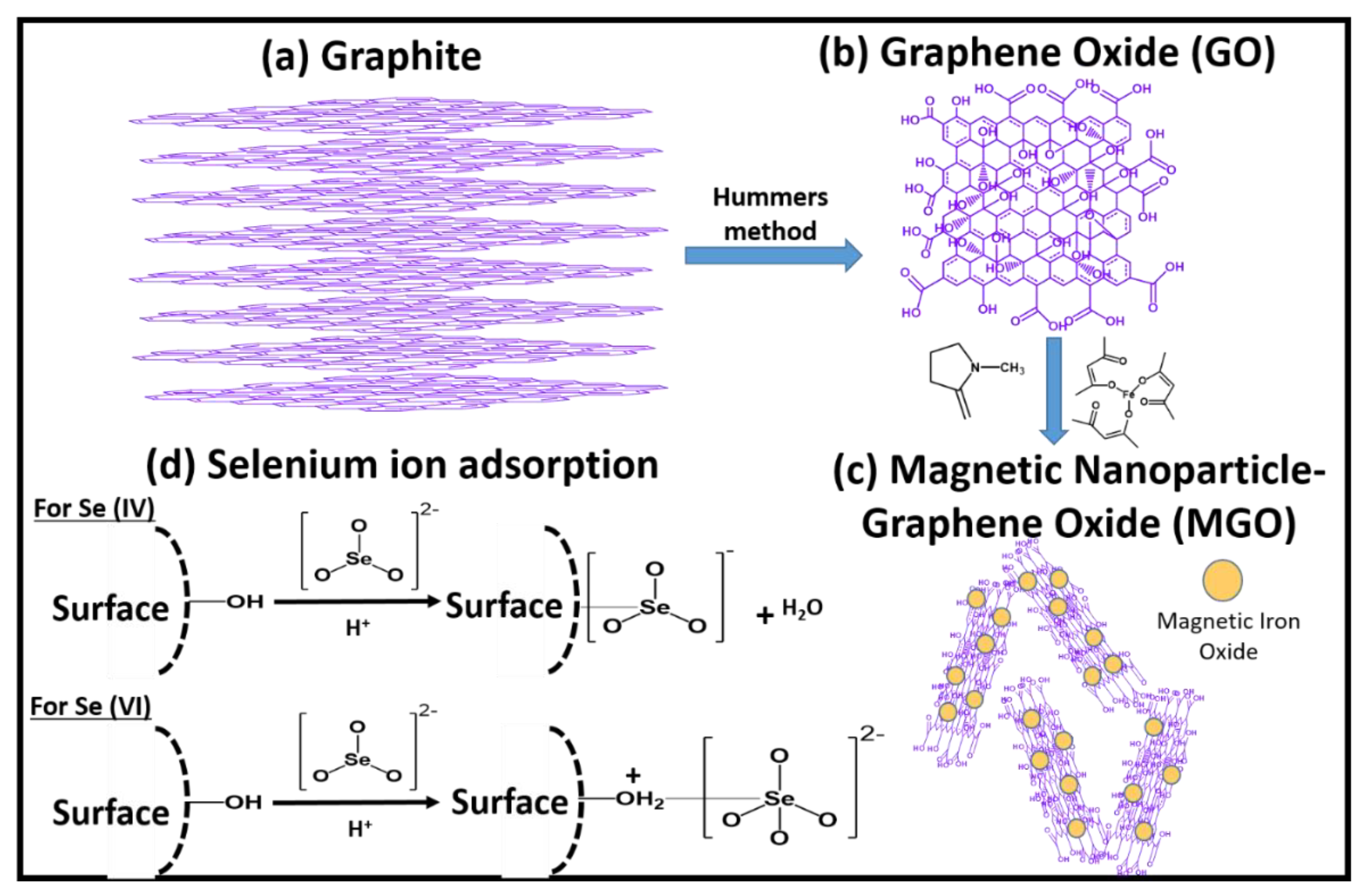
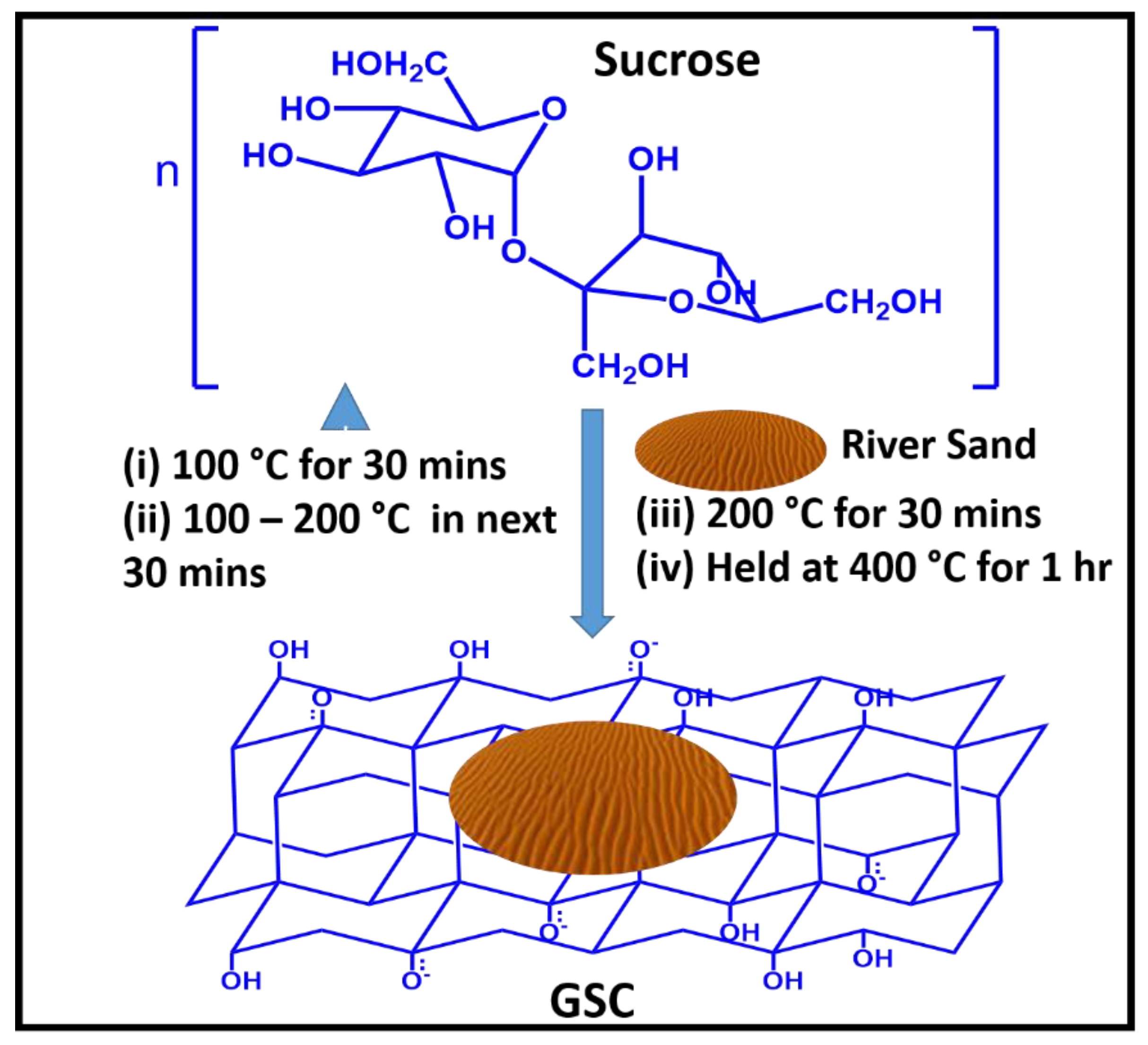
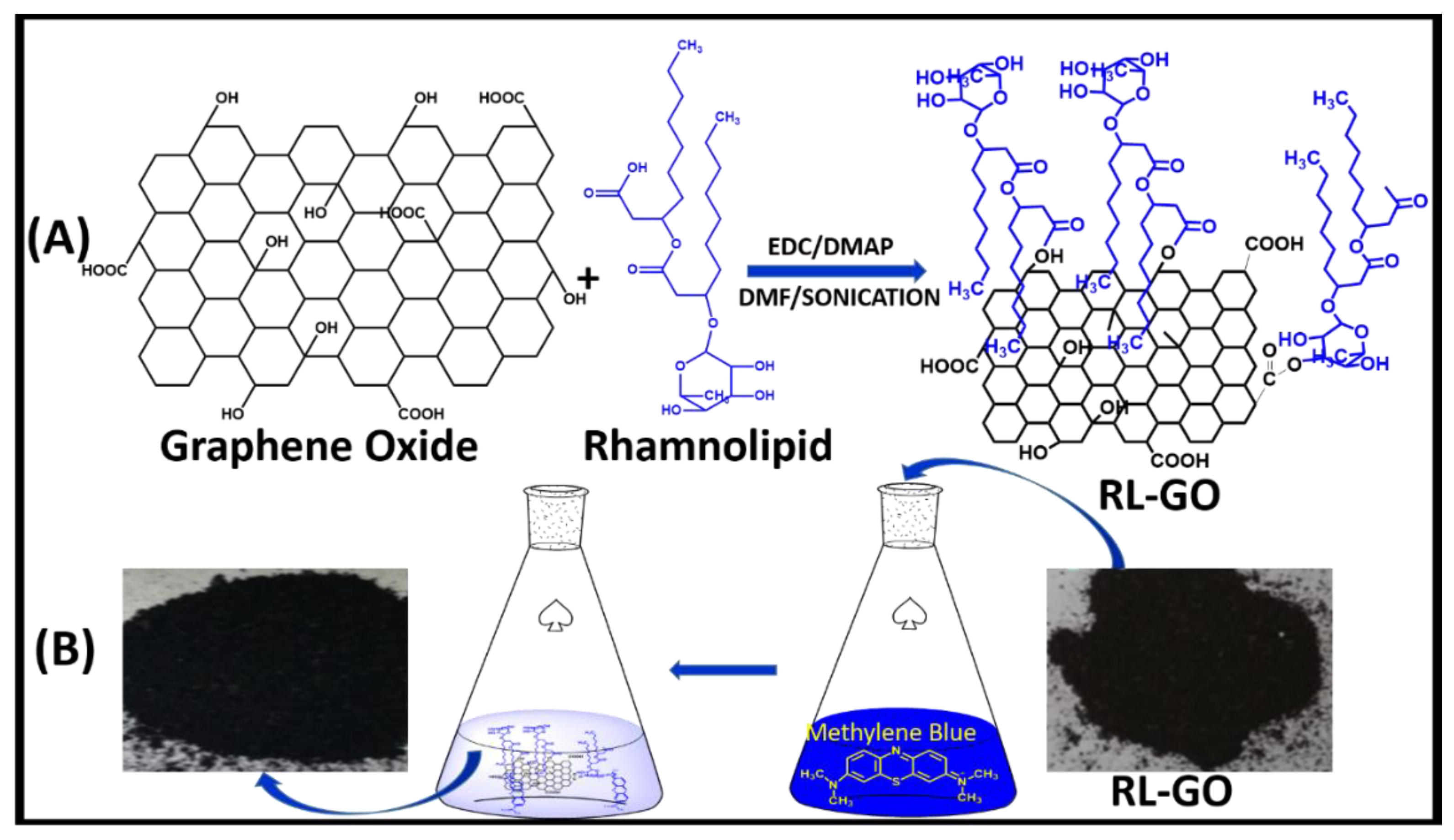


| Nanofiber Material | Target Pollutant | Pollutant Removal Efficiency | Method | pH | Ref. |
|---|---|---|---|---|---|
| Citric acid-modified acrylic fibers | Cu(II) and Ni(II) | Ni(II) = 2.55 mg/g at 250 mmol/g Cu(II) = 2.56 mg/g at 250 mmol/g | Grafting, Electrospinning | 6 | [46] |
| Polyacrylonitrile-polypyrrole (PAN/PPy) core/shell nanofibers | Cr (VI) | 28.5 mg/g at 100 mg/L in Cl− | Electrospinning, Adsorption | 2–5 | [34] |
| Chitin nanofibrils | Pb(II), Ni(II),Cd(II), Cu(II) | 303.49 mg/g, 134.72 mg/g 330.15 mg/g, 141.08 mg/g | Mechanical defibrillation | 5 | [47] |
| Poly(vinyl alcohol) (PVA)/NaX nanozeolite | Ni(II) and Cd(II) | 342.8 and 838.7 (mg/g) | Electrospinning | 5 | [48] |
| Phosphorylated PAN-based nanofiber (P-PAN) mat | Ag(I), and Cd(II), Pb(II), Cu(II) | 102.40 mg/g, and 18.89 mg/g, 98.06 mg/g, 78.03 mg/g | Electrospinning and chemical modification | 6 | [49] |
| Silk Fibroin/cellulose acetate-blend nanofibers | Cu(II) | 22.8 mg/g | Electrospinning | - | [50] |
| Nanofibers with sodium titanate (TNF-A), potassium/sodium titanate (TNF-B), potassium titanate (TNF-C) | Ba(II) (as substitute of 226Ra(II)) and Pb(II) | Ba(II)+—1.93 mmol (TFN-A), 1.67 (TFN-B), 1.74 (TFN-C) Pb(II)—1.91 mmol (TFN-A), 1.88 (TFN-B), 1.79 (TFN-C) | Chemical approaches | 6–7 | [51] |
| Cysteine-modified polypyrrole propylic acid nanofibers | Cr(III) | 1.75 g Cr/g of polymer | Electrochemical template-directed method | 3.28 | [42] |
| Iron oxide–alumina mixed nanocomposite fibers using Polyvinylpyrrolidone (PVP) | Ni(II), Hg(II), Cu(II), Pb(II) | 32.36 mg/g, 63.69 mg/g, 4.98 mg/g, 23.75 mg/g | Electrospinning, Sol gel | 6 | [52] |
| Sodium titanate nanofibers | (a) Ni(II), Zn(II), Cu(II), and Cd(II) (b) Pb(II) | (a) 60, 83, 115, and 149 mg/g (b) 250 mg/g | Hydrothermal | (a)6.5 (b) 4 | [53] |
| Rhodanine and poly(methyl methacrylate) | Ag(I) and Pb(II) | 140 and 115 mg/m2 | Electrospinning | - | [40] |
| S. No | Metal Oxide | Target Dye | Removal Efficiency | pH | References |
|---|---|---|---|---|---|
| 1 | Cobalt (Co) and nickel (Ni) oxide (Co0.4Ni0.4O0.2) | Methylene blue (MB) and Procion red (PR) | MB—20% PR—70% | 9.5 | [86] |
| 2 | Pectin-thorium (IV) tungstomolybdate (Pc/TWM) | Methylene Blue | 76% | - | [87] |
| 3 | Horseradish peroxidase (HRP) was encapsulated in phospholipid-template titania particles | Direct Black-38 | 79.72% | 6–9 | [88] |
| 4 | Fe3O4-ZnO magnetic semiconductor nanocomposite | Methylene Blue, Methyl Orange, Rhodamine B | - | Photo degradation | [89] |
| 5 | Magnetic Fe3O4/ZnCr-LDH (Layered double hydroxide) | Methyl Orange (MO) | 81.23% | 6.4–7.3 | [90] |
| 6. | BiFeO3/a-Fe2O3 core/shell composite | MO | 80% | 5.2 | [91] |
| 7 | Fe-Al binary oxide (5:5) | AB25, AO7, MB | 100%, 98%, 88% | - | [92] |
| 8 | Chromium spheres | Congo Red | 75% | - | [93] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabeela Nasreen, S.A.A.; Sundarrajan, S.; Syed Nizar, S.A.; Ramakrishna, S. Nanomaterials: Solutions to Water-Concomitant Challenges. Membranes 2019, 9, 40. https://doi.org/10.3390/membranes9030040
Nabeela Nasreen SAA, Sundarrajan S, Syed Nizar SA, Ramakrishna S. Nanomaterials: Solutions to Water-Concomitant Challenges. Membranes. 2019; 9(3):40. https://doi.org/10.3390/membranes9030040
Chicago/Turabian StyleNabeela Nasreen, Shaik Anwar Ahamed, Subramanian Sundarrajan, Syed Abdulrahim Syed Nizar, and Seeram Ramakrishna. 2019. "Nanomaterials: Solutions to Water-Concomitant Challenges" Membranes 9, no. 3: 40. https://doi.org/10.3390/membranes9030040
APA StyleNabeela Nasreen, S. A. A., Sundarrajan, S., Syed Nizar, S. A., & Ramakrishna, S. (2019). Nanomaterials: Solutions to Water-Concomitant Challenges. Membranes, 9(3), 40. https://doi.org/10.3390/membranes9030040







